Abstract
Background
Alcohol consumption is typically correlated with the alcohol use behaviors of one’s peers. Previous research has suggested that this positive relationship could be due to social selection, social influence, or a combination of both processes. However, few studies have considered the role of shared genetic and environmental influences in conjunction with causal processes.
Methods
The current study uses data from a sample of male twins (N=1790) who provided retrospective reports of their own alcohol consumption and their peers’ alcohol related behaviors, from adolescence into young adulthood (ages 12–25). Structural equation modeling was employed to compare three plausible models of genetic and environmental influences on the relationship between phenotypes over time.
Results
Model fitting indicated that one’s own alcohol consumption and the alcohol use of one’s peers are related through both genetic and shared environmental factors and through unique environmental causal influences. The relative magnitude of these factors, and their contribution to covariation, changed over time, with genetic factors becoming more meaningful later in development.
Conclusions
Peers’ alcohol use behaviors and one’s own alcohol consumption are related through a complex combination of genetic and environmental factors that act via correlated factors and the complementary causal mechanisms of social selection and influence. Understanding these processes can inform risk assessment as well as improve our ability to model the development of alcohol use.
Keywords: social selection, social influence, alcohol use, twin modeling
Introduction
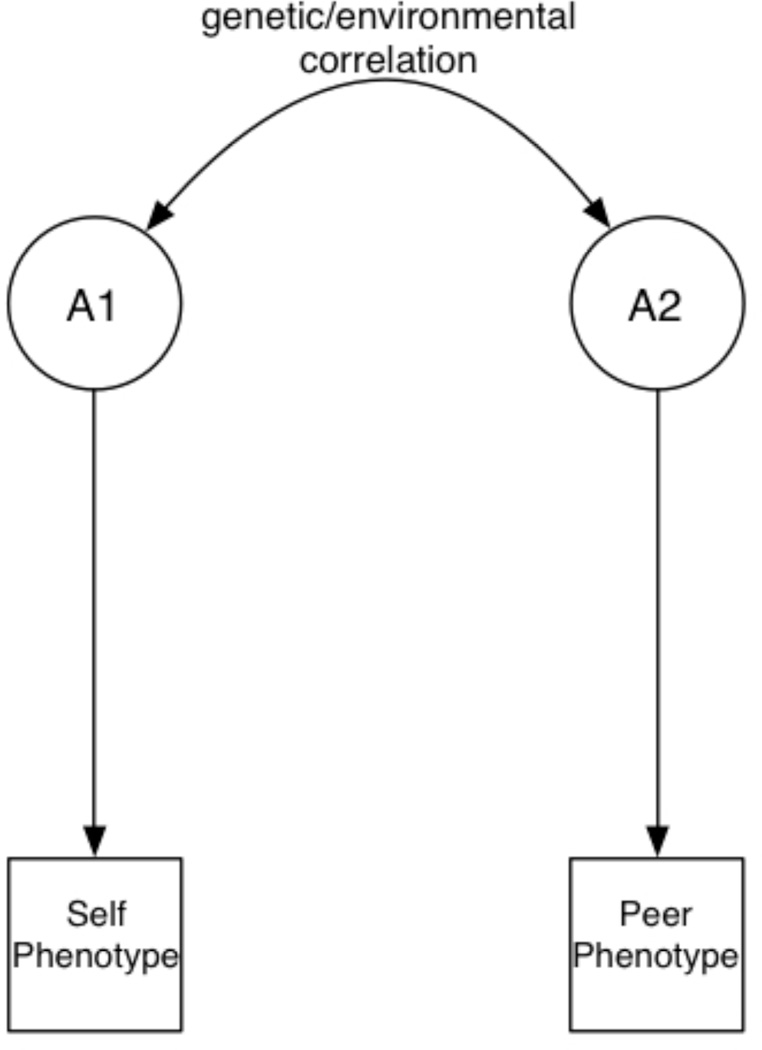
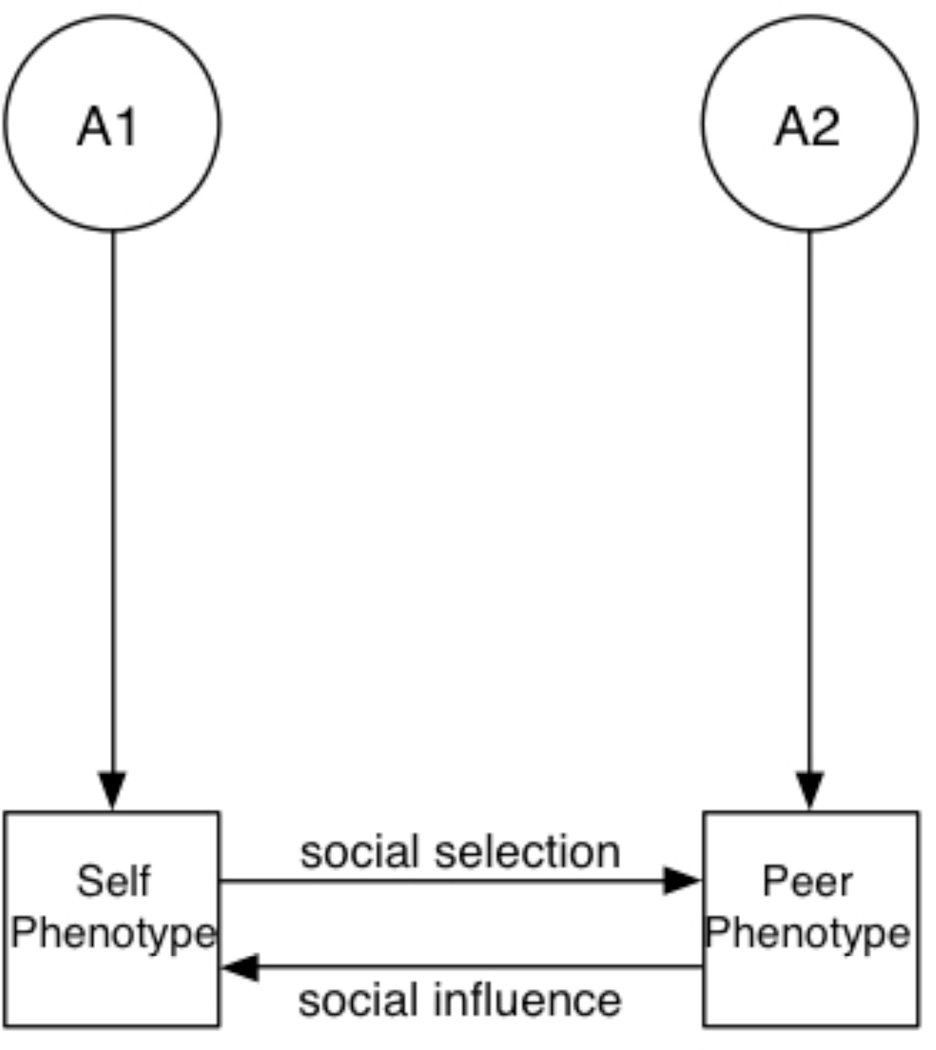
Alcohol use typically begins during adolescence, with over 70% of US high school students (grades 9–12) reporting that they have consumed at least one drink, and over 20% reporting that they first drank alcohol by age 13 (Centers for Disease Control and Prevention, 2012). These early experiences with alcohol typically occur in social contexts (Ennett et al., 2006; Henry et al., 2005; Strycker et al., 2003). The influence of peers on one’s drinking behaviors has been widely studied. Two basic, complementary causal mechanisms (Caspi, 2002) have been proposed to explain the association between one’s own drinking and that of one’s peers: social selection and social influence. Individuals are capable of selecting peers based on their own drinking behaviors; they also might modify their own drinking behavior in response to peer influences (Bauman and Ennett, 1994; Cullum et al., 2012; Henry et al., 2005; Kiuru et al., 2010). The terms selection and influence have different denotations across studies. In this study, we will use them to refer to causal processes that are distinct from latent genetic and environmental correlations (Figure 1). We note that, while our use of the term “causal” is consistent with much of the literature, the “causal” processes described herein should be interpreted as theoretically causal; the nature of the data, we cannot formally ascribe causation. The implications of distinct mechanisms will be discussed.
Figure 1.
Multiple potential relationships underlie phenotypic associations between one’s own phenotype and that of one’s peers. As depicted in panel A, these phenotypes could be genetically or environmentally correlated: some of the genes that influence liability to alcohol consumption could also influence affiliation with peers who have alcohol problems. Similarly, environmental factors could be shared across phenotypes. In the current study such factors are unmeasured, but examples include parental monitoring and neighborhood quality. As stated elsewhere, because genetic and environmental correlations exist at a level beyond the manifested phenotypes, a change in one phenotype will not necessarily result in a change to the other.
Panel B depicts the causal processes of social selection and social influence. In the former, an individual’s alcohol consumption directly influences his peers’ alcohol phenotype, because he is selecting those peers to match his own alcohol use or because their use changes to match his. Social influence operates in the other direction: peers’ alcohol use changes one’s own alcohol use, potentially through overt or implied peer pressure or social modeling. Unlike genetic/environmental correlation, the critical implication of these causal paths is that a change in one phenotype will necessarily result in a change to the downstream phenotype. For example, if one’s peers’ alcohol use declines, one’s own alcohol will also decline.
Evidence of social influence has been reported among longitudinal studies of college students (Cullum et al., 2012) and adolescents (Urberg et al., 1997; Wills and Cleary, 1999). Others have reported reciprocal effects between one’s own drinking and that of one’s peers. Two longitudinal studies of Finnish adolescents found evidence of both selection and influence (Kiuru et al., 2010; Mercken et al., 2012). In community-based samples of US adolescents followed longitudinally, initial levels of peer alcohol use were predictive of later adolescent alcohol use and vice versa (Curran et al., 1997; Simons-Morton and Chen, 2006; Stappenbeck et al., 2010). Still other research suggests that, when controlling for social selection, social influence is largely inconsequential (Mundt et al., 2012). Not all studies explicitly model both selection and influence (e.g., (Cullum et al., 2012), and interpretation of results is complicated if selection is not controlled for when examining influence (Bauman and Ennett, 1994; Bauman and Ennett, 1996; Jaccard et al., 2005; Madden et al., 2002; Urberg et al., 1997).
Cruz and colleagues (2012) examined social influence using a genetically informative twin and family sample. Such studies allow the partitioning of variance into that attributable to genetic versus environmental factors, and they enable the researcher to control for genetic/environmental correlation (Figure 1A, also known as shared liability). They found that, after controlling for the effects of genetic and shared environmental correlations, which they refer to as selection, peer network substance use predicted drinking behavior in adolescents. Likewise, another genetically informative study (Harden et al., 2008) found that genetic factors influencing the target’s substance use were also related to the substance use of the target’s peers. Once these influences were accounted for, peer behavior predicted target substance use. Thus, there is prior evidence from genetically informative studies that both genetic/environmental correlation and social influence play a role in determining an individual’s substance use. However, these studies did not test whether social selection (Figure 1B) contributed to the association between one’s own substance use and that of their peers.
The current study examines how a person’s alcohol consumption is related to their peers’ alcohol use from early adolescence through early adulthood, in a population-based sample of male twins. We fit three longitudinal models that represent alternative causative and correlative relationships between individual and peer alcohol use. These models capitalized on the genetically informative nature of twin samples in that we were able to investigate whether different sources of covariance – genetic and/or environmental – were operating in a causal or correlated manner. Unless otherwise noted, for the purposes of this report, we restrict our use of the terms social influence and social selection to theoretically causal processes that arise apart from correlation/shared liability processes. In other words, we explicitly examine both social influence and social selection, while controlling for genetic and environmental influences on one’s own alcohol use and perceived peer alcohol use. This is accomplished by including causal pathways in both directions in a subset of our models (available in the Supplementary Material). A critical implication of causal processes is that, if the expressed phenotype changes, the downstream phenotype will be impacted. In contrast, if only genetic/environmental correlation is at play, factors that modify one’s own alcohol use will not necessary impact the alcohol use of one’s peers. Elucidating the mechanistic etiology of alcohol consumption and peer alcohol use – causal or otherwise - could potentially improve prevention and treatment efforts in that the impact of external social processes and that of genetic/environmental factors can be explicitly evaluated and, in some cases, modified.
Methods
Sample
The current report is based on data collected in wave 3 of structured clinical interviews with adult Caucasian male twins participating in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD, Kendler & Prescott, 2006). Participants were originally ascertained from the population-based Virginia Twin Registry, were born between 1940 and 1974, and were 24–62 years old (mean=40.3, SD=9.0) at the time of their interview. Much of the sample (39%) had completed an associate’s degree or higher; 24% attended vocational school or some college; 29% had a high school diploma or GED; and 8% did not complete high school. Most were married (65%) or cohabitating (4%); 12% were divorced/separated/widowed. Informed consent was obtained after an explanation of the research protocol was provided; the Institutional Review Board at Virginia Commonwealth University approved the project. Interviews were conducted face-to-face or over the phone by trained interviewers. Zygosity was determined using a combination of self-report measures, photographs, and genotyping. In the current study, partial or complete data were available for N=1790 individuals (including 466 complete monozygotic [MZ] and 283 complete dizygotic [DZ] twin pairs).
Measures
Alcohol consumption
Participants took part in a life history calendar interview (Cohen et al., 2003; Freedman et al., 1988). They were asked to report their average monthly alcohol consumption (frequency and quantity) at different ages. These data were then combined into ages that correspond to meaningful developmental age ranges, as reported previously in Edwards and Kendler (2013): the ages used for the current report were 12–14, 15–17, 18–21, and 22–25. Mean alcohol consumption was calculated for these age ranges, with abstainers assigned values of 0. To each average consumption score, 1 was added, followed by a natural log-transformation to adjust for non-normality. Thus, the lowest possible log-transformed score was 0, corresponding to abstainers. These variables will be referred to as SELF1-SELF4.
Perceived peer alcohol use
Participants also reported whether, during each of the above age ranges, none, a few, some, most, or all of their peers drank alcohol, got drunk, or had problems with alcohol (e.g., had accidents, got hangovers, etc.). As the peers themselves did not report these behaviors, they are strictly perceived peer alcohol use. Peers were defined as “people you would have seen regularly and spent time with.” These data were subjected to a factor analysis to construct a single score meant to capture the extent to which an individual was exposed to peers’ alcohol use behaviors. These factor scores were used in all subsequent analyses, and will be referred to as PEER1-PEER4.
Statistical Analyses
Descriptive statistics were obtained using JMP Pro 10.0.2 (Cary, NC) and SAS 9.2 (Cary, NC). Twin modeling was conducted with OpenMx (Boker et al., 2011), using full information maximum likelihood. We tested three plausible models of how additive genetic (A), shared environmental (C), and unique environmental (E) factors might explain covariation between SELF and PEER across time. These models have been described previously (Kendler et al., 2008) and are provided in the Supplementary Material. Briefly, model 1 (Supplementary Figure 1A) is a simple model representing a causal mechanism, with three key components: i) latent factors (A1self-A8peer) loading (via La1–La8) onto each of 8 observed variables (4 measures each of SELF and PEER); ii) forward transmission (e.g., B12 from SELF1 to PEER1) of genetic/environmental influences across time within a phenotype (SELF or PEER); and iii) bidirectional causation within time across phenotypes, such that SELF1 has a causal effect on PEER1 (via B15), and vice versa (via B51). Model 2, a correlated factors model, postulates that the within- and cross-time correlations between SELF and PEER result from two correlated latent factors, the first of which loads directly onto SELF1-SELF4 and the second of which loads onto PEER1-PEER4. Time-specific factors also load onto each phenotype, but no forward transmission pathways are included in this model. Model 3 is a hybrid between Models 1 and 2, and has three key components: i) two uncorrelated latent factors, with the first loading on SELF1-SELF4 and the second loading onto PEER1-PEER4; ii) forward transmission within phenotype; and iii) bidirectional causal paths within time across phenotypes. Time-specific factors also load onto each phenotype. More detailed information is provided in the Supplementary Material.
We employed a two-stage modeling approach. In the first stage, we tested the three models described above, and selected a structure based on agreement between Akaike’s Information Criterion (AIC) and Bayesian Information Criterion (BIC), where a lower AIC/BIC optimizes the balance between explanatory power and parsimony. We began testing with the structure of C, leaving A and E in a hypothesis-free state (i.e., a Cholesky decomposition). Once the structure for C was selected, it was fixed and the three possible A structures were compared; once the A structure was selected, it too was fixed and the three possible E structures were compared. After the higher-order structures were fixed for A, C, and E, we conducted the second stage of model fitting. This involved testing hypothesis-based models nested within the previously identified ACE structure, again selecting submodels based on AIC/BIC as well as the p-value of the χ2 test resulting from a comparison of −2 times the log likelihoods (−2LL) of the full and nested models. Our selection of the factor structure within each source of variance was based on agreement between AIC and BIC. This approach balances fit and parsimony, though we prioritized testing specific hypotheses rather than specifying the most parsimonious model possible. Different approaches could result in a distinct series of model-fitting decisions.
Results
Descriptive statistics
Mean monthly alcohol consumption, prior to transformation, ranged from 0.67 (SD=5.35) drinks at ages 12–14 to 39.55 (SD=75.66) at ages 22–25. After transformation, the total phenotypic variance of SELF1–4 increased from 0.27 to 2.84 between ages 12–14 and ages 22–25. The variance of PEER1–4 increased from 0.85 to 1.29. Phenotypic twin correlations (Supplementary Table 1) were similar for MZ and DZ twins at age 12–14, but at older ages, MZ correlations were modestly to substantially higher than DZ correlations. This was the case for both within-phenotype and cross-phenotype correlations, suggesting that genetic factors become increasingly relevant to phenotypic covariation over time.
Testing the overall factor structure of environmental and genetic influences
We first compared the three structures for shared environmental (C) sources of variance, while the genetic (A) and unique environmental (E) variance structures were in a hypothesis-free state (i.e., in a Cholesky decomposition). Table 1 provides AIC/BIC values and −2 times the log likelihoods (−2LL) of fit for the three hypothesized latent factor structures. For both C and A, based on the best AIC/BIC values, we selected the structure shown in Supplementary Figure 1b, in which one common factor loads onto SELF1-SELF4, and a second common factor loads onto PEER1-PEER4; these two factors are correlated (rC=0.99, which was the upper bound of this path in the model, but see below; rA=0.83). The best-fitting model for E was the 8-factor model described previously, and depicted in Supplementary Figure 1a, with forward transmission within phenotype across time, and both social influence and social selection paths.
Table 1.
Model fitting for overall structure of genetic and environmental factors.
| # | Description | Comparison | −2LL | df | AIC | BIC |
|---|---|---|---|---|---|---|
| TEST C | ||||||
| 1 | 8 factors for C; bidirectional causation; forward transmission; Cholesky for A and E | n/a | 36530.08 | 14192 | 8146.08 | −62075.04 |
| 2 | 2 correlated latent C factors; C specifics; Cholesky for A and E | n/a | 36533.34 | 14197 | 8139.339 | −62106.523 |
| 3 | 2 uncorrelated latent C factors; C specifics; forward transmission; reciprocal causation; Cholesky for A and E | n/a | 36526.63 | 14184 | 8158.627 | −62022.912 |
| TEST A | ||||||
| 4 | 8 factors for A; bidirectional causation; forward transmission; 2 correlated C factors with specifics; Cholesky for E | within Model 2 | 36548.66 | 14211 | 8126.662 | −62188.471 |
| 5 | 2 correlated latent A factors; A specifics; Cholesky for E | within Model 2 | 36555.31 | 14216 | 8123.311 | −62216.562 |
| 6 | 2 uncorrelated latent A factors; A specifics; forward transmission; reciprocal causation; Cholesky for E | within Model 2 | 36548.66 | 14203 | 8142.655 | −62132.895 |
| TEST E | ||||||
| 7 | 8 factors for E; bidirectional causation; forward transmission; 2 correlated factors with specifics for A and C | within Model 5 | 36574.59 | 14230 | 8114.586 | −62294.558 |
| 8 | 2 correlated E factors; E specifics | within Model 5 | 36951.58 | 14235 | 8481.576 | −61952.309 |
| 9 | 2 uncorrelated E factors; E specifics; forward transmission; reciprocal causation; | within Model 5 | 36893.47 | 14222 | 8449.475 | −61920.086 |
C=shared environmental variance; A=genetic variance; E=unique environmental variance; −2LL=−2 times log likelihood; df=degrees of freedom; AIC=Akaike’s Information Criterion; BIC=Bayes Information Criterion
Italic text indicates the selected model for each source of variance.
Testing components of model substructure
After selecting the structures described above for A, C, and E, we tested a series of models nested within that structure. These submodels and the corresponding statistics are described in Table 2. Social selection pathways from SELF to concurrent PEER were constrained to be equal across the different ages (Model 15). In addition, the correlation between common C factors could be fixed to 1 (Model 20). Other nested models resulted in a decline in fit and their constraints were not included in the final model. The final models are presented in Figure 2A–C.
Table 2.
Model fitting procedure for submodels.
| # | Description of Nested Model | Comparison | −2LL | Δdf | Δ Χ2 |
p-value | ΔAIC | ΔBIC |
|---|---|---|---|---|---|---|---|---|
| 10 | Set C common factor loadings on SELF to be equal over time | vs. model 7* | 36647.45 | 3 | 72.86 | 0 | 66.86 | 52.021 |
| 11 | Set C common factor loadings on PEER to be equal over time | vs. model 7 | 36594.26 | 3 | 19.67 | 0 | 13.67 | −1.175 |
| 12 | Set A common factor loadings on SELF to be equal over time | vs. model 7 | 36665.49 | 3 | 90.91 | 0 | 84.91 | 70.061 |
| 13 | Set A common factor loadings on PEER to be equal over time | vs. model 7 | 36609.58 | 3 | 34.99 | 0 | 28.99 | 14.151 |
| 14 | Within E, drop causation from SELF to PEER | vs. model 7 | 36588.8 | 4 | 14.21 | 0.01 | 6.21 | −13.582 |
| 15 | Within E, constrain causation from SELF to PEER to be equal across waves | vs. model 7 | 36575.62 | 3 | 1.03 | 0.79 | −4.97 | −19.814 |
| 16 | Within E, drop causation from PEER to SELF | vs. model 15 | 36608.27 | 4 | 32.65 | 0 | 26.65 | 4.860 |
| 17 | Within E, constrain causation from PEER to SELF to be equal across waves | vs. model 15 | 36582.11 | 3 | 6.49 | 0.09 | 0.49 | −14.35 |
| 18 | Within E, equate forward transmission within SELF | vs. model 15 | 36625.65 | 2 | 50.04 | 0 | 46.04 | 36.140 |
| 19 | Within E, equate forward transmission within PEER | vs. model 15 | 36588.26 | 2 | 12.65 | 0 | 8.65 | −1.250 |
| 20 | Set rC to 1 | vs. model 15 | 36575.46 | 1 | −0.15 | 1 | −2.15 | −7.101 |
| 21 | Set rG to 1 | vs. model 20 | 36592.52 | 1 | 17.05 | 0 | 15.05 | 10.104 |
Model 7 refers to Model 7 in Table 1.
Abbreviations as in Table 1, plus the following: Δdf=change in degrees of freedom between the nested model and its comparison model; ΔΧ2=chi-square statistic, based on change in −2LL; ΔAIC/ΔBIC =change in AIC/BIC value between the nested model and its comparison model.
Italic text indicates that the nested model was selected, and subsequent models were fit within the context of that model.
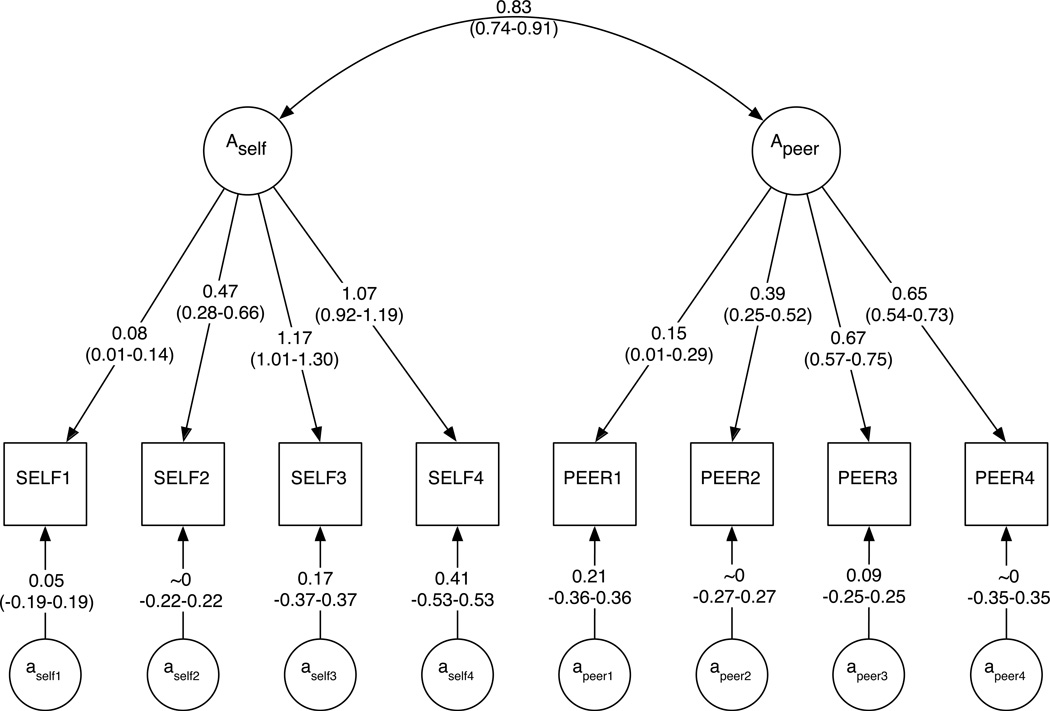
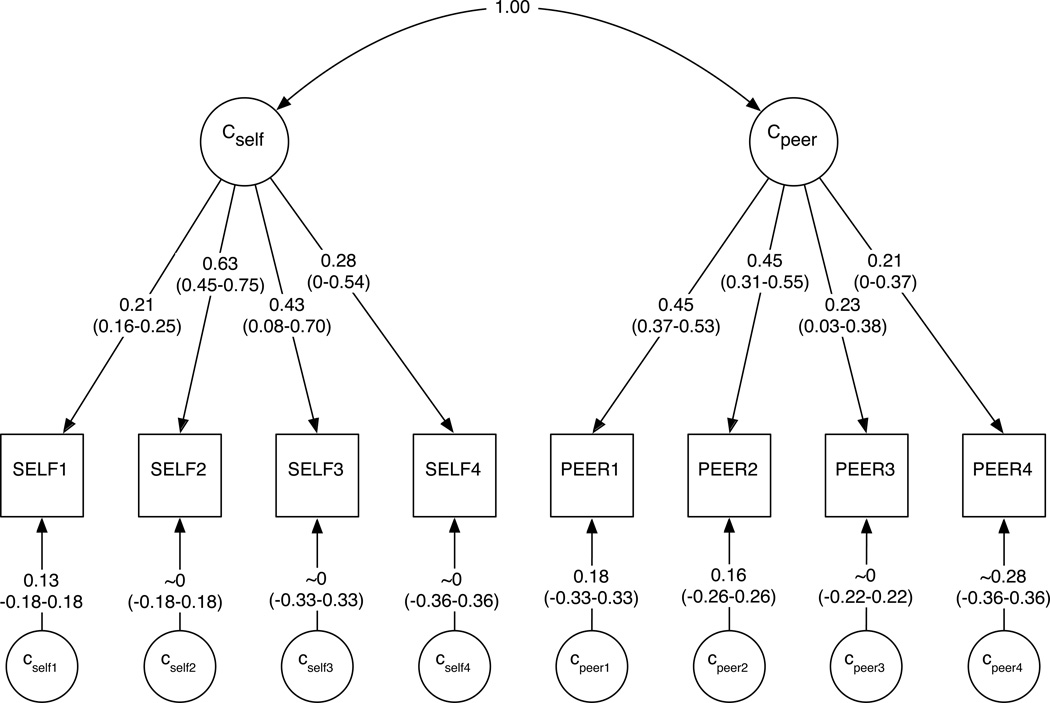
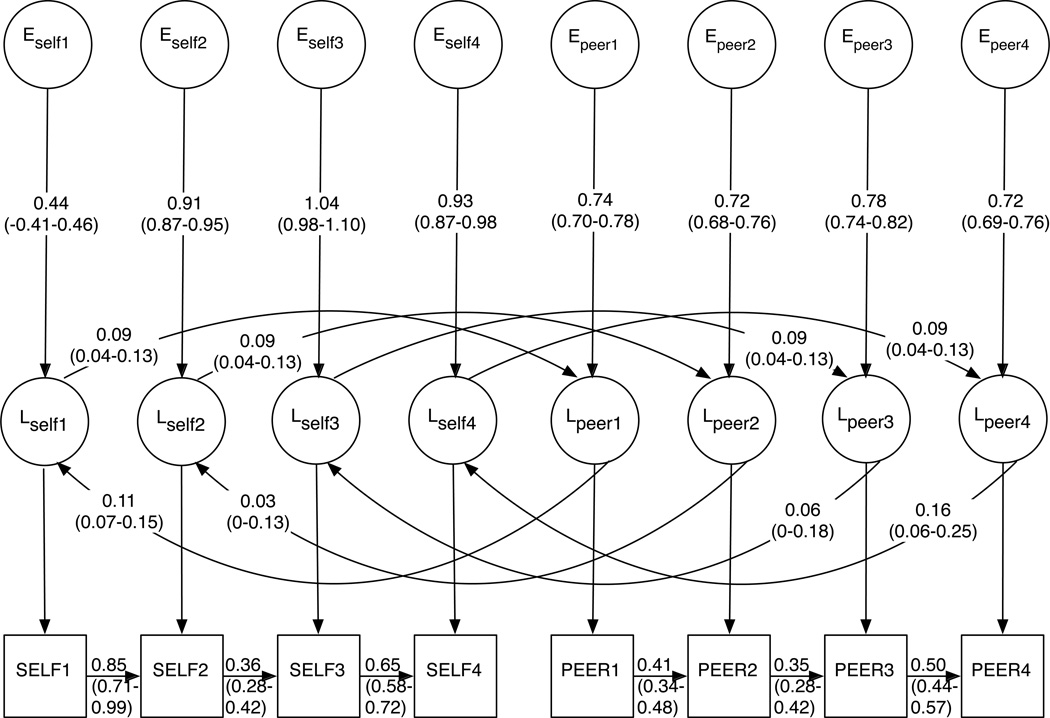
Figure 2.
Panels A–C depict final path estimates (95% confidence intervals) for genetic, shared environmental, and unique environmental components of the model, respectively. Confidence intervals for all paths are provided in Supplementary Table 4. In Figure 2C, the Lself/peervariables enable E-specific causation pathways, which are bidirectional. Further details on model components are available in the Supplementary Material.
Sources of variance and covariance in the final model
Unstandardized and standardized variance/covariance matrices are available in Supplementary Table 2. The heritability of SELF increased from 0.03 at ages 12–14 to 0.46 at ages 22–25. Shared environmental influences correspondingly decreased over time, from 0.22 to 0.03. Unique environmental factors account for a substantial proportion of the total variance at each age range (0.43–0.75). Results for PEER were similar, though its heritability in early adulthood (0.33) was lower than for SELF. Shared environmental influences became less relevant over time (from 0.28 down to 0.09), while unique environmental influences were temporally stable (0.58–0.62).
As stated above, A and C influences were significantly correlated across SELF and PEER (rA=0.83 and rC=1.00 in Figure 2A and Figure 2B, respectively), and the correlated common factors accounted for the majority of A and C variance. However, phenotype- and time-specific factors also accounted for a proportion of the variance (e.g., path estimates of 0–0.41 for SELF in Figure 2A; see Supplementary Tables 2 and 3). In all but two cases (specific A influences on PEER1 and specific C influences on PEER4), the majority of genetic and shared environmental variance for SELF and PEER was derived from correlated common factors. Phenotype- and time-specific A and C factors accounted for 6% or less of the total phenotypic variance.
As there were no correlated common E factors, unique environmental covariance between SELF and PEER was limited to that derived from causal pathways. Estimates for these paths were low, and in some cases were not significantly different from 0, though tests indicated that causal paths in either direction could not be removed completely from the model (Table 2, Models 14 and 16). While the final model demonstrated that causal influences are relevant with regards to non-shared environmental factors, the majority of covariation between SELF and PEER was attributable to the correlation between the genetic and shared environmental common factors (Supplementary Table 2). Overall, A covariance increased over time (alongside heritability), while C covariance decreased.
Discussion
These analyses evaluated the extent to which one’s own alcohol consumption (SELF) and the perceived drinking behavior of one’s peers (PEER) are phenotypically related due to theoretically causal relationships (social selection and social influence) versus shared genetic and/or environmental liabilities. The current findings indicate that both shared liability and causal factors are relevant. Most covariation is attributable to shared liability, and only non-shared environmental influences acted via causal pathways.
We found that genetic and shared environmental influences on alcohol use and perceived peer alcohol use are strongly correlated from early adolescence into early adulthood, and these influences account for the majority of covariation (56–91%) between the two phenotypes. The influence of unique environmental factors – those factors that twins do not share – impact the relationship between PEER and SELF via reciprocal causal pathways. Specifically, non-shared environmental factors influencing one’s own drinking also indirectly impacted exposure to peers’ drinking behaviors via social selection. This effect was reciprocal, in that non-shared environmental factors influencing peer affiliation – and thus exposure to peers’ drinking behaviors – indirectly influenced one’s own concurrent (and subsequent) drinking through social influence. Thus, while we observed modest evidence of theoretically causal social selection and social influence, genetic and environmental correlation dominated the etiology underlying the association between alcohol consumption and peer alcohol problems.
The genetic correlation between alcohol use and peer alcohol use (rA=0.83, Figure 2A) is effectively gene-environment correlation (rGE). However, it is not clear from these analyses whether this is active vs. evocative rGE (Jaffee and Price, 2008), or a combination thereof. In the former, one’s genotype not only affects their own phenotype, it also makes them more likely to select a particular environment (e.g., an individual’s genetically influenced impulsive behavior might result in exposure to dangerous situations). In the latter, one’s genetically influenced phenotype evokes a reaction among others (e.g., a child who exhibits problem behavior might experience high levels of physical discipline). Here, it seems plausible that individuals with a genetic liability toward high alcohol consumption might also have a genetic propensity to seek out peers who use alcohol excessively (active rGE), and previous research in this sample has identified a strong genetic component to choice of peers (Kendler et al., 2007). Evocative rGE cannot be ruled out, though how it would be operating in this context in a way that is not accounted for by other components of the model is not clear. Regardless of the underlying mechanism, genetic influences on alcohol use and perceived peer alcohol use become stronger over time: not only does the heritability of each phenotype increase over time, the genetic covariance between phenotypes also increases.
The observed shared environmental correlation (rC) indicates that those family-level factors (or other environmental factors which increase the similarity of both members of a twin pair) that increase one’s own drinking also impact alcohol-based peer affiliation. The influence of shared environmental factors – on SELF and PEER themselves, and on their covariation – decreases from adolescence into young adulthood. Thus, in adulthood, genetic correlation rather than shared environmental liability accounts for the majority of covariation.
Genetic and shared environmental correlations between phenotypes indicate that these latent factors contribute directly not just to one’s own alcohol consumption, but also to affiliation with peers who exhibit particular alcohol-related behaviors. Cruz and colleagues (2012) referred to this process as genetic and environmental selection, though here we have used selection to refer to a distinct process. One implication of this shared liability is that a change in one’s own alcohol use would not necessarily correspond to a change in peers’ alcohol use. In other words, the model suggests that an individual with a genetic liability toward high levels of alcohol consumption is also genetically liable to select peers who drink to excess. Even if one’s own drinking changed in response to an external factor, the genetic liability toward selecting heavy-drinking peers would remain unchanged. This contrasts with the implications of social influence and selection in the form of causal pathways, described below.
Unique environmental influences on the covariation between these phenotypes are complicated. These causal pathways from PEER to SELF potentially represent social influence: one’s peer’s drinking behaviors impact one’s own consumption. This effect is specific to non-shared (unique, or E) environmental factors contributing to affiliation with drinking peers. Specific examples of such factors were not measured or included in the current models, but could include exposure to different peers in school, or perhaps stressful life events experienced by only one member of a twin pair. The causal pathways from SELF to PEER suggest a different relationship: rather than an individual being influenced by his peers, in this case the individual is the source of influence on his peers, or his alcohol consumption influences what kind of peers he affiliates with. These paths can be conceptualized as a form of social selection. A critical implication of these causal paths is that changes in non-shared environmental influences on peer alcohol use would necessarily result in corresponding changes in one’s own use, and vice versa.
Social selection and influence account for little of the total phenotypic variation in SELF and PEER. “New” unique environmental factors become relevant at each successive age range, and the impact of earlier non-shared environmental factors is continued via forward transmission. The low parameter estimates for the social influence paths mean that little of the total phenotypic variance in SELF1–4 originates from E5-E8 (see Figure 2C); similarly, little of the total variance in PEER1–4 originates from E1–E4. Still, these causal influences could not be removed from the model. The fact that social selection paths could be constrained to be equal over time suggests that one’s influence on one’s E-related affiliation with peers is not highly variable, even though one’s peer group might change. In contrast, we could not constrain the social influence paths to be equal, indicating that the extent to which peers’ drinking influences one’s own drinking varies from adolescence into adulthood.
The current findings are broadly comparable to those reported by others who have employed genetically informative samples to control for genetic and environmental selection when exploring social influence. Cruz et al. (2012) found that genetic and shared environmental correlation contributed to the association between peer and adolescent drinking, and was supplemented by causal influences – specifically, peers’ drinking had a causal impact on adolescents’ own drinking. Similarly, Harden and colleagues (2008) reported support for both rGE and social influence; shared environmental factors were not found to be significant in that sample, which focused on adolescent substance use and peer groups. Finally, Hill and colleagues (2008) found evidence of genetic correlation, but not of social influence. All these reports used different modeling approaches than those employed in the current study.
Few other studies have controlled for genetic/environmental correlation while examining causal processes between self and peer alcohol use phenotypes. Nevertheless, previous research has identified genetic influences on peer substance use (Agrawal et al., 2010; Dick et al., 2007); there is support for rGE in the context of peer and self substance use (Fowler et al., 2007; Loehlin, 2010; Wills and Carey, 2013); and others have utilized genetically informative samples when exploring the relationship between peer and self alcohol use (Poelen et al., 2007; Scholte et al., 2008) without explicitly modeling selection and influence. Our results are also consistent with previous studies that have found that the relative impact of social influence is mitigated once selection processes are accounted for (Jaccard et al., 2005; Poelen et al., 2007), though in the current study those influences remain statistically significant. Evidence from additional genetically informative samples is needed to determine the extent to which genetic/environmental correlation, social selection, and social influence contribute to the phenotypic association between individual and peer substance use.
In summary, these results indicate that the covariation between one’s alcohol consumption and the drinking behaviors of one’s peers is the result of a complex combination of genetic and environmental influences, some of which are shared directly, while others operate in a causal fashion. Molecular genetic studies could be designed to identify specific genetic variants underlying these processes, though as with any complex trait, genetic effect sizes are likely to be quite small, and are potentially susceptible to moderation by other variants and by environmental factors. Perhaps more plausibly, potential environmental risk factors – e.g., parental monitoring or neighborhood characteristics (Chuang et al., 2005) – could be explicitly included in a model to determine the extent to which they influence alcohol related outcomes.
Limitations
We rely on retrospective reports, though the use of a life history calendar has been shown to mitigate problems with accurate recollection. Variation in age at interview potentially introduces bias into the accuracy of recall; however, tests wherein age at interview was included as a covariate resulted in the same model structure presented here. We are also limited to self-reports, which might be subject to bias. Similarly, some evidence suggests that self-reports of peer behavior overestimate the similarity between one’s own behavior and that of their peers (Hill et al., 2008). Data were only available for men, and there is potential for gender differences in the role of peer influences on drinking (Dick et al., 2007).
Our treatment of lifetime abstainers has implications for the interpretation of heritability estimates. Lifetime abstainers are coded as “0” at each wave rather than as missing. Because we did not explicitly model alcohol use initiation, the heritability estimates presented are potentially inflated: some proportion of the heritability of alcohol use is transmitted through a contingency pathway from initiation (as described in Kendler et al., 1999), but that source of genetic variation is not parsed out in the current study. Finally, as noted elsewhere, the processes and paths we refer to as “causal” are theoretically so, but given the nature of the study, we cannot ascribe causality with certainty.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants AA021399, AA107828, AA011408, and DA025109. The Virginia Twin Registry (now part of the Mid-Atlantic Twin Registry) directed by L. Corey and Lindon Eaves, provided subject access. Frank Butera and Karen Hough supervised data collection, Indrani Ray and Steven Aggen provided programming and database management. Other collaborators include Patrick Sullivan and Lenn Murrelle. Additional funding was provided by the Carman Trust and W. Keck, John Templeton, and Robert Wood Johnson Foundations.
References
- Agrawal A, Balasubramanian S, Smith EK, Madden PA, Bucholz KK, Heath AC, Lynskey MT. Peer substance involvement modifies genetic influences on regular substance involvement in young women. Addiction. 2010;105:1844–1853. doi: 10.1111/j.1360-0443.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman KE, Ennett ST. Peer influence on adolescent drug use. Am Psychol. 1994;49:820–822. doi: 10.1037//0003-066x.49.9.820. [DOI] [PubMed] [Google Scholar]
- Bauman KE, Ennett ST. On the importance of peer influence for adolescent drug use: commonly neglected considerations. Addiction. 1996;91:185–198. [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi V. Social selection, social causation, and developmental pathways: Empirical strategies for better understanding how individuals and environments are linked across the life-course. In: Pulkkinen L, Caspi V, editors. Paths to successful development. Personality in the life course. USA: Cambridge University Press; 2002. [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance - United States, 2011. Atlanta, GA: MMWR; 2012. [Google Scholar]
- Chuang YC, Ennett ST, Bauman KE, Foshee VA. Neighborhood influences on adolescent cigarette and alcohol use: mediating effects through parent and peer behaviors. J Health Soc Behav. 2005;46:187–204. doi: 10.1177/002214650504600205. [DOI] [PubMed] [Google Scholar]
- Cohen P, Kasen S, Chen H, Hartmark C, Gordon K. Variations in patterns of developmental transitions in the emerging adulthood period. Dev Psychol. 2003;39:657–669. doi: 10.1037/0012-1649.39.4.657. [DOI] [PubMed] [Google Scholar]
- Cruz JE, Emery RE, Turkheimer E. Peer network drinking predicts increased alcohol use from adolescence to early adulthood after controlling for genetic and shared environmental selection. Dev Psychol. 2012;48:1390–1402. doi: 10.1037/a0027515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullum J, O'grady M, Armeli S, Tennen H. Change and Stability in Active and Passive Social Influence Dynamics during Natural Drinking Events: A Longitudinal Measurement-Burst Study. J Soc Clin Psychol. 2012;31:51–80. doi: 10.1521/jscp.2012.31.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Stice E, Chassin L. The relation between adolescent alcohol use and peer alcohol use: a longitudinal random coefficients model. J Consult Clin Psychol. 1997;65:130–140. doi: 10.1037//0022-006x.65.1.130. [DOI] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends' influences on adolescent drinking: a genetic epidemiological study. Alcohol Clin Exp Res. 2007;31:2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychol Med. 2013;43:1857–1868. doi: 10.1017/S0033291712002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennett ST, Bauman KE, Hussong A, Faris R, Foshee VA, Cai L. The peer contect of adolescent substance use: findings from social network analysis. Journal of Research on Adolescence. 2006;16:159–186. [Google Scholar]
- Fowler T, Shelton K, Lifford K, Rice F, Mcbride A, Nikolov I, Neale MC, Harold G, Thapar A, Van Den Bree MB. Genetic and environmental influences on the relationship between peer alcohol use and own alcohol use in adolescents. Addiction. 2007;102:894–903. doi: 10.1111/j.1360-0443.2007.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-Demarco L. The life history calendar: a technique for collecting retrospective data. Sociol Methodol. 1988;18:37–68. [PubMed] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behav Genet. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KL, Slater MD, Oetting ER. Alcohol use in early adolescence: the effect of changes in risk taking, perceived harm and friends' alcohol use. J Stud Alcohol. 2005;66:275–283. doi: 10.15288/jsa.2005.66.275. [DOI] [PubMed] [Google Scholar]
- Hill J, Emery RE, Harden KP, Mendle J, Turkheimer E. Alcohol use in adolescent twins and affiliation with substance using peers. J Abnorm Child Psychol. 2008;36:81–94. doi: 10.1007/s10802-007-9161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard J, Blanton H, Dodge T. Peer influences on risk behavior: an analysis of the effects of a close friend. Dev Psychol. 2005;41:135–147. doi: 10.1037/0012-1649.41.1.135. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Genotype-environment correlations: implications for determining the relationship between environmental exposures and psychiatric illness. Psychiatry. 2008;7:496–499. doi: 10.1016/j.mppsy.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson K, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychol Med. 2008;38:1001–1011. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: a developmental twin study of peer-group deviance. Arch Gen Psychiatry. 2007;64:958–965. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kiuru N, Burk WJ, Laursen B, Salmela-Aro K, Nurmi JE. Pressure to drink but not to smoke: disentangling selection and socialization in adolescent peer networks and peer groups. J Adolesc. 2010;33:801–812. doi: 10.1016/j.adolescence.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Is there an active gene-environment correlation in adolescent drinking behavior? Behav Genet. 2010;40:447–451. doi: 10.1007/s10519-010-9347-z. [DOI] [PubMed] [Google Scholar]
- Madden PA, Bucholz KK, Todorov AA, Grant JD, Heath AC. The assessment of peer selection and peer environmental influences on behavior using pairs of siblings or twins. Twin Res. 2002;5:38–43. doi: 10.1375/1369052022884. [DOI] [PubMed] [Google Scholar]
- Mercken L, Steglich C, Knibbe R, Vries H. Dynamics of friendship networks and alcohol use in early and mid-adolescence. J Stud Alcohol Drugs. 2012;73:99–110. doi: 10.15288/jsad.2012.73.99. [DOI] [PubMed] [Google Scholar]
- Mundt MP, Mercken L, Zakletskaia L. Peer selection and influence effects on adolescent alcohol use: a stochastic actor-based model. BMC Pediatr. 2012;12:115. doi: 10.1186/1471-2431-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelen EA, Scholte RH, Willemsen G, Boomsma DI, Engels RC. Drinking by parents, siblings, and friends as predictors of regular alcohol use in adolescents and young adults: a longitudinal twin-family study. Alcohol Alcohol. 2007;42:362–369. doi: 10.1093/alcalc/agm042. [DOI] [PubMed] [Google Scholar]
- Scholte RH, Poelen EA, Willemsen G, Boomsma DI, Engels RC. Relative risks of adolescent and young adult alcohol use: the role of drinking fathers, mothers, siblings, and friends. Addict Behav. 2008;33:1–14. doi: 10.1016/j.addbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B, Chen RS. Over time relationships between early adolescent and peer substance use. Addict Behav. 2006;31:1211–1223. doi: 10.1016/j.addbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Stappenbeck CA, Quinn PD, Wetherill RR, Fromme K. Perceived norms for drinking in the transition from high school to college and beyond. J Stud Alcohol Drugs. 2010;71:895–903. doi: 10.15288/jsad.2010.71.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strycker LA, Duncan SC, Pickering MA. The social context of alcohol initiation among African American and White youth. Journal of Ethnicity in Substance Abuse. 2003;2:35–42. [Google Scholar]
- Urberg KA, Degirmencioglu SM, Pilgrim C. Close friend and group influence on adolescent cigarette smoking and alcohol use. Dev Psychol. 1997;33:834–844. doi: 10.1037//0012-1649.33.5.834. [DOI] [PubMed] [Google Scholar]
- Wills AG, Carey G. Adolescent Peer Choice and Cigarette Smoking: Evidence of Active Gene-Environment Correlation? Twin Res Hum Genet. 2013:1–7. doi: 10.1017/thg.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Cleary SD. Peer and adolescent substance use among 6th–9th graders: latent growth analyses of influence versus selection mechanisms. Health Psychology. 1999;18:453–463. doi: 10.1037//0278-6133.18.5.453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.