Abstract
Objective
Cancer-related fatigue (CRF) is one of the most common, persistent, and disabling symptoms associated with cancer and its treatment. Evidence-based treatments that are acceptable to patients are critically needed. This study examined the efficacy of Mindfulness-Based Stress Reduction (MBSR) for CRF and related symptoms.
Method
A sample of 35 cancer survivors with clinically-significant CRF was randomly assigned to a 7-week MBSR-based intervention or wait-list control group. The intervention group received training in mindfulness meditation, yoga, and self-regulatory responses to stress. Fatigue interference (primary outcome) and a variety of secondary outcomes (e.g., fatigue severity, vitality, disability, depression, anxiety, sleep disturbance) were assessed at baseline, post-intervention, and 1-month follow-up. Bonferroni correction was employed to account for multiple comparisons. Controls received the intervention after the 1-month follow-up. Participants in both groups were followed for 6 months after completing their respective MBSR courses to assess maintenance of effects.
Results
Compared to controls, the MBSR group reported large post-intervention reductions as assessed by effect sizes (d) in the primary outcome, fatigue interference (d= −1.43, p<.001), along with fatigue severity (d= −1.55, p<.001), vitality (d= 1.29, p<.001), depression (d= −1.30, p<.001), and sleep disturbance (d= −0.74, p=.001). Results were maintained or strengthened at 1-month follow-up, the point at which significant improvements in disability (d= −1.22, p<.002) and anxiety (d= −0.98, p=.002) occurred. Improvements in all outcomes were maintained 6 months after completing the course. MBSR adherence was high, with 90% attendance across groups and high rates of participant-reported home practice of mindfulness.
Conclusions
MBSR is a promising treatment for CRF and associated symptoms.
Keywords: cancer, oncology, cancer-related fatigue, MBSR, meditation, mindfulness
Fatigue is a highly prevalent and bothersome symptom for patients with cancer [1, 2]. Survivors have identified it as the most distressing [3] and debilitating [4] of all their symptoms in research; yet it is under-reported in the clinic and is seldom diagnosed or treated. Across studies, fatigue prevalence rates range from 59 to 100% [5, 6], and from 9 to 56% when syndromal diagnostic criteria for cancer-related fatigue (CRF) are applied [7]. CRF causes interference in quality of life across the cancer trajectory that has been characterized as profound and pervasive [4], sometimes persisting long after treatment has ended even in patients believed to be disease-free [5].
While research related to CRF has intensified recently [5], no “gold standard” treatment for it exists [8]. National Comprehensive Cancer Network (NCCN) treatment guidelines suggest that in response to a complaint of fatigue, providers should direct attention to potential contributing factors that may be correctable, such as anemia and pain [2]. For many patients, however, no specific treatable cause will be known. Pharmacologic interventions such as psychostimulants are considered “investigational” and secondary to nonpharmacologic interventions in the NCCN guidelines. A recent meta-analysis suggested that exercise-based treatments are helpful in addressing CRF [9]. In a comprehensive meta-analysis of 57 non-pharmacological interventions for cancer patients and survivors, Kangas and colleagues [8] concluded that exercise and psychosocial therapies each show potential for effectively ameliorating CRF.
Extant evidence suggests that integrative therapeutic approaches combining exercise and psychosocial interventions may best serve those suffering with CRF [2, 8, 10]. Mindfulness-Based Stress Reduction (MBSR) is an integrative intervention that has been identified as promising for CRF and worthy of further study [10, 11]. Within a group framework of experiential and didactic learning that includes meditation and yoga, participants cultivate the innate human quality of mindfulness [12]. Mindfulness has been defined as intentionally directing attention to one's present moment experience without judging that experience as positive or negative [13]. MBSR participants learn less reactive, healthier responses to stressful situations. The gentle hatha yoga included in MBSR as a practice of mindfulness in movement may serve to counteract deconditioning due to physical inactivity that is common among those with CRF.
Several meta-analyses and systematic reviews have suggested MBSR has promise in the cancer context [14-17]. Three randomized controlled trials (RCTs) of MBSR for cancer patients have included measures of fatigue, vigor, or vitality among the outcomes in their trials and found positive effects [18-20]. Fatigue was not the primary outcome in these trials, however, and none enrolled participants based on the presence of clinically significant fatigue. Only one study tested a mindfulness-based intervention specifically targeting fatigue in cancer survivors [21]. Investigators compared modified Mindfulness-Based Cognitive Therapy to a wait-list control and found the intervention group had significantly lower fatigue scores at the end of the 8-week class compared to controls.
The current pilot study targeted cancer-related fatigue interference as the primary outcome and included a wait-list control group for comparison at post-intervention and 1-month follow-up. We hypothesized that mindfulness training would reduce patients’ perception of the interference of fatigue and that improvements would be sustained through 1-month follow-up. Similarly, we hypothesized that mindfulness training would reduce fatigue severity, functional disability, depression, anxiety, and sleep disturbance, while improving vitality. We followed both groups to six months after participating in MBSR.
Methods
Design
A randomized controlled design was used to enroll a heterogeneous sample of 35 cancer survivors in a 1:1 ratio to either a 7-week MBSR course or a wait-list control condition. A wait-list control was utilized since MBSR had not been established as an effective intervention for CRF when the study began, and we wanted to see if there was a significant effect before comparing to attention control or an active comparator. Participants completed self-report measures at baseline (T1) and then were randomized. Subsequent assessments were completed at the end of the intervention (T2) and at 1-month follow-up (T3), which served as the end of the randomized portion of the trial. The wait-list participants were offered the 7-week MBSR course following completion of the T3 assessment, and all elected to participate. Wait-list participants completed the self-report measures immediately after the MBSR course (T4). Both groups completed a final assessment 6 months after completing their respective MBSR courses (T5). The study was approved by the Indiana University institutional review board (IRB) and is registered with ClinicalTrials.gov (NCT01247532).
Participants
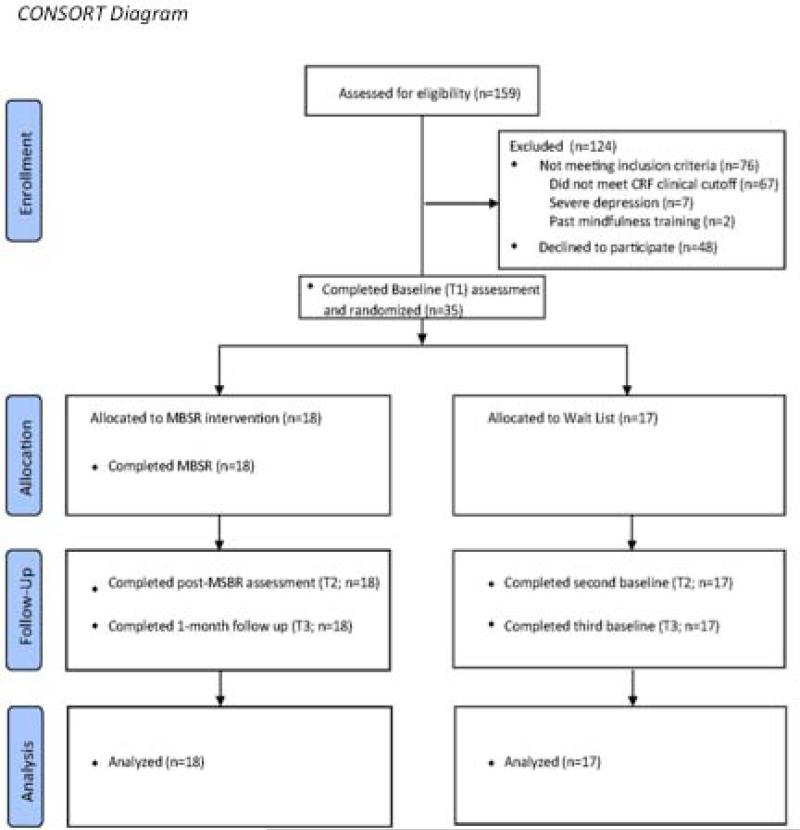
Individuals were considered eligible if they were at least 18, had a cancer diagnosis, reported experiencing persistent CRF for the previous 8 weeks or longer, and reported clinically significant CRF at the time of eligibility screening. Clinically significant CRF was defined by a cutoff mean score of ≥ 4 across the 3-item Fatigue Symptom Inventory severity composite [FSI composite; 22]. Participants were excluded if they had cancer treatment (other than endocrine therapy for breast cancer) in the prior 3 months, were enrolled in hospice care, had severe hearing impairment, were experiencing severe depression (PHQ-8 ≥ 20), had previously participated in a mindfulness meditation class, or did not understand English. Figure 1 represents the participant flow of the study.
Figure 1.
CONSORT Diagram
Baseline characteristics are presented by intervention arm in Table 1. Breast cancer was the most frequent diagnosis (85.7%), and the sample was predominantly female (94%), white (80%), and college educated (71%). About half were employed (49%), 60% were married, and 60% reported having a comfortable income. All had completed chemotherapy and/or radiation therapy at least 9 months before randomization, and the average time since completion was 51.3 months (SD = 39.3 months). Most (94.3%) were in an early stage of disease (stage 0 to III) at diagnosis.
Table 1.
Baseline Characteristics by Intervention Arm
| Baseline Characteristics | MBSR n = 18 | Wait-List Control n = 17 | p |
|---|---|---|---|
| Age, mean (SD) | 58.8 (9.3) | 55.7 (9.3) | .33 |
| Female, n (%) | 17 (94) | 16 (94) | 1.00 |
| White, n (%) | 15 (83) | 13 (76) | .69 |
| College education, n (%) | 12 (67) | 13 (77) | .71 |
| Married, n (%) | 11 (61) | 10 (59) | .89 |
| Employed, n (%) | 9 (50) | 8 (47) | .86 |
| Comfortable income, n (%) | 9 (50) | 12 (71) | .21 |
| Recent mental health treatment, n (%) | 1 (5) | 7 (41) | .01* |
| Symptom measures, mean (SD) | |||
| FSI-Interference | 4.35 (2.18) | 4.46 (2.02) | .88 |
| FSI-Severity | 5.57 (1.58) | 4.78 (1.30) | .12 |
| SF-36 Vitality | 36.6 (18.9) | 29.3 (17.1) | .24 |
| PHQ-8 Depression | 7.89 (5.41) | 8.94 (5.17) | .56 |
| GAD-7 Anxiety | 5.83 (4.57) | 8.06 (4.90) | .17 |
| ISI Sleep Disturbance | 11.17 (6.67) | 13.29 (7.05) | .37 |
| Five Facet Mindfulness Questionnaire | |||
| Observing | 28.11 (5.29) | 24.35 (5.28) | .049* |
| Describing | 29.94 (5.87) | 27.06 (7.91) | .24 |
| Acting with Awareness | 26.78 (6.32) | 22.00 (8.66) | .07 |
| Non-judging of inner experience | 31.61 (6.09) | 28.35 (7.75) | .18 |
| Non-reactivity of inner experience | 23.78 (3.57) | 20.65 (3.72) | .02* |
| Type of cancer, n (%) | .68 | ||
| Breast | 15 (83.3) | 15 (88.2) | |
| Esophageal | 1 (5.56) | 0 (0) | |
| Hematologic malignancies | 2 (11.11) | 2 (11.76) | |
| Type of cancer treatment, n (%) | |||
| Chemotherapy | 11 (31) | 12 (34) | .56 |
| Radiation therapy | 10 (29) | 12 (34) | .36 |
| Chemotherapy + Radiation | 7 (20) | 8 (23) | .63 |
| Endocrine therapy | 12 (34) | 8 (23) | .24 |
| Cancer stage, n | .20 | ||
| I | 5 | 7 | |
| II | 5 | 7 | |
| III | 4 | 2 | |
| IV | 2 | 1 |
Note.
Groups differed significantly at p < .05 on these variables. Each variable with significant differences was controlled for in subsequent analyses.
Procedures
The sample of 35 participants in this trial was recruited over 6 weeks in the spring of 2010. Participants were consecutively recruited through: (1) clinics affiliated with a National Cancer Institute-designated cancer center, (2) an urban oncology clinic affiliated with a public teaching hospital in the Midwest, and (3) a breast cancer survivor registry. Eligible and interested individuals were invited to attend one of two group enrollment sessions. The enrollment sessions included informed consent, baseline assessment, randomization, and—for those randomized to the intervention arm—orientation to the MBSR class. The randomization sequence was generated by coin toss in blocks of four by the principal investigator. Research assistants and participants were blinded to the randomization sequence using sequentially-numbered and sealed envelopes. All outcomes were self-reported on study questionnaires and therefore not subject to bias by assessor interpretation. Participants completed baseline and post-intervention questionnaires at the study site, and follow-up assessments were completed either at the study site or by mail according to participant preference. The 6-month follow-up assessments were completed in early 2011.
Intervention
The MBSR-CRF program tested in this study maintained fidelity to standard MBSR [13]. It featured training in the mindfulness practices of the body scan, sitting meditation, gentle hatha yoga, walking meditation, and compassion meditation. The protocol was adapted for the cancer context, a practice which has precedent in previous studies [23]. MBSR-CRF adaptations included 2-hour classes, seven classes instead of eight, no retreat, brief psycho-education related to CRF, and shorter guided home practices (20 minutes) to accommodate fatigued participants; however, all of the core content of the standard MBSR curriculum was included. Recordings of guided meditations of body scan, sitting meditation, gentle hatha yoga with chair adaptations, and compassion meditation were created by the facilitator for home practice.
For participants whose cancer diagnosis and treatment stimulated reactivity in attention to particular body areas (e.g., during the body scan), guidance was to acknowledge associated thoughts, emotions, and sensations in non-judgmental compassion, while offering the possibility of grounding in sensations of lesser valence such as those of the breath or contact with body support (e.g., chair, floor). Class discussion included the contrast between catastrophizing and being willing to connect with present moment experience of transient thoughts, emotions, and sensations. Given the high rates of sleep disturbance in the sample, an optional 8-minute bedtime body scan variant, “Arriving for Sleep,” was provided to lessen pre-sleep rumination and difficulties initiating sleep.
Information on the human stress reaction routinely presented in MBSR was expanded to include evidence of the relationship of stress and fatigue [24]. Information regarding the influence of the perception of exhaustion on subsequent diminished physical activity [25] as well as ample evidence that physical activity is helpful with CRF [9] were included. Mindful communication practice based in insight dialogue [26] was used as a vehicle for participants to explore how newly-developing strategies learned in mindfulness meet the interpersonal challenges of CRF.
Participants logged their daily home meditation practice, including number of minutes per day and type of practice (i.e., body scan, sitting meditation, yoga) on a diary card. Participants received $5 for each weekly diary card submitted, regardless of the logged amount of home practice. The course instructor was blinded to patient logs and outcomes during the class. The instructor had six years of MBSR teaching experience, completing all components of professional training leading to eligibility for MBSR Teacher Certification Review (Phase 4, Oasis Institute at the Center for Mindfulness in Medicine, Health Care and Society; [13].
Measures
Fatigue
The interference subscale of the Fatigue Symptom Inventory (FSI) was the primary outcome measure. The FSI is a 13-item self-report scale assessing the degree to which fatigue interferes with quality of life (7 items) as well as the severity (4 items) and frequency (2 items) of fatigue [27]. Interference is measured on 11-point scales that assess the degree fatigue interfered with general level of activity, ability to bathe and dress, normal work activity, ability to concentrate, relations with others, enjoyment of life, and mood. FSI severity is measured on 11-point scales that assess most, least, and average fatigue in the past week as well as current fatigue. FSI frequency is measured with two items assessing the number of days and the percentage of the average day over the past week the respondent felt fatigued.
The 4-item vitality scale of the SF-36 Health Survey served as a secondary fatigue measure [28]. Standardized subscale scores range from 0 to 100, with higher scores indicating greater vitality. Vitality scores ≤ 45 are indicative of clinically-significant CRF [22].
Secondary Outcomes
Functional status was assessed with the 3-item Sheehan Disability Scale [SDS; 29] which asks respondents to what extent on a 0 to 10 scale their health has interfered with their work, family life, and social life in the previous week. The SDS score is the mean of the three items and higher scores reflect greater disability. Depression severity was measured with the Patient Health Questionnaire 8-item depression scale (PHQ-8). PHQ-8 scores range from 0 to 24, with scores of 5, 10, 15, and 20 representing mild, moderate, moderately severe, and severe depression, respectively [30]. Anxiety was measured with the 7-item Patient Health Questionnaire Generalized Anxiety Disorder Scale (GAD-7) [31]. Scores range from 0 to 21, with cut-points of 5, 10, and 15 representing mild, moderate, and severe levels of anxiety. Sleep disturbance was measured with the 7-item Insomnia Severity Index [ISI; 32], which evaluates the perceived severity of insomnia and the impact of sleep difficulties over the course of the previous two weeks. The ISI has been found to be a reliable and valid instrument to assess primary insomnia [33], as well as insomnia secondary to cancer [34].
Feasibility and Adherence. Retention through the 6-month follow-up period was chosen as the main feasibility measure. To measure adherence to the MBSR program, class attendance was tracked, along with number of home practice logs submitted, and the total number of days and minutes per day of mindfulness practice reported. At the end of the course, participants were asked to report the average number of days per week they had continued to participate in formal and informal mindfulness practice.
Analysis
The randomized groups were compared on T1 characteristics (e.g., demographic characteristics, medical comorbidity, recent mental health treatment, and self-reported mindfulness) to determine whether to adjust for any of these variables in subsequent analyses due to potentially confounding effects on outcomes. The only significant T1 differences between groups were in recent participation in mental health treatment (p = . 02) and degree of mindfulness on two subscales of the Five Facet Mindfulness Questionnaire [Observing, p=.04; Non-Reactivity, p=.02; 35]. These differences were controlled in subsequent analyses.
An ANCOVA model was used to test efficacy by comparing the MBSR and control groups on all outcomes immediately after the intervention (T2) and 1 month later (T3), while adjusting for baseline scale scores for each variable. A Bonferroni correction was used to maintain the family-wise Type I error rate <0.05 across the 18 comparisons in the randomized portion of the trial (9 comparisons each at T2 and T3; see Table 2). Thus a conservative two-tailed p-value of <0.00278 (=0.05/18) was considered statistically significant. Effect sizes for each outcome variable were calculated as the standardized mean difference between the MBSR and wait-list control groups at T2 and T3 in fatigue and other outcomes, divided by the pooled baseline standard deviation of the particular outcome variable.
Table 2.
Efficacy of MBSR at Time 2 and Time 3
| Time 2 Outcomes | Adjusted Means | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variables | MBSR (N=18) | Control (N=17) | Diff | SE diff | p* | Pooled SD | Effect Size | 95% CI Effect Size |
| FSI Interference | 2.11 | 4.58 | −2.47 | 0.47 | <0.001 | 1.73 | −1.43 | −1.96, −0.90 |
| FSI Severity | 3.03 | 5.57 | −2.54 | 0.45 | <0.001 | 1.64 | −1.55 | −2.09, −1.01 |
| FSI Fatigue Days (0-7 scale) | 3.36 | 5.56 | −2.20 | 0.53 | <0.001 | 2.03 | −1.08 | −1.60, −0.57 |
| FSI Percent of Day Fatigued | 2.34 | 5.65 | −3.31 | 0.53 | <0.001 | 1.81 | −1.83 | −2.41, −1.25 |
| SF-36 Vitality | 52.96 | 33.22 | 19.75 | 4.54 | <0.001 | 15.35 | 1.29 | 0.71, 1.87 |
| Sheehan Disability Scale | 2.60 | 3.49 | −1.12 | 0.66 | 0.25 | 2.51 | −0.45 | −0.96, 0.07 |
| PHQ-8 Depression | 4.58 | 10.03 | −5.46 | 1.10 | <0.001 | 4.18 | −1.30 | −1.82, −0.79 |
| GAD-7 Anxiety | 3.91 | 5.92 | −2.00 | 1.20 | 0.104 | 4.24 | −0.47 | −1.02, 0.08 |
| ISI Sleep Disturbance | 7.72 | 12.76 | −5.04 | 1.41 | 0.001 | 6.81 | −0.74 | −1.15, −0.33 |
| Time 3 Outcomes | Adjusted Means | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variables | MBSR (N=18) | Control (N=17) | Diff | SE diff | P* | Pooled SD | Effect Size | 95% CI Effect Size |
| FSI Interference | 1.88 | 4.59 | −2.70 | 0.55 | <0.001 | 2.01 | −1.34 | −1.88, −0.81 |
| FSI Severity | 3.22 | 5.54 | −2.32 | 0.44 | <0.001 | 1.51 | −1.54 | −2.10, −0.97 |
| FSI Fatigue Days (0-7 scale) | 3.62 | 6.05 | −2.44 | 0.57 | <0.001 | 2.00 | −1.22 | −1.77, −0.66 |
| FSI Percent of Day Fatigued | 2.48 | 5.79 | −3.31 | 0.63 | <0.001 | 1.92 | −1.73 | −2.37, −1.08 |
| SF-36 Vitality | 56.49 | 30.42 | 26.08 | 4.76 | <0.001 | 15.09 | 1.73 | 1.11, 2.35 |
| Sheehan Disability Scale | 2.09 | 4.69 | −2.60 | 0.62 | <0.002 | 2.13 | −1.22 | −1.79, −0.65 |
| PHQ-8 Depression | 3.59 | 11.91 | −8.32 | 1.26 | <0.001 | 4.86 | −1.71 | −2.22, −1.20 |
| GAD-7 Anxiety | 3.39 | 7.82 | −4.43 | 1.29 | 0.002 | 4.54 | −0.98 | −1.53, −0.42 |
| ISI Sleep Disturbance | 6.57 | 13.36 | −6.78 | 1.74 | <0.001 | 6.76 | −1.00 | −1.51, −0.50 |
Note. Results are based on ANCOVA models comparing MBSR and controls at T2, and separately at T3, adjusting for T1 measure of the outcome variables, baseline mental health treatment, and the observing and describing subscales of the Five Facet Mindfulness Questionnaire. All Fatigue Symptom Inventory (FSI) subscales are rated on 0-10 scales except where indicated. The effect size for the SF-36 Vitality scale is in the opposite direction than the FSI effect sizes because the SF-36 Vitality scale is scored such that a higher score represents better vitality.
Using Bonferroni correction for multiple comparisons, only p-values < 0.00278 (=.05/18) are considered significant.
Paired t-tests were used to assess for within-group improvement on all outcomes for each group after completing the MBSR course, as well as to assess for maintenance of intervention benefits from immediate post-intervention to 6 months post-intervention. Analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina).
Results
Randomized Controlled Trial to Test Efficacy of MBSR
Primary outcome
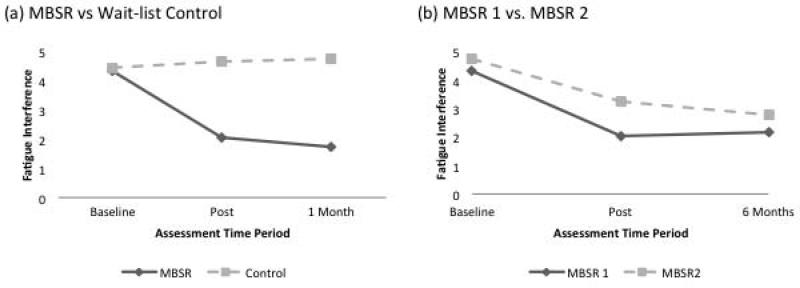
As shown in Table 2, the MBSR group demonstrated significantly greater improvement than the control group in fatigue interference as measured against the Bonferroni-corrected significance level of p < .00278 at T2 and T3. Effect sizes (d) for group differences (adjusted for baseline levels) in fatigue interference were large at both time points, ranging from −1.43 at T2 to −1.34 at T3. The post-intervention effect on fatigue interference for each group can be observed visually in Figure 2a.
Figure 2.
Figure 2(a) represents the randomized portion of the trial, comparing MBSR to wait-list control at two time points adjusted for baseline differences. Figure 2(b) represents the non-randomized portion of the trial in which the wait-list controls received the MBSR training at the end of the 1-month follow-up. MBSR 1 represents the intervention group, and MBSR 2 represents the wait-list control group. Each group's FSI fatigue interference score immediately before they began the MBSR course represents their baseline score, and the baseline score for each group is compared to their respective post-MBSR and 6-month follow-up scores.
Secondary outcomes
The MBSR group demonstrated significantly greater improvement than the control group on all secondary fatigue measures (i.e., fatigue severity, fatigue days, and percent of day fatigued) and vitality at T2 and T3, as shown in Table 2. Effect sizes on all outcomes were large, ranging from −1.08 to −1.83 for T2 measures and from −1.22 to −1.73 for T3 measures. Functional disability scores were lower in the MBSR group at T2 (d = −0.45), although not statistically different (p = 0.25); however, at T3 the MBSR group demonstrated significantly lower functional disability scores than controls (p = .0013) with a large effect size (d = −1.22).
Depression scores were significantly lower (p < .001) for MBSR than controls with large differences at T2 (d = −1.30) and T3 (d = −1.71). Sleep disturbance was significantly improved for MBSR compared to the control condition at both T2 (d = −0.74) and T3 (d = −1.00). Anxiety scores were lower in the intervention group at T2 than for the control group (d = −0.47), although not statistically different (p = 0.10). By T3, however, the MBSR group demonstrated significantly lower anxiety scores than the control group (p = 0.002) with a large effect size (d = −0.98).
Analysis of Wait-List Controls
The wait-list control group received the MBSR intervention immediately after their T3 assessment and was assessed again immediately after, and 6 months after, completion of the intervention. The post-intervention effect on fatigue interference for each group after completing MBSR can be observed visually in Figure 2b. Both groups experienced significant within-group improvements in all outcomes after completing the MBSR course.
Maintenance of Post-Intervention Effects in Both Groups
Improvements after MBSR in all outcomes were sustained or strengthened at the 6-month follow-up in each group. Paired t-tests demonstrated that none of the outcomes changed significantly for either group between their post-MBSR assessment and the 6-month follow-up. In fact, Figure 2b shows that 6 months after completing the MBSR course, both groups had improved similar amounts from their baseline fatigue interference score.
Feasibility and Adherence
All participants (N=35) completed the study through T3, with one member of the control group dropping out at the 6-month follow-up. Attendance rates were 88% in the intervention group and 91% when the control group received the MBSR course. No adverse events were reported, and the intervention was well-tolerated by all participants. As for home practice participation, 16 of 18 participants randomized to MBSR turned in practice logs each week and reported practicing the body scan, yoga, or sitting meditation an average of 28 out of 36 recommended days of home practice during the program. Average number of minutes of practice daily was 35 (SD = 15). Number of practice logs submitted and practice time was similar for the wait-list group when they participated in MBSR. Among the 34 who completed the 6-month follow-up, 74% reported continued “formal” mindfulness practice and 88% reported continued “informal” mindfulness practice since the completion of the MBSR course. Participants reported engaging in “formal” mindfulness practices (e.g., body scan, sitting meditation) 2 days per week for 20 minutes per day on average over the preceding 6 months. Participants reported “informal” mindfulness practice (e.g., doing everyday activities mindfully) 3.8 days per week on average.
Discussion
This study has four important findings. First, MBSR participants demonstrated significantly greater improvements in fatigue interference than wait-list controls, which supported the primary hypothesis. The magnitude of the effect of MBSR on this and other fatigue outcomes including fatigue severity and vitality was large at the end of the intervention and one month later. Second, MBSR resulted in significant and sustained improvements in depression and sleep disturbance at both time points, with significant improvements in anxiety and functional disability emerging at 1 month. In total, 16 of 18 comparisons on primary and secondary outcomes across T2 and T3 were statistically significant after adjusting for multiple comparisons. Third, improvements in all symptoms were maintained for at least 6 months beyond the completion of the MBSR course for both groups after their respective courses. Fourth, MBSR proved acceptable to fatigued cancer survivors, evidenced by high rates of attendance and mindfulness practice during the course and moderate amounts of continued mindfulness practice, particularly informal practice, through the 6-month follow-up period.
The current findings are generally consistent with the four published RCTs of mindfulness-based interventions in cancer that included fatigue, vigor, or vitality among the outcome measures [18-21]. Although only one of these trials [21] was testing an intervention to help with CRF specifically, each found evidence to suggest fatigue, vigor, and/or vitality improved after a mindfulness course. The only null finding for a fatigue outcome across these four trials was reported by Speca and colleagues (2000). In their trial of a 10.5-hour adaptation of MBSR, the change in fatigue was non-significant, which is not surprising since participants were not enrolled based on a fatigue eligibility criterion; however, vigor improved significantly.
The previous study most analogous to the present trial is that of van der Lee and Garssen [21]. Both trials included a heterogeneous sample of post-treatment cancer survivors enrolled based on the presence of clinically significant fatigue and randomized to either a mindfulness-based intervention for CRF or a wait-list control group. van der Lee and Garssen tested an adaptation of Mindfulness-Based Cognitive Therapy (MBCT) for 59 adults with CRF compared to 24 assigned to the wait list. MBCT and MBSR are similar courses, with a principal difference being that MBCT includes cognitive therapy components which are not part of MBSR, and which are particularly relevant for people vulnerable to depression. The MBCT intervention included 26 hours of class time plus a 2.5-hour booster compared to our 15-hour MBSR course with no booster. Another difference is that van der Lee and Garssen invited their wait-list controls to participate in the MBCT program immediately after the post-intervention assessment, whereas randomization was maintained in the current trial through the 1-month follow-up. Participants in the MBCT trial had significantly reduced post-intervention fatigue compared to controls, with a rather large effect size favoring MBCT (d = 0.74). In the present study, MBSR produced similar but larger effect sizes on fatigue interference, fatigue severity, and vitality. Improvements in fatigue were maintained for at least 6 months in both trials.
In summary, the current study is the first RCT of MBSR in cancer to use fatigue as the primary outcome, limit eligibility to adults with clinically significant levels of fatigue, and compare MBSR to controls at 1-month follow-up. Lack of an eligibility criterion related to heightened fatigue has been problematic in previous CRF studies, reducing the likelihood of detecting intervention effects and being inconsistent with how interventions are delivered in clinical practice [36]. Moreover, although there is no gold standard measure of CRF , a recent psychometric analysis of 18 CRF questionnaires recommended the Fatigue Symptom Inventory (FSI) as one of only three “excellent” measures [37]. No previously published MBSR study in cancer has included any of the “excellent” measures; however, the FSI was the measure used in the present trial.
Clinical Implications
Present findings substantially strengthen evidence supporting MBSR as a treatment for CRF. In 2014, the National Comprehensive Cancer Network (NCCN) added MBSR as an evidence-based intervention for fatigued post-treatment cancer survivors [2]. MBSR is listed as having “category 1” evidence, indicating NCCN consensus that the intervention is appropriate for use based on high-level evidence. However, the evidence cited was based on several quasi-experimental studies coupled with two RCTs comparing MBSR to wait-list control [20, 38]. Thus, the present study not only strengthens the evidence for MBSR as an efficacious intervention for CRF but adds new evidence that the beneficial effects are maintained at least up to 6 months. Positive findings related to feasibility and adherence in the present study may have particularly salient clinical implications, helping to answer questions about whether fatigued cancer survivors are willing and able to participate in a weekly meditation and yoga class that includes daily home practice.
Limitations
Study limitations include a small sample that yielded limited statistical power. In spite of this, 77.8% and 100% of the outcomes assessed at T2 and T3, respectively, were statistically significant, even after using a conservative Bonferroni correction for multiple comparisons. The sample was also from a single institution and not representative of the general population of people with cancer: most were women, the majority was white and college-educated, and the majority had breast cancer. The heterogeneity in type and stage of cancer and anti-neoplastic treatments received in this sample precludes precise estimates of treatment effect in specific groups; however, it increases the generalizability of findings to real-world practice.
The potential for selection bias exists because the study included only patients who were willing to enroll in a clinical trial; therefore, bias could arise from unmeasured differences between patients who declined participation compared to those who agreed to participate. Even with such limits to generalizability, the influence of these biases on internal validity of the study was minimized by random assignment to groups. Furthermore, as demonstrated in Table 1, randomization resulted in comparable groups with respect to potentially confounding variables, except for the three variables adjusted for in subsequent analyses.
Lack of an active comparison treatment or attention control is an important limitation, although use of a wait-list control condition was considered appropriate for this initial pilot study. Also, blinding to group assignment was not feasible, as is often the case in behavioral interventions—especially those using a wait-list control design.
Future Directions
Although the results of the present trial are not definitive, documenting feasibility, acceptability, and preliminary efficacy is an important step before proceeding to a larger RCT for efficacy. Before conclusive statements of efficacy are possible, adequately powered RCTs comparing MBSR to attention control are needed to account for time, attention, and outcome expectancies. Ideally, an attention control condition would utilize a group format and be structurally equivalent to MBSR in number and duration of sessions and amount of home practice. For the attention control intervention to have face value, minimize drop out, and address ethical concerns that might arise if an inert attention condition is offered, it may be useful to focus on topics relevant to cancer survivorship including surveillance and prevention of new or recurrent cancers, nutrition and weight management, and facts related to symptoms common in cancer survivors (e.g., sleep disturbance, depression, anxiety, pain, cognitive impairment, fatigue). Effectiveness trials comparing MBSR to exercise or cognitive behavioral therapy are also needed. Examination of the pre-post intervention change in various biomarkers in behavioral trials for CRF could shed light on our understanding of CRF and how integrative interventions such as MBSR may effectively address this complex symptom. One hypothesized pathway through which mindfulness-based interventions may work is through reductions in pro-inflammatory cytokines, which have been linked to the onset and persistence of fatigue in cancer survivors [39]. Mindfulness has been shown to reduce inflammatory markers in cancer populations [40].
Conclusion
In undertaking this pilot study, we were responding to suggestions in extant literature that MBSR may be an intervention that is particularly well suited to help with clinically significant CRF. Study hypotheses were well supported—suggesting that MBSR may be both efficacious and acceptable—thereby providing compelling impetus to test this intervention in RCTs with larger samples of cancer patients suffering from persistent fatigue during and after treatment.
Acknowledgments
Research reported in this publication was supported by grants from the Walther Cancer Foundation, Inc. and the National Cancer Institute (R25 CA 117865-01A11). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Walther Cancer Foundation or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Shelley A. Johns, Indiana University School of Medicine Regenstrief Institute, Inc..
Linda F. Brown, Indiana University School of Medicine
Kathleen Beck-Coon, Indiana University School of Nursing.
Patrick O. Monahan, Indiana University School of Medicine
Yan Tong, Indiana University School of Medicine.
Kurt Kroenke, Indiana University School of Medicine; Regenstrief Institute, Inc..
References
- 1.Kroenke K, Johns S, Theobald D, Wu J, Tu W. Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Supportive Care in Cancer. 2013;21(3):765–773. doi: 10.1007/s00520-012-1578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology: Cancer-related Fatigue
- 3.Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. Journal of the National Comprehensive Cancer Network. 2008;6(1):3–13. doi: 10.6004/jnccn.2008.0002. [DOI] [PubMed] [Google Scholar]
- 4.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 5.Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Review of Pharmacoeconomics & Outcomes Research. 2011;11(4):441–446. doi: 10.1586/erp.11.44. [DOI] [PubMed] [Google Scholar]
- 6.Kim JE, Dodd MJ, Aouizera BE, Jahan T, Miaskowski C. A review of the prevalence of multiple symptoms in oncology patients. Journal of Pain and Symptom Management. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan KA, McGinty HL, Jacobsen PB. A systematic review of research using the diagnostic criteria for cancer-related fatigue. Psycho-Oncology. 2013;22(4):737–744. doi: 10.1002/pon.3085. [DOI] [PubMed] [Google Scholar]
- 8.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychological Bulletin. 2008;134(5):700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 9.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: A meta-analysis. American Journal of Preventive Medicine. 2012;43(2):e1–e24. doi: 10.1016/j.amepre.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Mustian KM, Morrow GR, Carroll JK, Figueroa-Moseley CD, Jean-Pierre P, Williams GC. Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. The Oncologist. 2007;12(Supplement 1):52–67. doi: 10.1634/theoncologist.12-S1-52. [DOI] [PubMed] [Google Scholar]
- 11.Sood A, Barton DL, Bauer BA, Loprinzi CL. A critical review of complementary therapies for cancer-related fatigue. Integrative cancer therapies. 2007;6(1):8–13. doi: 10.1177/1534735406298143. [DOI] [PubMed] [Google Scholar]
- 12.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 13.Santorelli S, Kabat-Zinn J, editors. Mindfulness-based stress reduction professional education and training resource manual: MBSR Standards of practice, curriculum, and supporting materials, Revised March 2013 edn. Center for Mindfulness in Medicine, Health Care, and Society; 2013. [Google Scholar]
- 14.Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psycho-Oncology. 2009;18:571–579. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- 15.Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? Psycho-Oncology. 2011;20:681–697. doi: 10.1002/pon.1819. [DOI] [PubMed] [Google Scholar]
- 16.Piet J, Würtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology. 2012;80(6):1007–1020. doi: 10.1037/a0028329. [DOI] [PubMed] [Google Scholar]
- 17.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: A meta-analysis. Psycho-Oncology. 2013;22(7):1457–1465. doi: 10.1002/pon.3171. [DOI] [PubMed] [Google Scholar]
- 18.Lengacher CA, Johnson-Mallard V, Post-White J, Moscoso MS, Jacobsen PB, Klein TW, Widen RH, Fitzgerald SG, Shelton M, Barta M, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncology. 2009 doi: 10.1002/pon.1529. 10(DOI: 10.1002) [DOI] [PubMed] [Google Scholar]
- 19.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic Medicine. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(12):1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 21.van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: a treatment study. Psycho-Oncology. 2012;21(3):264–272. doi: 10.1002/pon.1890. [DOI] [PubMed] [Google Scholar]
- 22.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. Journal of Pain and Symptom Management. 2008;36(5):480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson LE. Mindfulness-based interventions in oncology. In: Didonna F, editor. Clinical handbook of mindfulness. Springer; New York: 2009. pp. 383–404. [Google Scholar]
- 24.Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 25.Marcora S, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. Journal of Applied Physiology. 2009;106(3):857–864. doi: 10.1152/japplphysiol.91324.2008. [DOI] [PubMed] [Google Scholar]
- 26.Kramer G. Insight Dialogue: The Interpersonal Path to Freedom. Shambala; 2007. [Google Scholar]
- 27.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: Further validation of the Fatigue Symptom Inventory. Quality of Life Research. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: Manual and interpretation guide. The Health Institute, New England Medical Center; Boston: 1993. [Google Scholar]
- 29.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM. Insomnia: Psychological assessment and management. Guilford Press; New York: 1993. [Google Scholar]
- 33.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 34.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psycho-Oncology. 2005;14:429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 35.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2007;26(6):660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agasi-Idenburg C, Velthuis M, Wittink H. Quality criteria and user-friendliness in self-reported questionnaires on cancer-related fatigue: a review. Journal of clinical epidemiology. 2010;63(7):705–711. doi: 10.1016/j.jclinepi.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Lengacher CA, Reich R, Post-White J, Moscoso M, Shelton M, Barta M, Le N, Budhrani P. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. Journal of Behavioral Medicine. 2012;35(1):86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- 39.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment applications. Brain, Behavior, and Immunity. 2013;(30 Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo R, Mathews HL. Effect of mindfulness-based stress reduction on immune function, quality of life, and coping in women newly diagnosed with early stage breast cancer. Brain, Behavior, and Immunity. 2008;22(6):969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]