Abstract
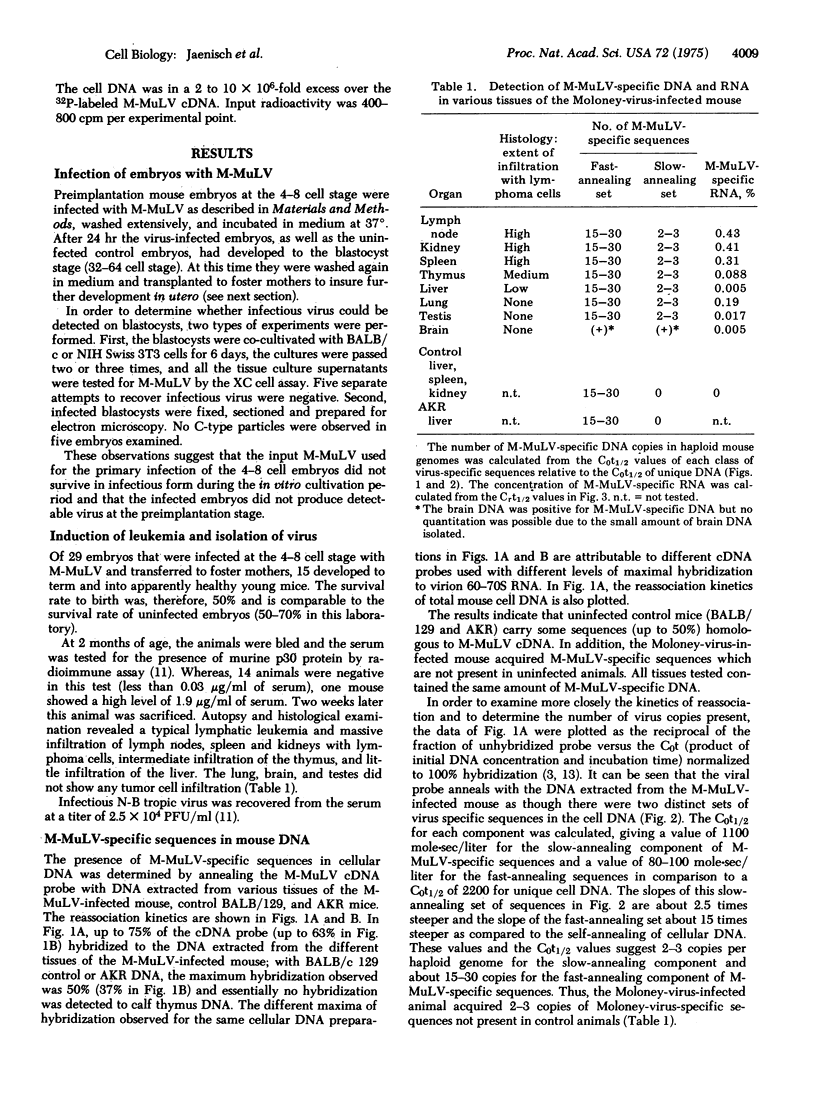
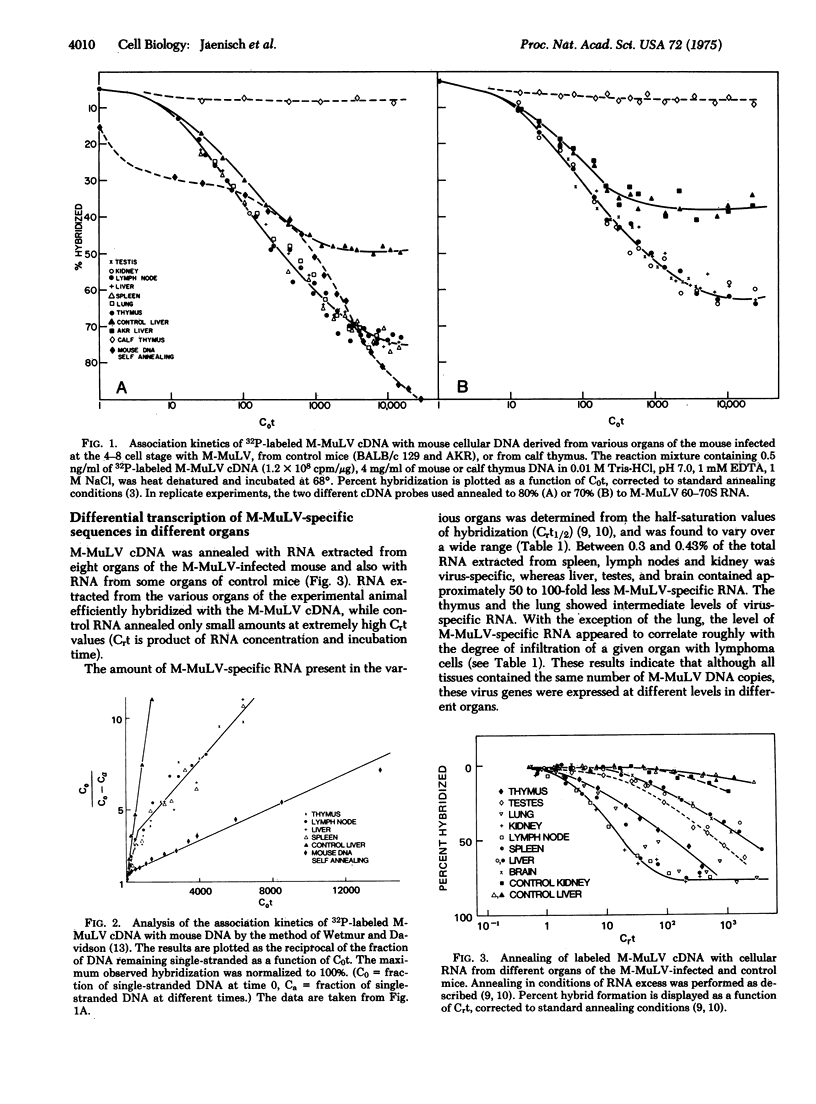
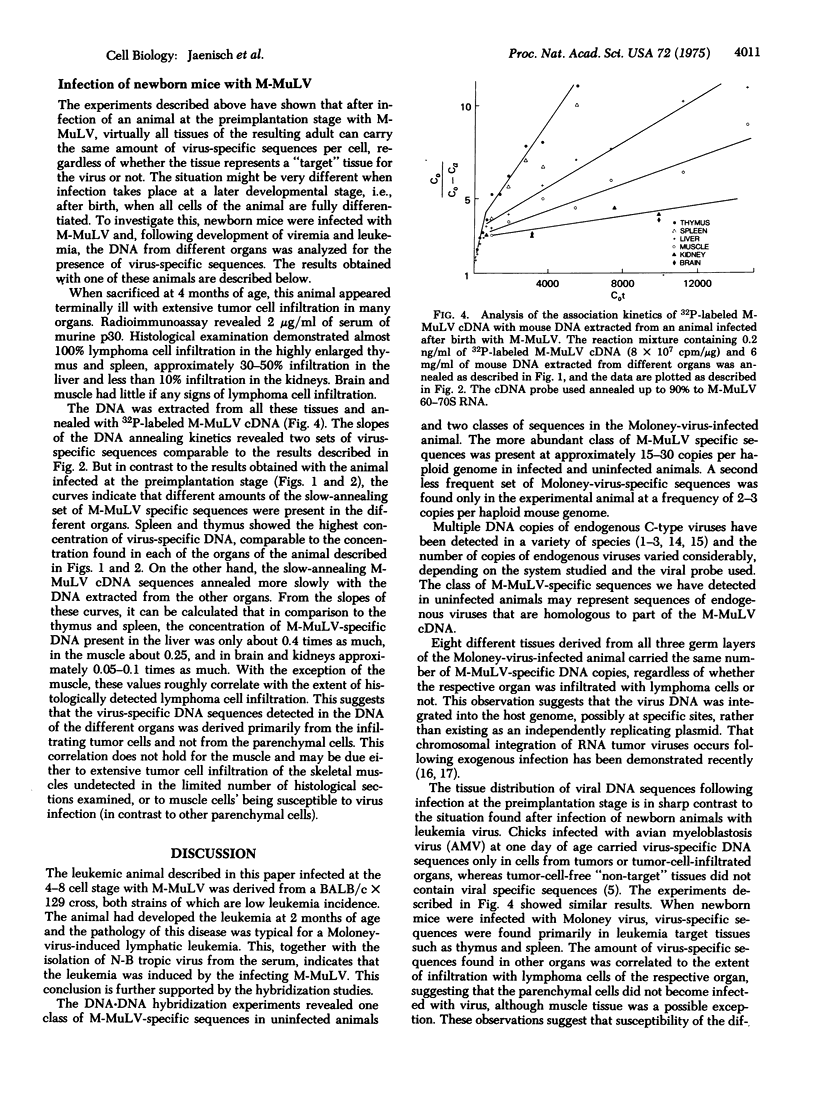
Explanted mouse embryos derived from low leukemia incidence strains were infected with Moloney murine leukemia virus (M-MuLV) at the 4-8 cell stage of development. After cultivation in vitro to the blastocyst stage, the embryos were surgically transferred to the uteri of pseudo-pregnant surrogate mothers. Of 15 animals born, one developed a leukemia at 8 weeks of age. When autopsied, this leukemia was found to be of the lymphatic type, as is typical for the M-MuLV-induced disease. In addition, infectious M-MuLV virus was isolated from the serum. Molecular hybridization tests for the presence of M-MuLV-specific sequences were conducted on DNA and RNA extracted from eight different organs. The DNA-DNA reannealing experiments revealed the presence of two classes of M-MuLV-specific sequences in equal concentrations in all tissues tested. The less abundant class of M-MuLV-specific sequences was not detected in tissues from uninfected animals or in non-target tissues of leukemic animals infected at birth. The results are consistent with the working hypothesis that the virus was integrated in all cells of the animal, possibly including the germ line. Fifty to 100 times more M-MuLV-specific RNA was detected in tumor tissues than was found in non-target organs such as liver, brain, and testes. Since all organs contained the same amount of virus-specific DNA, these results indicate that the M-MuLV-specific DNA can be differentially expressed in different tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Croker B. P., Jr, del Villano B. C., Jensen F. C., Lerner R. A., Dixon F. J. Immunopathogenicity and oncogenicity of murine leukemia viruses. I. Induction of immunologic disease and lymphoma in (BALB-c times NZB)F1 mice by Scripps leukemia virus. J Exp Med. 1974 Oct 1;140(4):1028–1048. doi: 10.1084/jem.140.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L. Facts and theories on viruses causing cancer and leukemia. Proc Natl Acad Sci U S A. 1974 May;71(5):2013–2017. doi: 10.1073/pnas.71.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Infection of mouse blastocysts with SV40 DNA: normal development of the infected embryos and persistence of SV40-specific DNA sequences in the adult animals. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):375–380. doi: 10.1101/sqb.1974.039.01.049. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW L. W., MOLONEY J. B. Studies of congenital transmission of a leukemia virus in mice. Proc Soc Exp Biol Med. 1961 Dec;108:715–723. doi: 10.3181/00379727-108-27045. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Callahan R., Lieber M. M., Sherr C. J. Endogenous primate and feline type C viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1159–1168. doi: 10.1101/sqb.1974.039.01.133. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



