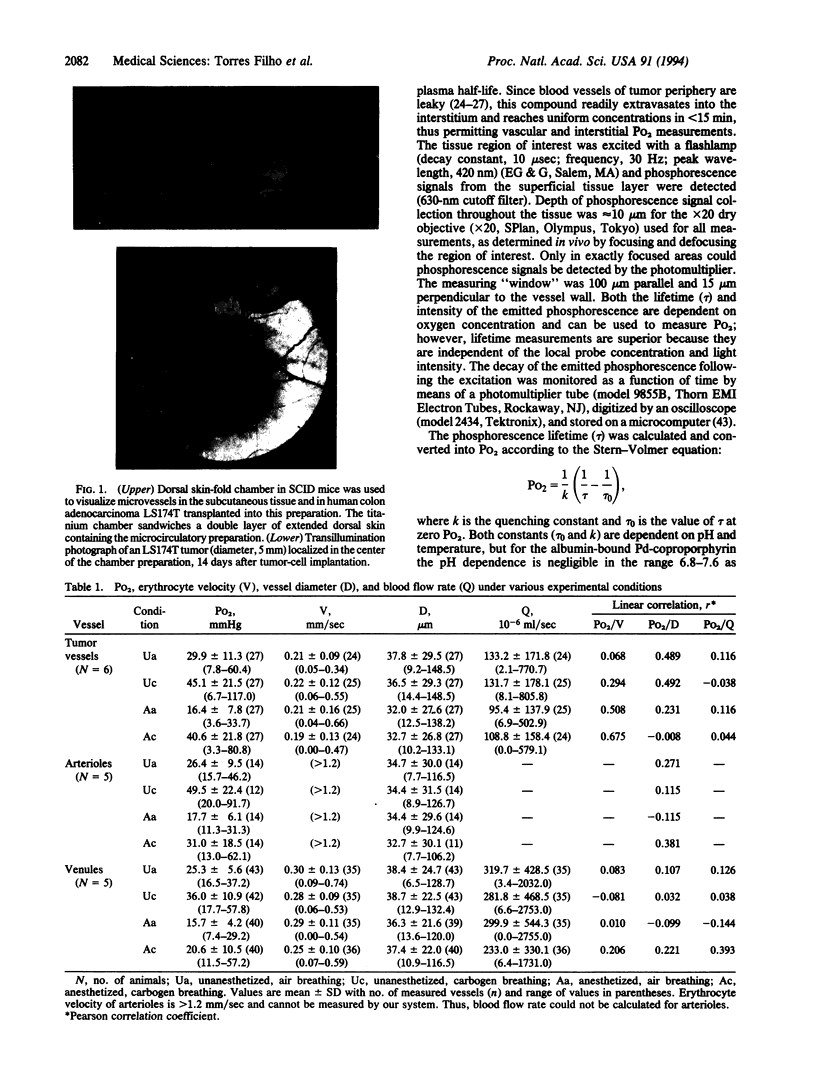
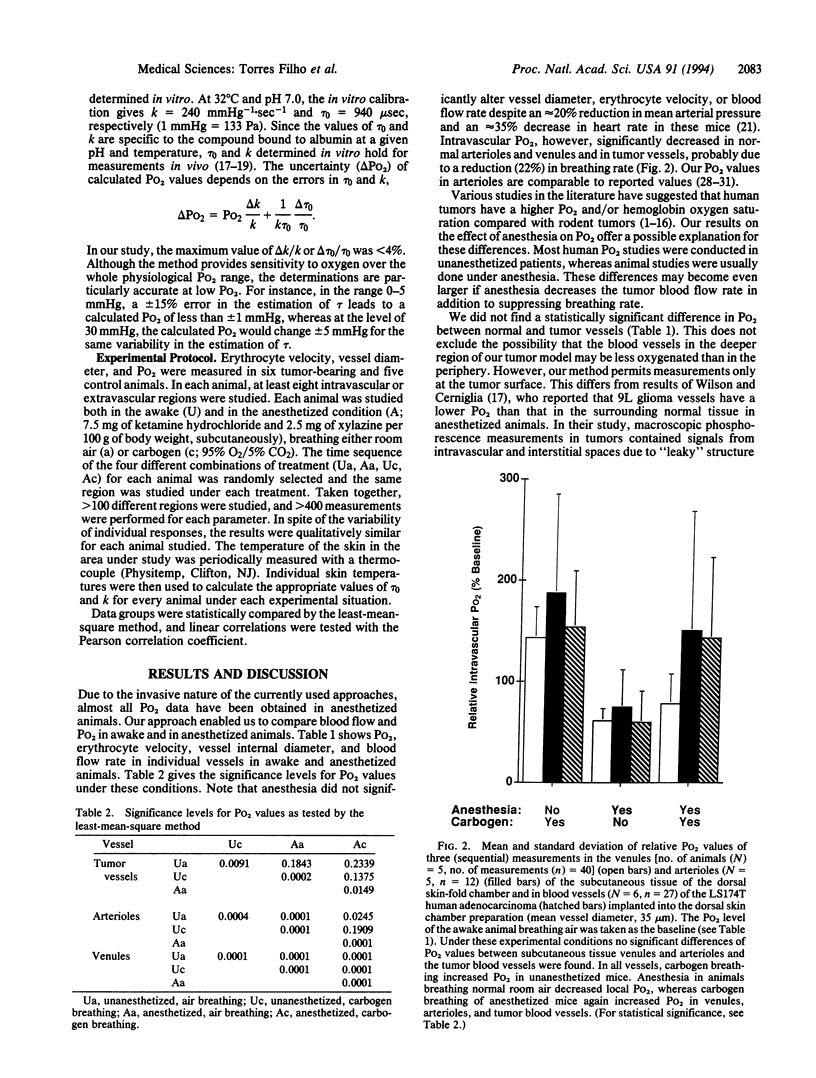
Abstract
Simultaneous measurements of intravascular and interstitial oxygen partial pressure (PO2) in any tissue have not previously been reported, despite the importance of oxygen in health and in disease. This is due to the limitations of current techniques, both invasive and noninvasive. We have optically measured microscopic profiles of PO2 with high spatial resolution in subcutaneous tissue and transplanted tumors in mice by combining an oxygen-dependent phosphorescence quenching method and a transparent tissue preparation. The strengths of our approach include the ability to follow PO2 in the same location for several weeks and to relate these measurements to local blood flow and vascular architecture. Our results show that (i) PO2 values in blood vessels in well-vascularized regions of a human colon adenocarcinoma xenograft are comparable to those in surrounding arterioles and venules, (ii) carbogen (95% O2/5% CO2) breathing increases microvascular PO2 in tumors, and (iii) in unanesthetized and anesthetized mice PO2 drops to hypoxic values at < 200 microns from isolated vessels but drops by < 5 mmHg (1 mmHg = 133 Pa) in highly vascularized tumor regions. Our method should permit noninvasive evaluations of oxygen-modifying agents and offer further mechanistic information about tumor pathophysiology in tissue preparations where the surface of the tissue can be observed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter L. T., Jain R. K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989 Jan;37(1):77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- CATER D. B., SILVER I. A. Quantitative measurements of oxygen tension in normal tissues and in the tumours of patients before and after radiotherapy. Acta radiol. 1960 Mar;53:233–256. doi: 10.3109/00016926009171671. [DOI] [PubMed] [Google Scholar]
- Chapman J. D. Measurement of tumor hypoxia by invasive and non-invasive procedures: a review of recent clinical studies. Radiother Oncol. 1991;20 (Suppl 1):13–19. doi: 10.1016/0167-8140(91)90181-f. [DOI] [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Degner F. L., Sutherland R. M. Mathematical modelling of oxygen supply and oxygenation in tumor tissues: prognostic, therapeutic, and experimental implications. Int J Radiat Oncol Biol Phys. 1988 Aug;15(2):391–397. doi: 10.1016/s0360-3016(98)90021-9. [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Ong E. T., Klitzman B., Secomb T. W., Vinuya R. Z., Dodge R., Brizel D., Gross J. F. Perivascular oxygen tensions in a transplantable mammary tumor growing in a dorsal flap window chamber. Radiat Res. 1992 May;130(2):171–182. [PubMed] [Google Scholar]
- Duling B. R., Berne R. M. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970 Nov;27(5):669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- Duling B. R. Changes in microvascular diameter and oxygen tension induced by carbon dioxide. Circ Res. 1973 Mar;32(3):370–376. doi: 10.1161/01.res.32.3.370. [DOI] [PubMed] [Google Scholar]
- Duling B. R., Kuschinsky W., Wahl M. Measurements of the perivascular PO2 in the vicinity of the pial vessels of the cat. Pflugers Arch. 1979 Dec;383(1):29–34. doi: 10.1007/BF00584471. [DOI] [PubMed] [Google Scholar]
- Eddy H. A., Casarett G. W. Development of the vascular system in the hamster malignant neurilemmoma. Microvasc Res. 1973 Jul;6(1):63–82. doi: 10.1016/0026-2862(73)90007-1. [DOI] [PubMed] [Google Scholar]
- Eskey C. J., Koretsky A. P., Domach M. M., Jain R. K. Role of oxygen vs. glucose in energy metabolism in a mammary carcinoma perfused ex vivo: direct measurement by 31P NMR. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2646–2650. doi: 10.1073/pnas.90.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S. J., Ward R., Bleehen N. M. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised p02 histography. Br J Cancer. 1992 Nov;66(5):919–924. doi: 10.1038/bjc.1992.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986 May;31(3):288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- Groebe K., Vaupel P. Evaluation of oxygen diffusion distances in human breast cancer xenografts using tumor-specific in vivo data: role of various mechanisms in the development of tumor hypoxia. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):691–697. doi: 10.1016/0360-3016(88)90313-6. [DOI] [PubMed] [Google Scholar]
- Höckel M., Schlenger K., Knoop C., Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991 Nov 15;51(22):6098–6102. [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. OXYGEN TENSION IN HUMAN MALIGNANT DISEASE UNDER HYPERBARIC CONDITIONS. Br J Cancer. 1965 Mar;19:139–150. doi: 10.1038/bjc.1965.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- Kreuzer F. Oxygen supply to tissues: the Krogh model and its assumptions. Experientia. 1982 Dec 15;38(12):1415–1426. doi: 10.1007/BF01955753. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwock L., Gill M., McMurry H. L., Beckman W., Raleigh J. A., Joseph A. P. Evaluation of a fluorinated 2-nitroimidazole binding to hypoxic cells in tumor-bearing rats by 19F magnetic resonance spectroscopy and immunohistochemistry. Radiat Res. 1992 Jan;129(1):71–78. [PubMed] [Google Scholar]
- Leunig M., Yuan F., Menger M. D., Boucher Y., Goetz A. E., Messmer K., Jain R. K. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992 Dec 1;52(23):6553–6560. [PubMed] [Google Scholar]
- Rasey J. S., Koh W. J., Grierson J. R., Grunbaum Z., Krohn K. A. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int J Radiat Oncol Biol Phys. 1989 Nov;17(5):985–991. doi: 10.1016/0360-3016(89)90146-6. [DOI] [PubMed] [Google Scholar]
- Rumsey W. L., Vanderkooi J. M., Wilson D. F. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988 Sep 23;241(4873):1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- Secomb T. W., Hsu R., Dewhirst M. W., Klitzman B., Gross J. F. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993 Feb 15;25(3):481–489. doi: 10.1016/0360-3016(93)90070-c. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Swartz H. M., Boyer S., Gast P., Glockner J. F., Hu H., Liu K. J., Moussavi M., Norby S. W., Vahidi N., Walczak T. Measurements of pertinent concentrations of oxygen in vivo. Magn Reson Med. 1991 Aug;20(2):333–339. doi: 10.1002/mrm.1910200217. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- Torres Filho I. P., Intaglietta M. Microvessel PO2 measurements by phosphorescence decay method. Am J Physiol. 1993 Oct;265(4 Pt 2):H1434–H1438. doi: 10.1152/ajpheart.1993.265.4.H1434. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977 May;13(3):399–408. doi: 10.1016/0026-2862(77)90106-6. [DOI] [PubMed] [Google Scholar]
- Weerappuli D. P., Pittman R. N., Popel A. S. Effect of convection in capillaries on oxygen removal from arterioles in striated muscle. J Theor Biol. 1990 Nov 21;147(2):275–288. doi: 10.1016/s0022-5193(05)80057-8. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Cerniglia G. J. Localization of tumors and evaluation of their state of oxygenation by phosphorescence imaging. Cancer Res. 1992 Jul 15;52(14):3988–3993. [PubMed] [Google Scholar]
- Wilson D. F., Pastuszko A., DiGiacomo J. E., Pawlowski M., Schneiderman R., Delivoria-Papadopoulos M. Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J Appl Physiol (1985) 1991 Jun;70(6):2691–2696. doi: 10.1152/jappl.1991.70.6.2691. [DOI] [PubMed] [Google Scholar]
- Yuan F., Leunig M., Berk D. A., Jain R. K. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res. 1993 May;45(3):269–289. doi: 10.1006/mvre.1993.1024. [DOI] [PubMed] [Google Scholar]