Abstract
Studies in a number of countries have reported associations between exposure to ambient air pollutants and adverse birth outcomes, including low birth weight, preterm birth (PTB) and, less commonly, small for gestational age (SGA). Despite their growing number, the available studies have significant limitations, e.g., incomplete control of temporal trends in exposure, modest sample sizes, and a lack of information regarding individual risk factors such as smoking. No study has yet examined large numbers of susceptible individuals. We investigated the association between ambient air pollutant concentrations and term SGA and PTB outcomes among 164,905 singleton births in Detroit, Michigan occurring between 1990 and 2001. SO2, CO, NO2, O3 and PM10 exposures were used in single and multiple pollutant logistic regression models to estimate odds ratios (OR) for these outcomes, adjusted for the infant’s sex and gestational age, the mother’s race, age group, education level, smoking status and prenatal care, birth season, site of residence, and long-term exposure trends.
Term SGA was associated with CO levels exceeding 0.75 ppm (OR=1.14, 95% confidence interval=1.02–1.27) and NO2 exceeding 6.8 ppb (1.11, 1.03–1.21) exposures in the first month, and with PM10 exceeding 35 μg/m3 (1.22, 1.03–1.46) and O3 (1.11, 1.02–1.20) exposure in the third trimester. PTB was associated with SO2 (1.07, 1.01–1.14) exposure in the last month, and with (hourly) O3 exceeding 92 ppb (1.08, 1.02–1.14) exposure in the first month.
Exposure to several air pollutants at modest concentrations was associated with adverse birth outcomes. This study, which included a large Black population, suggests the importance of the early period of pregnancy for associations between term SGA with CO and NO2, and between O3 with PTB; and the late pregnancy period for associations between term SGA and O3 and PM10, and between SO2 with PTB. It also highlights the importance of accounting for individual risk factors such as maternal smoking, maternal race, and long-term trends in air pollutant levels and adverse birth outcomes in evaluating relationships between pollutant exposures and adverse birth outcomes.
Keywords: Ambient air pollution, Term low birth weight, Term small-for-gestational-age, Preterm birth
1. Introduction
Indicators of adverse birth outcomes and fetal health during pregnancy are predictors of infant mortality and morbidity (Goldenberge et al., 2008; Haram et al., 2006; McIntire et al., 1999). Common indicators include growth restriction, often defined as small for gestational age (SGA) and measured by a birth weight below the 10th percentile for the same gestational age by sex; preterm birth (PTB), defined as a live birth before the 37th week of gestation; and low birth weight (LBW), defined as a birth weight less than 2500 g. Air pollutants have been associated with adverse impacts on fetal development in both animal and human studies (Veras et al., 2010). Epidemiological studies have focused on LBW outcomes (Bignami et al., 1994; Falkner, 1986; Glinianaia et al., 2004; Kavlock and Grabowski, 1980; Kavlock et al., 1979; Maisonet et al., 2001; Ritz and Yu, 1999, Shah and Balkhair, 2011; Sram et al., 2005), and PTB and SGA outcomes in California (Huynh et al., 2006; Parker et al., 2005; Ritz et al., 2000, 2007; Salam et al., 2005; Wilhelm and Ritz, 2005). A recent meta-analysis has linked exposure to particulate matter (PM) to LBW and PTB (Sapkota et al., 2010); another multi-study shows adverse birth outcomes at high indoor PM concentrations caused by solid fuel cookstoves in developing countries (Pope et al., 2010). Other pollutants, e.g., sulfur dioxide (SO2) and carbon monoxide (CO), also have been associated with adverse birth outcomes. Key gaps in the growing adverse birth effects literature include the effects of pollutants at current exposures, the critical exposure windows during pregnancy, and most fundamentally, the underlying mechanisms (Bosetti et al., 2010; Sapkota et al., 2010; Shah and Balkhair, 2011; Slama et al., 2008).
The present study examines associations between ambient air pollutant concentrations and adverse birth outcomes occurring in three industrialized and urban areas in Detroit, Michigan, U.S. These areas contain a very high proportion of Blacks, a susceptible population that has not been previously examined for such associations. We report on models that evaluate associations between term SGA and PTB outcomes using several pollutants and exposure windows. A parallel analysis examines associations between term LBW and air pollutants in the same population, which is reported in the supplemental materials.
2. Material and methods
2.1. Study group, health outcomes, and covariates
The study group consisted of all live, singleton births for mothers living at time of birth in three areas of Detroit between January 1, 1990 and December 31, 2001. Birth certificate data, obtained from the Michigan Department of Community Health, were used to determine individual level covariates, which included gestational age, infant sex, date of birth, maternal age, race, smoking status, education level, and level of prenatal care. Eligible mothers resided in postal (ZIP) codes that were wholly or partially contained within a 4 km radius surrounding an air quality monitoring station, a distance that has shown stronger risk estimates for subjects than larger radii and presumed to better represent community level exposure (U.S. EPA, 1998; Wilhelm and Ritz, 2005). Eligible births were restricted in weight from 750 to 4000 g, gestational age from 22 to 42 weeks, and maternal age from 16 to 45 years. Birth weights exceeding 4000 g may result from poorly controlled maternal diabetes, and birth weights below 750 g are rarely viable and unlikely to be affected by air pollutant exposure. Gestational age was based on the date of the last menstrual period (LMP), if available, or the estimated week of gestation as estimated by the mother’s physician.
Term SGA births were defined as infants whose birth weights fell below the 10th percentile by sex and gestational week, based on study population’s distribution and restricted to gestational ages between 37 and 42weeks. A PTB was defined as a birth occurring before 37 weeks gestation. Term LBW was defined for infants born at or after 37 weeks of gestation and weighing less than 2500 g, but was not a focus of the present analysis because, by definition, it overlaps with term SGA. The LBW analysis is shown, however, in the supplemental materials.
2.2. Exposure assessment
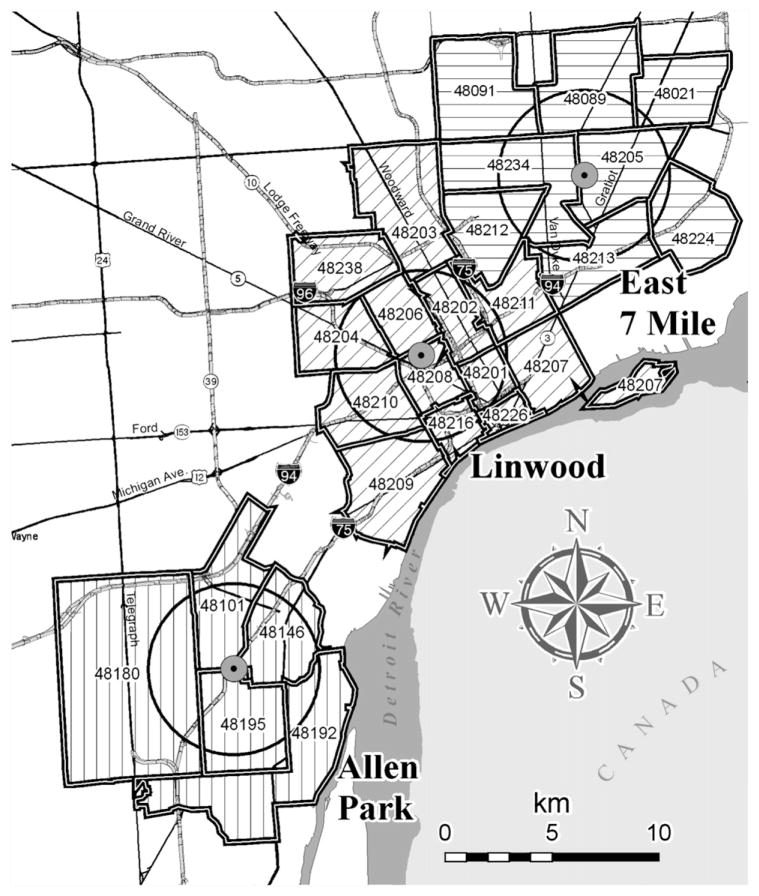
Three fixed site ambient air monitors located in densely populated, urban areas that measured multiple air pollutants over extended periods were chosen. These sites were approximately 20 km apart (Fig. 1), and were selected because they were the only sites that sampled CO, nitrogen dioxide (NO2), SO2 and particulate matter with aerodynamic diameter below 10 μm (PM10) in Detroit during the study period. The ambient data were collected by Michigan Department of Environmental Quality (MDEQ) with oversight by U.S. EPA using federal reference methods and MDEQ protocols (U.S. EPA, 2006). A total of 5, 8 and 13 zip codes were within the 4 km radius of the Allen Park, East Seven Mile, and Linwood monitors, respectively, out of a total of 30 zip codes in Detroit (Fig. 1). CO measurements were available at the Allen Park and Linwood sites for the entire study period; however, due to vandalism in July 1997 and quality assurance (QA) issues, CO data at Linwood were restricted to 1990–1996. SO2 measurements were available at East Seven Mile and Linwood sites for the entire study period, but only up to 1997 at Allen Park. NO2 was available for the entire study period at the East Seven Mile and Linwood sites; however, several months (September 1996 at Linwood; March, April and September through November 1997 at East Seven Mile) were omitted due to QA concerns. Ozone (O3) was monitored only during the high O3 season (April to September) in Detroit, however, year-round monitoring was conducted at downtown Windsor, Canada (approximately 20 km east), and these measurements were highly correlated to the Detroit data (r>0.83, high O3 season only). PM10 was measured every sixth day at Allen Park only. PM2.5 (PM less than 2.5 μm) was collected every third day from only May 1999. Due to the short time record, PM2.5 was excluded from the present analysis.
Fig. 1.
Map of the Detroit area showing the three air quality monitoring sites and 4 km radius.
Hourly measurements below method detection limits (MDLs) were replaced by one-half the MDL to avoid erroneous zero values. For CO, NO2 and SO2, daily (24-h) averages were computed from hourly data, from which monthly and trimester (3 month) averages were computed. For O3, the daily 1-hmaximum was used to compute daily, monthly and trimester averages. Running monthly and trimester averages were computed from the every sixth day PM10 measurements at Allen Park. Daily, monthly and trimester averages each required the availability of ≥75% of all possible measurements, e.g., daily averages required at least 18 of 24 possible hourly values. Exposures were estimated for five windows (time periods) for each pregnancy based on the gestational period and LMP: the first and last gestational months, and each trimester using divisions of 1–13, 14–26, and 27 weeks to birth, respectively (Parker et al., 2005).
2.3. Statistical methods
Logistic regression models were used to estimate adjusted odds ratios (AORs) and 95% confidence intervals (CIs) for each outcome and each of the five exposure windows (first month, last month, and first, second and third trimesters, as described above). For PTB, only the first and last months’ exposures were examined since, by definition, PTBs do not reach three full trimesters and analyses using the final trimester exposure would reduce the sample size. Term SGA and PTB outcomes were analyzed as dichotomous variables. Pollutant concentrations were expressed in quartiles, and AORs were calculated as ratios of associations for the second, third and fourth exposure quartiles relative to the first exposure quartile.
Models for PTB were adjusted for infant sex, maternal race (Black, White, other), maternal education level (<12, 12, >12 years), maternal smoking status during pregnancy (yes/no), use of prenatal care (yes/no), late prenatal care (starting after the fourth month of pregnancy; yes/no) and residence location (Allen Park, Linwood, East Seven Mile). To control for possible seasonal or community-specific effects not captured by birth records (Ritz and Wilhelm, 2008), models included variables for birth season, defined as spring (March–May), summer (June–Aug.), fall (Sept.–Nov.), and winter (Dec.–Feb.). To examine long-term trends in pollutant levels, a locally-weighted regression smoother was applied to the air pollutant concentrations (Cleveland, 1979). To control for possible biases associated with temporal changes in the study population and environment, models were adjusted for birth year using consecutive 4-year periods (1990–1993–1994–1997 and 1998–2001). No adjustments for gestational age were used because PTB was already restricted to births with gestational age from 22 to <37 weeks. Models for term SGA used similar adjustments, except infant sex was excluded since, by definition, this outcome captures sex and gestational age.
Single pollutant models were constructed by pooling data across all sites, with analytic control for site in the models. Multiple pollutant models were restricted to Linwood where CO, SO2 and NO2 were measured. PM10 measured at Allen Park was assigned to Linwood in multiple pollutant models given the modest PM concentration gradients in the region (Heindorf, 2007; Stevens et al., 2006). O3 was not included in the multiple pollutant models due to the lack of data during low O3 season. Models including O3 were evaluated using both Detroit data for the high O3 season, as well as the year-round data collected at Windsor. As a single test across the exposure quartiles for each pollution-outcome comparison, Wald type tests were conducted for joint hypotheses with 3 degrees of freedom, and trend tests were conducted for pollution-outcome associations for p-values below 0.05. Lastly, possible effect measure modification by race and maternal smoking status was investigated using analyses stratified by these variables. This analysis excluded “other” races due to small sample sizes.
The supplemental materials present sensitivity analyses which show similar analyses, but with stratification by maternal race, smoking status and educational levels, as well as additional analyses for term LBW.
3. Results
3.1. Study population
A total of 186,214 births occurred in the study zip codes between 1990 and 2001, which was reduced to 173,492 births (93% of all births) due to the 4 km radius restriction, and then to 164,905 births due to the remaining eligibility criteria (95% of births with in the 4 km radius). Infant and maternal characteristics by birth outcome and race are shown in Table 1. Most mothers were Black. In comparison to Black mothers, White mothers had fewer births as teenagers (16–19 years of age), were more likely to have completed high school, were more likely to obtain prenatal care, and were more likely to be smokers. Unsurprisingly, maternal smoking and lack of perinatal care also were associated with large increases in adverse birth outcomes. Based on (unadjusted) odds ratios of term SGA and PTB, infants born to Black mothers had an approximately 2-fold higher risk of both term SGA and PTB than those born to White mothers. (Supplemental Table S-1 lists odds ratios for each covariate.)
Table 1.
Infant and maternal characteristics by birth outcomes and ethnicity, 1990–2001.
| Characteristics | All birthsa N=164,905 (%) |
Term SGA N=13,754 (%) |
PTB N=24,954 (%) |
Term births N=139,951 (%) |
Black N=93,078 (%) |
White N=68,164 (%) |
|---|---|---|---|---|---|---|
| Infant sex | ||||||
| Female | 49.1 | 49.1 | 47.8 | 49.3 | 49.3 | 48.8 |
| Male | 50.9 | 51.0 | 52.2 | 50.7 | 50.6 | 51.2 |
| Race | ||||||
| Black | 56.4 | 69.1 | 71.1 | 53.8 | – | – |
| White | 41.3 | 28.6 | 27.1 | 43.9 | – | – |
| Other | 2.2 | 2.3 | 1.8 | 2.3 | – | – |
| Age (yrs) | ||||||
| 16–19 | 17.4 | 19.8 | 19.8 | 17.0 | 22.0 | 11.6 |
| 20–29 | 58.1 | 55.4 | 54.0 | 58.8 | 57.4 | 59.1 |
| ≥30 | 24.5 | 24.8 | 26.2 | 24.2 | 20.6 | 29.3 |
| Education (yrs) | ||||||
| 0–11 | 32.9 | 40.5 | 39.5 | 31.7 | 36.2 | 28.4 |
| 12 | 40.0 | 38.5 | 38.9 | 40.2 | 39.0 | 41.9 |
| ≥13 | 27.1 | 21.0 | 21.7 | 28.1 | 24.8 | 29.7 |
| Tobacco use | ||||||
| Smoker | 21.8 | 35.7 | 27.0 | 20.9 | 19.3 | 26.0 |
| Prenatal care | ||||||
| None | 3.4 | 5.4 | 7.5 | 2.7 | 4.8 | 1.7 |
| Late (after 4th month) | 26.0 | 31.8 | 35.9 | 24.3 | 32.7 | 17.2 |
| Birth season | ||||||
| Spring (Mar–May) | 25.2 | 24.8 | 25.1 | 25.3 | 25.0 | 25.7 |
| Summer (Jun–Aug) | 26.1 | 26.2 | 25.7 | 26.2 | 26.0 | 26.3 |
| Fall (Sept–Nov) | 24.1 | 23.7 | 23.8 | 24.2 | 23.8 | 24.5 |
| Winter (Dec–Feb) | 24.6 | 25.3 | 25.5 | 24.4 | 25.2 | 23.6 |
| Birth period | ||||||
| 1990–1993 | 39.5 | 43.4 | 40.6 | 39.3 | 41.1 | 37.7 |
| 1994–1997 | 31.3 | 29.7 | 31.0 | 31.3 | 31.0 | 31.5 |
| 1998–2001 | 29.3 | 26.9 | 28.5 | 29.4 | 28.0 | 30.8 |
Abbreviations: SGA, small for gestational age; PTB, preterm births.
All births included Blacks, Whites and others.
Several long-term trends were observed (Supplemental Table S-2). First, the overall birth rate as well as the rate of adverse birth outcomes declined, with the greatest change occurring between the 1990–3 and 1994–7 periods. Second, most but not all risk factors also showed downward trends, with some difference by race. For example, the rate of teenage pregnancies declined from 19% in 1990 to 15% in 2001, largely due to decreases among Black mothers (from 25 to 18%), rather than among White mothers, which were relatively stable at 11%. Many of these patterns observed followed national trends, e.g., downward trends of teenage pregnancy and smoking during pregnancy (Hamilton et al., 2003; Mathews, 2001).
3.2. Air pollutant exposures
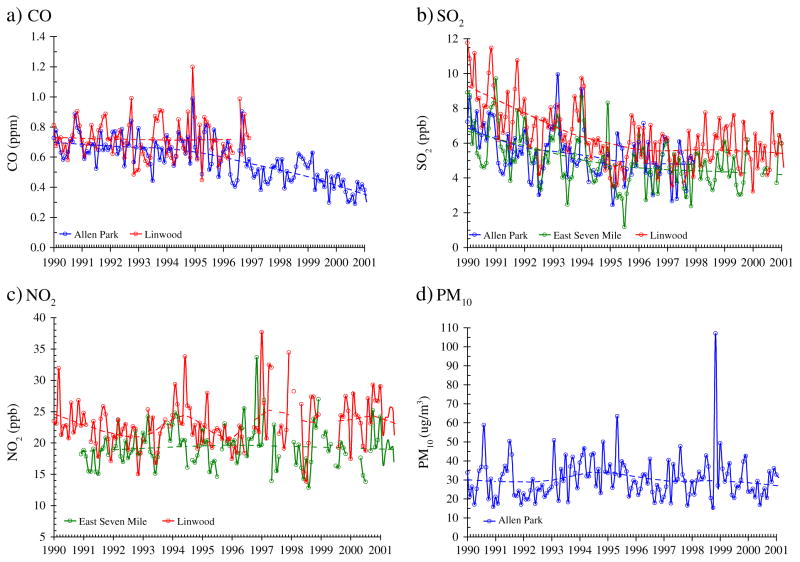
Exposure levels for 3-h, 24-h, first month and first trimester averaging periods are shown in Table 2. Concentrations were below the U.S. National Ambient Air Quality Standards (NAAQS) with the exception of O3, which exceeded the current 8-h NAAQS of 75 ppb. Over the 12-year study period, both the average concentration and the amplitude of concentration fluctuations declined for CO and SO2 (Fig. 2a, b), but not for NO2 and PM10 (Fig. 2c, d). For O3, the maximum 1-h concentration (April– September) slightly increased over the study period, and East 7 Mile had the highest levels (Fig. 3). Considering monthly averages, SO2 had low-to-moderate correlation with CO (r=0.35) and NO2 (r=0.27); CO and NO2 had low correlation (r≤0.27); and PM10 had low correlation with both CO and SO2 (r≤0.11). The correlation coefficients varied little when different exposure windows were used. Although they capture only pair-wise dependencies, the correlation coefficients suggest that collinearity would not be a problem in multi-pollutant models.
Table 2.
Summary of air pollutant concentrations.
| Pollutant | Average time | Site | N | Mean | SD | Min | Percentile
|
Max | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| CO (ppm) | 3-h | All | 6674 | 0.84 | 0.72 | 0.05 | 0.40 | 0.63 | 1.03 | 8.77 |
| AP | 4266 | 0.80 | 0.69 | 0.05 | 0.40 | 0.60 | 0.97 | 8.77 | ||
| LW | 2408 | 0.91 | 0.76 | 0.05 | 0.43 | 0.70 | 1.13 | 7.23 | ||
| 24-h | All | 6695 | 0.62 | 0.38 | 0.05 | 0.37 | 0.53 | 0.77 | 5.18 | |
| AP | 4278 | 0.56 | 0.33 | 0.05 | 0.35 | 0.49 | 0.70 | 4.01 | ||
| LW | 2417 | 0.72 | 0.44 | 0.05 | 0.43 | 0.62 | 0.90 | 5.18 | ||
| Montha | All | 66,182 | 0.66 | 0.15 | 0.26 | 0.57 | 0.67 | 0.76 | 1.18 | |
| Trimestera | All | 66,905 | 0.66 | 0.12 | 0.28 | 0.61 | 0.67 | 0.73 | 0.93 | |
| SO2 (ppb) | 24-h | All | 11,194 | 5.6 | 4.8 | 0.5 | 2.2 | 4.1 | 7.4 | 49.5 |
| AP | 2826 | 5.4 | 4.1 | 0.5 | 2.4 | 4.3 | 7.2 | 31.7 | ||
| E7M | 4108 | 4.9 | 4.1 | 0.5 | 2.0 | 3.7 | 6.5 | 31.4 | ||
| LW | 4260 | 6.3 | 5.7 | 0.5 | 2.2 | 4.5 | 8.7 | 49.5 | ||
| Montha | All | 140,092 | 5.8 | 1.8 | 1.0 | 4.5 | 5.5 | 6.8 | 12.5 | |
| Trimestera | All | 141,016 | 5.8 | 1.5 | 2.2 | 4.7 | 5.5 | 6.6 | 11.0 | |
| NO2 (ppb) | 24-h | All | 7169 | 21.2 | 9.3 | 0.5 | 14.7 | 20.1 | 26.3 | 76.7 |
| E7M | 3418 | 19.2 | 8.5 | 0.5 | 13.2 | 18.1 | 24.0 | 76.7 | ||
| LW | 3751 | 23.0 | 9.6 | 0.5 | 16.4 | 21.9 | 27.9 | 76.5 | ||
| Annual | All | 12 | 21.3 | 1.2 | 19.6 | 20.6 | 21.0 | 21.9 | 23.5 | |
| E7M | 12 | 19.1 | 1.1 | 17.6 | 18.6 | 18.9 | 19.4 | 21.6 | ||
| LW | 12 | 23.0 | 1.6 | 20.9 | 21.6 | 23.2 | 24.0 | 26.1 | ||
| Montha | All | 99,442 | 21.3 | 4.1 | 8.2 | 18.7 | 21.0 | 23.6 | 41.7 | |
| Trimestera | All | 100,163 | 21.2 | 3.1 | 14.1 | 19.1 | 21.0 | 23.2 | 30.8 | |
| PM10 (μg/m3) | 24-h | AP | 661 | 29.9 | 16.1 | 4.0 | 19.0 | 27.0 | 37.0 | 131.0 |
| Montha | AP | 27,178 | 30.0 | 9.3 | 12.8 | 23.0 | 29.0 | 35.8 | 63.4 | |
| Trimestera | AP | 27,376 | 30.0 | 6.4 | 17.5 | 24.3 | 30.1 | 35.2 | 46.0 | |
| O3 (ppb) | 8-h | All | 6366 | 41.99 | 17.93 | 0.50 | 29.25 | 39.88 | 52.75 | 113.50 |
| AP | 2173 | 39.37 | 16.56 | 2.25 | 27.25 | 37.38 | 49.50 | 113.50 | ||
| E7M | 2098 | 45.98 | 18.82 | 0.50 | 32.71 | 44.00 | 57.50 | 108.00 | ||
| LW | 2095 | 40.71 | 17.70 | 1.00 | 28.00 | 39.00 | 51.75 | 108.00 | ||
| Montha | All | 94,279 | 69.78 | 21.03 | 3.50 | 55.13 | 72.63 | 86.25 | 113.50 | |
| Trimestera | All | 124,267 | 76.56 | 21.58 | 3.50 | 62.00 | 81.50 | 92.50 | 113.50 | |
Abbreviations: AP, Allen Park; E7M, East Seven Mile; LW, Linwood; SD, standard deviation; 25th, 50th, 75th are percentiles.
N represents the numbers of 3-h, 24-h, and annual average concentrations, and the numbers of subjects who had month and trimester average concentrations.
Month and trimester averages are subjects’ exposure estimates for 1st month and 1st trimester.
Fig. 2.
Trends of monthly averages of pollutant concentrations (CO, NO2, SO2 and PM10) from 1990 to 2001. Results of LOESS smoother shown as dashed line.
Fig. 3.
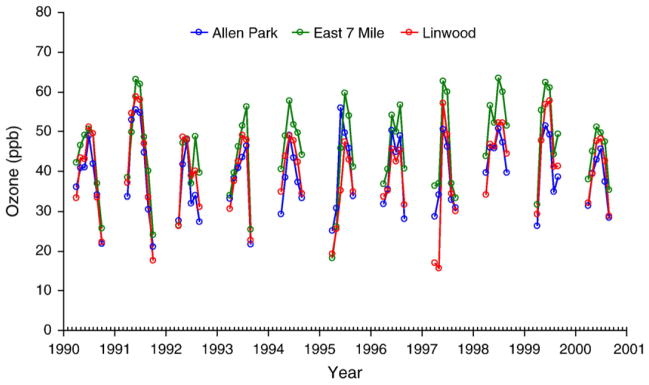
Trends of monthly averages of ozone concentrations from 1990 to 2001.
3.3. Single pollutant models
Results of single pollutant models for CO, SO2, NO2 and PM10 for term SGA and PTB outcomes are summarized in Table 3; results for O3 are shown in Table 4. We first describe results for term SGA. CO was positively associated with term SGA for all exposure windows, and the odds ratio of term SGA births increased from 1.05 to 1.20 for women with higher CO levels (>0.56 ppm; second through highest exposure quartiles). After adjusting for long-term trends, the strongest association between CO and term-SGA persisted for women in the highest quartile (>0.75 ppm) of first month CO exposures (AOR=1.14; 95% CI: 1.02–1.27; trend test p=0.04).
Table 3.
Adjusted odds ratio and 95% confident interval (95% CI) for each window of exposure to air pollutants (CO, SO2, NO2, and PM10) for term small for gestational age (SGA) and preterm birth (PTB).
| Windows and quartiles of exposures |
CO
|
SO2
|
NO2
|
PM10
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Adjusteda | Trend-adjustedb | N | Adjusteda | Trend-adjustedb | N | Adjusteda | Trend-adjustedb | N | Adjusteda | Trend-adjustedb | |||
| Term SGA | 1st month | 2nd | 51,545 | 1.17 (1.06–1.29) | 1.11 (1.00–1.24) | 103,576 | 0.98 (0.92–1.04) | 0.96 (0.90–1.03) | 70,918 | 1.06 (0.99–1.14) | 1.07 (0.99–1.14) | 23,929 | 1.06 (0.91–1.24) | 1.07 (0.92–1.25) |
| 3rd | 1.07 (0.97–1.18) | 1.00 (0.89–1.12) | 1.03 (0.97–1.10) | 1.00 (0.94–1.07) | 1.06 (0.98–1.14) | 1.06 (0.98–1.14) | 0.99 (0.84–1.16) | 1.01 (0.85–1.20) | ||||||
| 4th | 1.20 (1.09–1.33) | 1.14 (1.02–1.27) | 1.11 (1.04–1.18) | 1.04 (0.97–1.13) | 1.10 (1.01–1.19) | 1.11 (1.03–1.21) | 1.15 (0.98–1.36) | 1.16 (0.98–1.38) | ||||||
| Last month | 2nd | 56,916 | 1.06 (0.97–1.17) | 1.00 (0.90–1.11) | 111,480 | 1.03 (0.97–1.09) | 1.01 (0.95–1.07) | 77,264 | 1.00 (0.93–1.07) | 1.00 (0.93–1.07) | 25,905 | 0.99 (0.85–1.15) | 1.00 (0.86–1.17) | |
| 3rd | 1.10 (1.00–1.20) | 1.02 (0.91–1.13) | 0.98 (0.92–1.04) | 0.94 (0.88–1.01) | 1.00 (0.93–1.07) | 1.00 (0.93–1.07) | 1.07 (0.92–1.25) | 1.09 (0.93–1.28) | ||||||
| 4th | 1.05 (0.96–1.16) | 0.98 (0.88–1.09) | 1.07 (1.00–1.14) | 0.98 (0.91–1.05) | 0.93 (0.86–1.01) | 0.95 (0.88–1.03) | 1.08 (0.92–1.26) | 1.07 (0.91–1.26) | ||||||
| 1st trimester | 2nd | 52,254 | 1.15 (1.04–1.27) | 1.11 (0.98–1.25) | 104,362 | 1.04 (0.98–1.11) | 1.02 (0.96–1.09) | 71,449 | 1.02 (0.95–1.10) | 1.03 (0.96–1.11) | 24,096 | 1.02 (0.87–1.19) | 1.06 (0.90–1.25) | |
| 3rd | 1.17 (1.06–1.29) | 1.10 (0.98–1.24) | 1.04 (0.97–1.11) | 1.01 (0.94–1.09) | 1.04 (0.95–1.12) | 1.05 (0.97–1.14) | 1.01 (0.86–1.20) | 1.06 (0.89–1.27) | ||||||
| 4th | 1.16 (1.04–1.28) | 1.10 (0.97–1.25) | 1.15 (1.08–1.23) | 1.09 (1.00–1.18) | 1.02 (0.93–1.12) | 1.06 (0.97–1.16) | 1.11 (0.94–1.32) | 1.14 (0.95–1.36) | ||||||
| 2nd trimester | 2nd | 53,805 | 1.07 (0.97–1.18) | 1.01 (0.90–1.13) | 107,681 | 1.00 (0.94–1.06) | 0.98 (0.92–1.05) | 74,384 | 0.98 (0.91–1.05) | 0.98 (0.91–1.06) | 24,248 | 1.16 (0.99–1.37) | 1.23 (1.04–1.45) | |
| 3rd | 1.10 (1.00–1.21) | 1.01 (0.90–1.14) | 0.97 (0.91–1.03) | 0.94 (0.88–1.01) | 0.97 (0.90–1.06) | 0.99 (0.91–1.08) | 1.17 (0.99–1.38) | 1.22 (1.02–1.45) | ||||||
| 4th | 1.10 (0.99–1.23) | 1.02 (0.90–1.15) | 1.12 (1.05–1.20) | 1.05 (0.96–1.14) | 0.96 (0.88–1.05) | 1.01 (0.92–1.11) | 1.04 (0.88–1.23) | 1.05 (0.87–1.26) | ||||||
| 3rd trimester | 2nd | 51,607 | 1.08 (0.98–1.19) | 1.00 (0.90–1.11) | 112,609 | 1.07 (1.01–1.13) | 1.04 (0.98–1.11) | 82,359 | 0.93 (0.87–1.00) | 0.94 (0.88–1.01) | 24,193 | 1.03 (0.88–1.22) | 1.05 (0.89–1.25) | |
| 3rd | 1.05 (0.95–1.15) | 0.96 (0.86–1.07) | 1.02 (0.95–1.08) | 0.98 (0.92–1.05) | 0.98 (0.91–1.06) | 0.99 (0.92–1.07) | 1.20 (1.02–1.42) | 1.25 (1.05–1.49) | ||||||
| 4th | 1.06 (0.96–1.17) | 0.97 (0.87–1.09) | 1.12 (1.05–1.20) | 1.03 (0.96–1.12) | 0.98 (0.90–1.06) | 1.01 (0.93–1.09) | 1.22 (1.04–1.44) | 1.22 (1.03–1.46) | ||||||
| PTB | 1st month | 2nd | 59,876 | 0.90 (0.84–0.97) | 0.96 (0.88–1.04) | 121,870 | 1.00 (0.96–1.05) | 0.99 (0.94–1.04) | 84,760 | 1.03 (0.97–1.08) | 1.03 (0.97–1.09) | 23,929 | 0.99 (0.87–1.12) | 0.97 (0.86–1.10) |
| 3rd | 0.92 (0.86–0.99) | 0.98 (0.90–1.07) | 1.00 (0.95–1.05) | 0.97 (0.93–1.02) | 1.03 (0.97–1.09) | 1.03 (0.97–1.09) | 1.10 (0.97–1.24) | 1.07 (0.94–1.22) | ||||||
| 4th | 0.89 (0.83–0.97) | 0.95 (0.87–1.03) | 1.03 (0.98–1.09) | 0.98 (0.92–1.04) | 1.00 (0.95–1.07) | 1.02 (0.96–1.08) | 1.06 (0.93–1.20) | 1.05 (0.92–1.20) | ||||||
| Last month | 2nd | 66,001 | 0.97 (0.91–1.04) | 1.04 (0.96–1.13) | 111,480 | 1.08 (1.03–1.13) | 1.08 (1.03–1.13) | 92,315 | 0.98 (0.93–1.04) | 0.98 (0.93–1.03) | 28,406 | 0.98 (0.87–1.10) | 0.96 (0.85–1.08) | |
| 3rd | 0.89 (0.83–0.96) | 0.96 (0.89–1.04) | 1.11 (1.05–1.16) | 1.11 (1.05–1.16) | 0.99 (0.94–1.05) | 0.99 (0.94–1.05) | 0.91 (0.80–1.02) | 0.88 (0.78–1.00) | ||||||
| 4th | 0.96 (0.89–1.04) | 1.04 (0.95–1.13) | 1.07 (1.02–1.13) | 1.07 (1.01–1.14) | 0.98 (0.92–1.04) | 0.98 (0.92–1.04) | 0.95 (0.84–1.07) | 0.95 (0.84–1.08) | ||||||
Note: N = numbers of mothers who had pollutant exposures by exposure windows; term SGA models do not include infant sex.
Adjusted for infant sex, maternal race, age groups, education levels, tobacco use, prenatal care, birth seasons and site of residency.
Adjusted for variables above and birth periods.
Table 4.
Adjusted odds ratio and 95% confident interval (95% CI) for each window of exposure to ozone during high season (April–September) and all season for term small for gestational age (SGA) and preterm birth (PTB).
| Window and quartiles of exposures | N | High seasona
|
High seasonb
|
N | All seasona
|
All seasonb
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORs | (95% CI) | ORs | (95% CI) | ORs | (95% CI) | ORs | (95% CI) | |||||
| Term SGA | 1st month | 2nd | 49,468 | 0.91 | (0.83–0.99) | 0.89 | (0.81–0.97) | 113,509 | 1.01 | (0.95–1.07) | 1.02 | (0.96–1.09) |
| 3rd | 0.96 | (0.88–1.04) | 0.95 | (0.87–1.03) | 1.01 | (0.94–1.08) | 1.03 | (0.96–1.11) | ||||
| 4th | 0.99 | (0.90–1.07) | 0.97 | (0.89–1.06) | 1.00 | (0.92–1.09) | 1.02 | (0.94–1.11) | ||||
| Last month | 2nd | 57,230 | 1.10 | (1.02–1.19) | 1.10 | (1.01–1.19) | 123,386 | 0.98 | (0.91–1.05) | 1.00 | (0.93–1.08) | |
| 3rd | 1.06 | (0.98–1.15) | 1.06 | (0.98–1.16) | 0.97 | (0.90–1.06) | 1.00 | (0.92–1.08) | ||||
| 4th | 1.10 | (1.02–1.20) | 1.11 | (1.02–1.21) | 0.99 | (0.90–1.09) | 1.02 | (0.93–1.12) | ||||
| 1st trimester | 2nd | 40,840 | 1.09 | (0.99–1.21) | 1.07 | (0.97–1.19) | 114,279 | 1.05 | (0.99–1.12) | 1.07 | (1.01–1.13) | |
| 3rd | 1.12 | (1.01–1.24) | 1.12 | (1.01–1.24) | 0.99 | (0.91–1.07) | 0.99 | (0.91–1.07) | ||||
| 4th | 1.10 | (0.98–1.24) | 1.13 | (1.01–1.27) | 1.04 | (0.96–1.14) | 1.05 | (0.96–1.14) | ||||
| 2nd trimester | 2nd | 40,006 | 0.92 | (0.84–1.02) | 0.92 | (0.83–1.02) | 118,379 | 0.98 | (0.92–1.05) | 1.00 | (0.94–1.07) | |
| 3rd | 1.01 | (0.91–1.11) | 1.00 | (0.90–1.11) | 0.98 | (0.91–1.06) | 0.99 | (0.91–1.07) | ||||
| 4th | 0.97 | (0.87–1.08) | 0.97 | (0.87–1.09) | 0.94 | (0.87–1.02) | 0.97 | (0.89–1.05) | ||||
| 3rd trimester | 2nd | 62,424 | 1.04 | (0.96–1.12) | 1.04 | (0.96–1.13) | 122,758 | 0.98 | (0.92–1.05) | 1.01 | (0.94–1.07) | |
| 3rd | 1.07 | (0.99–1.16) | 1.08 | (1.00–1.17) | 0.98 | (0.90–1.07) | 1.00 | (0.92–1.09) | ||||
| 4th | 1.11 | (1.02–1.20) | 1.11 | (1.02–1.20) | 1.04 | (0.94–1.14) | 1.06 | (0.96–1.16) | ||||
| PTB | 1st month | 2nd | 57,980 | 1.10 | (1.04–1.17) | 1.10 | (1.04–1.16) | 133,088 | 1.04 | (1.01–1.08) | 1.05 | (1.01–1.09) |
| 3rd | 1.07 | (1.01–1.13) | 1.06 | (1.01–1.13) | 1.09 | (1.04–1.14) | 1.09 | (1.04–1.14) | ||||
| 4th | 1.08 | (1.02–1.15) | 1.08 | (1.02–1.14) | 1.09 | (1.04–1.15) | 1.09 | (1.04–1.15) | ||||
| Last month | 2nd | 66,749 | 0.88 | (0.84–0.93) | 0.88 | (0.83–0.93) | 144,445 | 1.03 | (0.98–1.08) | 1.03 | (0.98–1.08) | |
| 3rd | 0.91 | (0.87–0.96) | 0.91 | (0.86–0.95) | 1.00 | (0.95–1.06) | 1.00 | (0.95–1.05) | ||||
| 4th | 0.96 | (0.91–1.01) | 0.96 | (0.91–1.01) | 0.99 | (0.94–1.06) | 0.98 | (0.93–1.05) | ||||
Adjusted for infant sex, maternal race, age groups, education levels, tobacco use, prenatal care, birth seasons and site of residency.
Adjusted for variables above and birth periods. (Note: Term SGA models do not include infant sex.)
For SO2, only first trimester exposures showed consistent odds ratios of term SGA birth increasing with SO2 concentration without trend-adjustments, however, trend-adjusted AORs were attenuated toward the null. First month and first trimester NO2 exposures were positively associated with term SGA, and results were unchanged after trend adjustment. The odds ratio of term SGA birth increased from 1.02 to 1.10 for women with higher NO2 levels (>18.7 ppb; second through the highest exposure quartiles). Women with the highest quartile first-month NO2 exposures (>23.6 ppb) had the highest risk (AOR=1.10; 95% CI: 1.01–1.19).
Maternal exposure to PM10 during all three trimesters was positively associated with term SGA, and results were unaffected by trend adjustment. The odds ratio of term SGA birth and PM10 exposure increased from 1.01 to 1.22 for women with higher PM10 (>22.8 μg m−3; second through the highest exposure quartiles). The third trimester top quartile PM10 exposure (>35.8 μg m−3) gave the highest risk of a term SGA birth (AOR=1.22; 1.04–1.44; trend test p=0.01).
For the high O3 season, the last month, first trimester and third trimester exposures showed consistent patterns of increasing odds ratios for term SGA births as O3 concentrations increased, both with and without trend-adjustments. For example, the trend-adjusted odds ratios increased from 1.04 to 1.13 (>63 ppb; second through top exposure quartiles), and women with highest quartile first trimester O3 exposures (>97ppb) had the highest risks (AOR=1.13; 95% CI: 1.01–1.27). The year-round models using the Windsor data did not show similar or consistent associations.
Turning to PTB, SO2 exposure during the last month was positively associated with this outcome, both with and without adjustments for long-term trends. The odds ratio for PTB birth increased from 1.07 to 1.11 for women with SO2 levels >4.5 ppb (second through highest exposure quartiles). No associations were observed for PTB with CO, NO2 or PM10. First month O3 exposure was positively associated with PTB in both the high O3 season (trend test p=0.01) and the year-round analyses (the latter using the Windsor data), and the odds ratio of a PTB birth increased from 1.06 to 1.10 for women with high O3 levels (>63 ppb; second through highest exposure quartiles).
3.4. Multiple pollutant models
Table 5 summarizes results of the four-pollutant models (CO, SO2, NO2 and PM10) for term SGA and PTB outcomes. All models were adjusted for long-term trends and were restricted to mothers near the Linwood site for the 1990–1996 period (given the availability of CO data, as described earlier). In these models, consistent patterns were obtained for term SGA births for CO (first and second trimester), SO2 (all trimesters), NO2 (first month and all trimesters), and PM10 (first month and first trimester). For term PTB births, odds ratios were elevated during only first-month SO2 and NO2 exposures. Overall, these results did not differ greatly from the results of single-pollutant models for the Linwood area mothers (data not shown). Furthermore, the patterns of associations among infants of the Linwood mothers did not differ appreciably from those obtained using single-pollutant models that included mothers from Allen Park and East Seven Mile, which suggests that the multi-pollutant model results may be representative of the larger study population. However, risk estimates for the multipollutant models were less precise, a result of the smaller sample size (n ranged from 24,191 to 32,768).
Table 5.
Results of the multipollutant models (including CO, SO2, NO2 and PM10) for the Linwood site for term small for gestational age (SGA) and preterm birth (PTB).
| Windows and quartiles of exposures | N | CO
|
SO2
|
NO2
|
PM10
|
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Term SGA | 1st month | 2nd | 24,191 | 1.04 (0.89–1.21) | 1.00 (0.83–1.20) | 1.14 (0.97–1.33) | 0.99 (0.88–1.10) |
| 3rd | 0.90 (0.77–1.05) | 0.99 (0.83–1.17) | 1.12 (0.97–1.31) | 1.06 (0.94–1.20) | |||
| 4th | 1.02 (0.87–1.19) | 0.93 (0.78–1.11) | 1.28 (1.09–1.49) | 1.00 (0.88–1.13) | |||
| Last month | 2nd | 26,946 | 0.93 (0.80–1.08) | 0.99 (0.83–1.18) | 1.09 (0.93–1.26) | 1.04 (0.94–1.16) | |
| 3rd | 1.03 (0.89–1.20) | 0.97 (0.82–1.15) | 1.04 (0.90–1.20) | 0.99 (0.88–1.17) | |||
| 4th | 0.98 (0.84–1.14) | 1.03 (0.86–1.23) | 0.99 (0.85–1.16) | 1.01 (0.90–1.14) | |||
| 1st trimester | 2nd | 25,952 | 1.22 (1.02–1.46) | 1.18 (0.92–1.51) | 1.04 (0.83–1.31) | 1.12 (0.98–1.28) | |
| 3rd | 1.20 (1.00–1.45) | 1.01 (0.83–1.23) | 1.04 (0.83–1.31) | 1.06 (0.92–1.23) | |||
| 4th | 1.16 (0.96–1.41) | 1.05 (0.87–1.28) | 1.14 (0.91–1.44) | 1.09 (0.94–1.25) | |||
| 2nd trimester | 2nd | 25,272 | 1.14 (0.95–1.36) | 1.30 (1.01–1.69) | 1.06 (0.81–1.40) | 1.10 (0.95–1.27) | |
| 3rd | 1.19 (0.98–1.44) | 1.12 (0.91–1.37) | 1.03 (0.78–1.35) | 1.05 (0.91–1.21) | |||
| 4th | 1.22 (1.01–1.47) | 1.11 (0.90–1.36) | 1.12 (0.85–1.48) | 1.06 (0.91–1.24) | |||
| 3rd trimester | 2nd | 24,651 | 0.97 (0.83–1.14) | 1.17 (0.94–1.45) | 1.10 (0.92–1.33) | 0.92 (0.81–1.05) | |
| 3rd | 0.98 (0.83–1.16) | 1.24 (1.02–1.50) | 1.07 (0.88–1.29) | 0.97 (0.85–1.11) | |||
| 4th | 0.99 (0.84–1.17) | 1.31 (1.06–1.60) | 1.04 (0.85–1.26) | 0.92 (0.81–1.04) | |||
| PTB | 1st month | 2nd | 29,560 | 0.94 (0.84–1.06) | 1.27 (1.11–1.47) | 1.06 (0.94–1.19) | 1.00 (0.92–1.09) |
| 3rd | 1.00 (0.90–1.13) | 1.14 (0.99–1.30) | 1.08 (0.97–1.21) | 1.01 (0.91–1.11) | |||
| 4th | 0.95 (0.85–1.06) | 1.13 (0.98–1.30) | 1.05 (0.94–1.18) | 1.08 (0.98–1.19) | |||
| Last month | 2nd | 32,768 | 1.01 (0.90–1.13) | 1.04 (0.91–1.19) | 0.92 (0.82–1.03) | 1.06 (0.97–1.15) | |
| 3rd | 0.94 (0.84–1.05) | 1.06 (0.93–1.21) | 0.99 (0.89–1.11) | 0.98 (0.89–1.08) | |||
| 4th | 1.03 (0.92–1.16) | 0.99 (0.86–1.14) | 1.01 (0.90–1.14) | 0.92 (0.84–1.01) | |||
Adjusted for infant sex, maternal race, age groups, education levels, tobacco use, prenatal care, birth seasons, site of residency and birth periods.
Note: Term SGA models do not include infant sex.
4. Discussion
After controlling for trends and covariates, the estimated odds ratios for term SGA with CO, NO2, O3 and PM10 exposures, and for PTB with O3 and SO2 exposures, showed that low to moderate exposure to ambient air pollutants was associated with small, though consistent increases in the risk of adverse birth outcomes. A similar association between term LBW and SO2 exposure during the early pregnancy period in the same population was also found (Supplemental Tables S-3 to S-5). This is the first adverse birth effects study examining air pollutants in Detroit, an area with considerable industry and a large Black population. We did not always find monotonically increasing dose–response relationships between pollutant exposures and risks, which could reflect sample size and statistical power limitations, artifacts due to exposure misclassification or trend adjustments, non-logit-linear dose–response relationships, and other reasons discussed below.
4.1. Possible mechanisms
Biological pathways linking pollutant exposure to term SGA and PTB are not well understood (Demerjian, 2000; Liu et al., 2003; Xu et al., 1995). Term SGA may be triggered by an abnormal reaction between trophoblast and uterine tissues in the first few weeks of pregnancy, which is consistent with the timing of the CO-term-SGA and NO2-term-SGA associations found in this study. CO reduces the oxygen-carrying capacity of maternal hemoglobin, which decreases oxygen delivery to the fetus, and it crosses the placental barrier where it interferes with oxygen binding to fetal hemoglobin (Di Cera et al., 1989; Sangalli et al., 2003). Both effects may induce tissue hypoxia and reduce fetal growth. Alternatively, CO may be a proxy for PM emissions from vehicles and other combustion sources that contain polycyclic aromatic hydrocarbons (PAHs) that can induce DNA adducts, which have been associated with increased risks of LBW (Perera et al., 1998, 1999; Sram et al., 1999). NO2 exposure increases lipid peroxidation in both maternal and cord blood, which could interfere with normal intrauterine growth development via oxidative stress (Tabacova et al., 1998). PM is a complex toxicant, which includes mixtures of fine particles, metals and organic matter (e.g., PAHs), and compositions are source-specific (Dejmek et al., 1999). Several mechanisms have been proposed for PM10, including the DNA adduct pathway discussed above.
The operative mechanism determines the critical exposure period, and periods identified in previous studies may inform this understanding. The critical exposure period for PTB associated with coal combustion toxics was the first two months of pregnancy (Mohorovic, 2004). For CO and SO2, exposures during the last month of pregnancy have been associated with PTB (Liu et al., 2003). Potential etiologic factors for PTB (infection and stress) may be associated with air pollutant exposures in late pregnancy (Lockwood, 1994), and possibly include alveolar inflammation or systemic infection (Maisonet et al., 2004; Yang et al., 2004), in which case the induction period may be short and immediate, leading to an early birth after a relevant exposure (Maisonet et al., 2001). The present study focused on both early and late exposure periods during pregnancy that have been previously associated with PTB. We found that only the last month’s SO2 exposure was associated with PTB births.
4.2. Comparison with previous studies
Our results for term SGA are generally consistent with studies examining CO, O3 and PM2.5 in California, U.S. (Parker et al., 2005; Salam et al., 2005), NO2, PM2.5 and PM10 in Australia (Mannes et al., 2005), CO, NO2 and PM2.5 in Canada (Liu et al., 2003, 2007), and PM10 in the Czech Republic (Dejmek et al., 1999). Regarding PTB, our results are consistent with studies examining prenatal exposures to SO2 (Bobak, 2000; Leem et al., 2006; Liu et al., 2003; Sagiv et al., 2005; Xu et al., 1995) and O3 (Leem et al., 2006). Still, as discussed below, these studies used a variety of designs, populations, airsheds, study durations, sample sizes, exposure measures, covariates and model structures, and thus vary with respect to their statistical power, degree of exposure misclassification, and control of confounding.
4.3. Air pollutant sources and trends
Detroit is a diverse airshed that contains many emission sources, e.g., steel mills, waste water treatment plants and sewage sludge incinerators, automotive fabrication and assembly plants, a petroleum refinery and storage facilities, asphalt plants, chemical manufacturing, and a high density of mobile sources (including many diesel trucks and a major international truck crossing). This diversity, along with the long record and monthly averages used, helps to explain the modest correlations observed among the pollutant measures, which are lower than those reported in most other studies (e.g., Liu et al., 2003, 2007). In an exposure study, Williams et al. (2008) identified likely emission sources at the Allen Park site as industry and freeway traffic, while the Linwood and East Seven Mile sites were affected by diesel truck traffic and other industrial sources. Other factors contributing to variability include the long time period and different long-term trends among the pollutants, the significance of background or regional concentrations, and the effects of meteorology (e.g., wind speed and direction, inversions, and precipitation) that can alter the dispersion and fate of different pollutants and different emission sources.
4.4. Temporal trends and exposure levels
Concentrations of pollutants such as SO2 and CO have declined over the past several decades, as have several individual risk factors of adverse birth outcomes, at least in some countries (Ananth et al., 2003; Dugandzic et al., 2006; Martin et al., 2006). We demonstrated that accounting for long-term declines in CO and SO2 concentrations attenuated effect sizes in the CO-term-SGA and SO2-term-SGA associations. In analyses examining CO-term-LBW associations using trend-adjusted and de-trended CO data (data not shown), associations were also attenuated. Conversely, associations for NO2 and PM10 did not show such trends and results were insensitive to temporal adjustments. With a few exceptions, previous birth effect studies have been short in duration, and effects of long-term temporal trends have not been extensively examined. A 13 year-long study in Canada found that birth year confounded associations between SO2 and PM10 and LBW (Dugandzic et al., 2006). These results, and our findings, suggest the need for time trend adjustments when analyzing long periods.
Another factor contributing to differences among studies are the considerable differences in pollutant levels, cut-offs considered, averaging times, and mixtures among cities and countries examined. For example, a Canadian study (Liu et al., 2003) examined 1 ppm and 10 ppb increases in CO and NO2 exposures, respectively, while a southern California study (Salam et al., 2005) used inter-quartile ranges of 1.2 ppm and 25 ppb, respectively, for monthly average CO and NO2 concentrations.
We also note the lack of multi-pollutant models in the adverse births effect literature, which are essential for understanding effects of simultaneous exposure to several pollutants. Only the most recent Canadian study (Liu et al., 2007) used multi-pollutant models (NO2, CO and PM2.5).
4.5. Social economic status, race and smoking and effect modification
Both socio-economic status (SES) and maternal smoking are well-known risk factors for adverse birth outcomes, and this was clearly seen in the Detroit data (e.g., Supplemental Table S-1). However, SES and smoking may not have ideal indicators (we used maternal education level for SES), and they have been inconsistently treated in the literature, e.g., they were included in analyses in the southern California study (Salam et al., 2005), but not in the Canadian studies (Liu et al., 2003, 2007). In addition to being a covariate, Ponce et al. (2005) suggested that SES might modify exposures or interact with effects of air pollutants. In stratified analyses, we found that term SGA births were positively associated with first trimester CO exposure among mothers with fewer than 12 years of education, but not among mothers with more education (Supplemental Table S-6). While this suggests that SES is an effect modifier that acts to increase the vulnerability of low SES women to the effects of air pollution, the evidence is limited and does not support the broader hypothesis.
In addition to SES, maternal race and smoking may modify effects of pollutant exposure, e.g., stronger effects have been found associating CO exposure with LBW(Maisonet et al., 2001) and PM2.5 with LBW(Bell et al., 2007) among infants of Black mothers as compared to White mothers. We saw some evidence of stronger associations between CO and term-SGA, NO2 and term-SGA, SO2 and term-SGA, and SO2 and PTB for infants of Black mothers as compared to associations for White mothers (Supplemental Tables S-7 to S-10). Such heterogeneity could reflect susceptibility attributable to individual-level factors for which race is a proxy, but also neighborhood-level vulnerability factors correlated with race, e.g., a lack of access to health care or intra-group diffusion of harmful health behaviors (Huebner, 2006). Both the Linwood area where CO2, NO2 and SO2 were measured, and the East Seven Mile area where NO2 and SO2 were measured, were predominantly Black areas, while Allen Park where CO and SO2 were measured was predominantly White. While we do not know of any specific biases, this pattern of housing segregation could allow a neighborhood effect.
For maternal smoking, heterogeneity was observed in some associations for term SGA, e.g., positively associations with first month NO2 exposures among smokers, and first month CO exposures and third trimester PM10 exposures among non-smokers. The lack of associations for CO or PM10 among smokers may reflect the large CO and PM10 doses already received by smoking mothers, diminishing the effects of ambient exposures or increasing the variability of the responses.
In summary, although we examined stratum-specific estimates to check for heterogeneity by maternal education (a measure of maternal SES), race and smoking, only a handful of results were found that were consistent with underlying effect measure modification. These findings should be interpreted cautiously. A large number of associations were examined in the stratified analyses, and the few results consistent with heterogeneity do not provide strong evidence that the associations were heterogeneous with respect to these three factors, and they may have been spurious. Despite an overall large sample size, the stratified analyses may not have had sufficient power to detect true effect measure modification.
4.6. Strengths and limitations
Strengths of this study included a large sample size (n=164,905), a study duration adequate for trend assessment (7 to 12 years, depending on pollutant), and individual-level information on residence location, maternal race, smoking status, pregnancy outcomes and SES. The analysis accounted for time trends in pollutant concentrations, which affected SO2 and CO results, and considered simultaneous exposures to multiple pollutants. Additionally, the study included a large Black population, an understudied and susceptible population, which allowed examination of possible heterogeneity of exposures and effect by race.
We also recognize limitations to the analysis. Geocoding of individual residences was unavailable, thus residences (and subjects) were selected if their ZIP code area was within 4 km of an air quality monitor. In the worst case, a residence could have been as far as 12 km from the monitor, which would increase the likelihood of exposure misclassification and bias. However, most homes were much closer since most of the studied areas were densely populated. Further, air pollution exposures at the ZIP code level can yield reasonable exposure estimates (Basu et al., 2004). Over time, pollutant levels in Detroit fell below those recorded in most other studies, and the low exposures may be subject to greater exposure measurement errors. Exposure misclassification is especially likely for subjects living near major traffic routes (more likely near Linwood and East Seven Mile sites), which could increase exposures above levels measured at the monitoring sites. (Monitor sites were located in residential areas at least several blocks from major roads.) However, limiting study areas to a relatively small radius (4 km) around monitors should minimize such errors. Missing pollutant data may have influenced results, although the results using a single monitor (Linwood) were consistent with those using all three sites, suggesting any such bias was minimal. Additional information on potential covariates and confounders not contained in the birth certificate database might have been helpful, e.g., alcohol consumption, although effects of many such factors would likely be correlated with other individual-level risk factors that were available. Finally, measurements of personal, occupational, or indoor exposures were unavailable, a limitation of all studies that rely on ambient exposure measures (Alm et al., 2001; Brunekreef et al., 2005; Kim et al., 2006).
Continuous exposures of air pollutants were considered but are not presented in this study for several reasons: the biologic pathways of air pollution exposures that lead to adverse birth outcomes are still not well understood, and exposure cut-offs (or thresholds) reported in the literature are inconsistent; the range of each pollutant and sometimes its units can differ among studies, which makes comparisons among studies difficult, especially in multiple pollutant models; and the use of categorical exposures allows examination of the dose–response relationship.
Since a large number of associations were estimated, there exists the possibility of spurious associations. We report point and interval estimates without constructing joint confidence regions determined using Bonferroni or other adjustments, which have been criticized as being overly conservative, even when there is scientific interest in testing the global hypothesis that all associations are null (Rothman et al., 2008). In the present study, interest focuses on the individual relations between pollutants and outcomes. Due to the moderate correlations observed among the pollutants, conventional multiple comparison “adjustments” would not be appropriate; other methods, e.g., hierarchical modeling, are beyond the scope of this paper and were not explored.
5. Conclusions
Exposures to ambient air pollutants CO, NO2, O3 and PM10 were associated with increased risk of term SGA, and exposures to O3 and SO2 were associated with increased risk of PTB in the Detroit population, which contains a high percentage and number of Black women. This study suggests the importance of the early period of pregnancy for the CO-term-SGA, NO2-term-SGA and O3-PTB associations, and the late pregnancy period for O3-term-SGA (high season), PM10-term-SGA and SO2-PTB associations. Results accounted for individual risk factors including maternal race and smoking, as well as the long-term trends in ambient air pollutant exposures.
Supplementary Material
Acknowledgments
Funding sources
This study was in part supported by Cooperative Agreement Number 572305 from the Agency for Toxic Substances and Disease Registry.
We appreciate contributions of Kathy Humphreys, Division for Vital Records and Health Statistics, Michigan Department of Community Health, and the Michigan Department of Environmental Quality. The authors also thank Drs. Graciela Mentz and Marie O’Neill for their comments.
Footnotes
Supplementary materials related to this article can be found online at doi: 10.1016/j.envint.2012.01.003.
Disclaimer
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry.
References
- Alm S, Mukala K, Tiittanen P, Jantunen MJ. Personal carbon monoxide exposures of preschool children in Helsinki, Findland—comparison to ambient air concentrations. Atmos Environ. 2001;35:6259–66. [Google Scholar]
- Ananth CV, Demissie K, Kramer MS, Vintzileos AM. Small-for-gestational-age births among black and white women: temporal trends in the United States. Am J Public Health. 2003;93:577–9. doi: 10.2105/ajph.93.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Woodruff TJ, Parker JD, Saulnier L, Schoendorf KC. Comparing exposure metrics in the relationship between PM2.5 and birth weight in California. J. Exp. Anal. Environ. Epidemiol. 2004;14:391–6. doi: 10.1038/sj.jea.7500336. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belange K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–24. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami G, Musi B, Dell’Omo G, Laviola G, Alleva E. Limited effects of ozone exposure during pregnancy on physical and neurobehavioral development of CD-1 mice. Toxicol Appl Pharmacol. 1994;129:264–71. doi: 10.1006/taap.1994.1251. [DOI] [PubMed] [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–6. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C, Nieuwenhuijsen MJ, Gallus S, Cipriani S, Vecchia CL, Parazzini F. Ambient particulate matter and preterm birth or birth weight: a review of the literature. Arch Toxicol. 2010;84:447–60. doi: 10.1007/s00204-010-0514-z. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Janssen NA, de Hartog JJ, Oldenwening M, Meliefste K, Hoek G, et al. Personal, indoor, and outdoor exposures to PM2.5 and its components for groups of cardiovascular patients in Amsterdam and Helsinki. The Health Effects Institute; 2005. [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- Dejmek J, Selevan SG, Benes I, Solansky I, Sram RJ. Fetal growth and maternal exposure to particulate matter during pregnancy. Environ Health Perspect. 1999;107:475–80. doi: 10.1289/ehp.99107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerjian KL. A review of national monitoring networks in North America. Atmos Environ. 2000;34:1861–84. [Google Scholar]
- Di Cera E, Doyle ML, Morgan MS, De Cristofaro R, Landolfi R, Bizzi B, et al. Carbon monoxide and oxygen binding to human hemoglobin F0. Biochemistry. 1989;28:2631–8. doi: 10.1021/bi00432a041. [DOI] [PubMed] [Google Scholar]
- Dugandzic R, Dodds L, Stieb D, Smith-Doiron M. The association between low level exposures to ambient air pollution and term low birth weight: a retrospective cohort study. Environ Health. 2006:1–8. doi: 10.1186/1476-069X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. Developmental biology prenatal growth in human growth. New York: Plenum Press; 1986. [Google Scholar]
- Glinianaia S, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health—a systematic review of the epidemiologic evidence. Epidemiol. 2004;15:36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- Goldenberge RL, Culhane JF, Iams JD, Romero R. Preterm birth 1—epidemiology and causes of preterm birth. Lancet. 2008:371. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Sutton PD. National vital statistics reports. Hayattsville, Maryland: Centers for Disease Control and Prevention; 2003. Births: preliminary data for 2002. [PubMed] [Google Scholar]
- Haram K, Softeland E, Bukowski R. Intrauterine growth restriction. Int J Gynecol Obstet. 2006;93:5–12. doi: 10.1016/j.ijgo.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Heindorf MA. Personal communication with Mary Ann Heindorf at Michigan Department of Environmental Quality. Lansing: 2007. [Google Scholar]
- Huebner C. Birth outcomes among urban African-American women: a multilevel analysis of the role of racial residential segregation. Soc Sci Med. 2006;63:3030–45. doi: 10.1016/j.socscimed.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Huynh M, Woodruff TJ, Parker JD, Schoendorf KC. Relationships between air pollution and preterm birth in California. Environ Effects. 2006;20:454–61. doi: 10.1111/j.1365-3016.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- Kavlock RJ, Grabowski CT. Studies on the developmental toxicity of ozone: postnatal effects. Toxicol Letters. 1980;5:3–9. doi: 10.1016/0378-4274(80)90141-1. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Daston G, Grabowski CT. Studies on the developmental toxicity of ozone. I. Prenatal effects Toxicol. Appl Pharmacol. 1979;48:19–28. doi: 10.1016/s0041-008x(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Kim D, Sass-Kortsak A, Purdham JT, Dales RE, Brook JR. Associations between personal exposures and fixed-site ambient measurements of fine particulate matter, nitrogen dioxide, and carbon monoxide in Toronto, Canada. J Exp Anal Environ Epidemiol. 2006;16:172–83. doi: 10.1038/sj.jea.7500446. [DOI] [PubMed] [Google Scholar]
- Leem J-H, Kaplan BM, Shim YK, Pohl HR, Gotway CA, Bullard SM, et al. Exposure to air pollutants during pregnancy and preterm delivery. Environ Health Perspect. 2006;114:905–9. doi: 10.1289/ehp.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111:1773–8. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Exp Anal Environ Epidemiol. 2007;17:426–32. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ. Recent advances in elucidating the pathogenesis of preterm delivery, the detection of patients at risk, and preventative therapies. Curr Opinion Obstet Gynceol. 1994;6:7–18. [PubMed] [Google Scholar]
- Maisonet M, Bush TJ, Correa A, Kaakkola JJK. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environ Health Perspect. 2001;109:351–6. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Correa A, Misra D, Jaakkola JJK. A review of the literature on the effects of ambient air pollution on fetal growth. Environ Res. 2004;95:106–15. doi: 10.1016/j.envres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on birth weight in Sydney. Occup Environ Med. 2005;62:524–30. doi: 10.1136/oem.2004.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. National vital statistics reports. Hayattsville, Maryland: Centers for Disease Control and Prevention; 2006. Births: final data for 2004. [PubMed] [Google Scholar]
- Mathews TJ. National vital statistics reports. Hayattsville, Maryland: Centers for Disease Control and Prevention; 2001. Smoking during pregnancy in the 1990s. [PubMed] [Google Scholar]
- McIntire DD, Bloom SL, Casey BM, Leveno K. Birth weight in relation to morbidity and mortality among newborn infants. New Engl J Med. 1999;340:1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- Mohorovic L. First two months of pregnancy—critical time for preterm delivery and low birth weight caused by adverse effects of coal combustion toxics. Early Hum Develop. 2004;80:115–23. doi: 10.1016/j.earlhumdev.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–8. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM. Recent developments in molecular epidemiology: a study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–14. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107:451–60. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce NA, Hoggatt KJ, Wilhelm M, Ritz B. Preterm birth: the interaction of traffic-related air pollution with economic hardship in Los Angeles neighborhoods. Am J Epidemiol. 2005;162:140–8. doi: 10.1093/aje/kwi173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32:70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–90. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ Health Perspect. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in southern California between 1989 and 1993. Epidemiol. 2000;11:502–11. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JKC. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166:1045–52. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Sagiv SK, Mendola P, Loomis D, Herring AH, Neas LM, Savitz DA, et al. A time series analysis of air pollution and preterm birth in Pennsylvania, 1997–2001. Environ Health Perspect. 2005;113:602–6. doi: 10.1289/ehp.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam MT, Millstein J, Li Y-F, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–44. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangalli MR, McLean AJ, Peek MJ, Rivory LP, Le Couteur DG. Carbon monoxide disposition and permeability-surface area product in the foetal circulation of the perfused term human placenta. Placenta. 2003;24:8–11. doi: 10.1053/plac.2002.0877. [DOI] [PubMed] [Google Scholar]
- Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Health. 2010 doi: 10.1007/s11869-010-0106-3. [DOI] [Google Scholar]
- Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37:498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–8. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Rossner P, Rubes J, Topinka J, Dejmek J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat Res. 1999;428:203–15. doi: 10.1016/s1383-5742(99)00048-4. [DOI] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–82. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CD, Williams R, Vette A, Jones P. Urban scale variability of PM2.5 components. Research Triangle Park: U.S. EPA; 2006. [Google Scholar]
- Tabacova S, Baird DD, Balabaeva L. Exposure to oxidized nitrogen: lipid erozidation and neonatal health risk. Arch Environ Health. 1998;53:214–21. doi: 10.1080/00039899809605698. [DOI] [PubMed] [Google Scholar]
- US EPA. Quality assurance handbook for air pollution measurement systems. Vol II: Part 1—ambient air quality monitoring program quality system development. 1998. EPA-454/R-98-004. [Google Scholar]
- U.S. EPA. List of designated reference and equivalent methods. 2006. [Google Scholar]
- Veras MM, Caldini EG, Dolhnikoff M, Saldiva PHN. Air pollution and effects on reproductive-system functions globally with particular emphasis on the Brazilian population. J Toxicol Environ Health. 2010;13:1–15. doi: 10.1080/10937401003673800. (Part B) [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113:1212–21. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Rea A, Vette A, Croghan C, Whitaker D, Stevens C, et al. The design and field implementation of the Detroit Exposure and Aerosol Research Study. J Expo Sci Environ Epidemiol. 2008:1–17. doi: 10.1038/jes.2008.61. [DOI] [PubMed] [Google Scholar]
- Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery. A community-based cohort study. Arch Environ Health. 1995;50:407–15. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Chang C-C, Chuang H-Y, Ho C-K, Wu T-N, Chang P-Y. Increased risk of preterm delivery among people living near the three oil refineries in Taiwan. Environ Int. 2004;30:337–42. doi: 10.1016/S0160-4120(03)00180-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.