Abstract
Varenicline and bupropion each have been shown to significantly improve cessation of tobacco addiction in humans. They act through different mechanisms and the question about the potential added efficacy with their combined used has arisen. Preclinical animal models of nicotine addiction can help with the evaluation of this combined approach and what dose combinations of varenicline and bupropion may be useful for enhancing tobacco cessation. In this study, we investigated the interacting dose-effect functions of varenicline and bupropion in a rat model of nicotine self-administration. Young adult female Sprague-Dawley rats were allowed to self-administer nicotine in one-hr sessions under an FR1 reinforcement schedule. Varenicline (0.3, 1. 3 mg/kg) and bupropion (8.33, 25, 75 mg/kg) were administered alone or together 15 min before each session. The vehicle saline was the control. Higher doses of each drug alone reduced nicotine self-administration compared to control with reductions of 62% and 75% with 3 mg/kg varenicline and 75 mg/kg bupropion respectively. Lower dose varenicline which does not by itself reduce nicotine self-administration, significantly augmented bupropion effects. The 0.3 mg/kg varenicline dose combined with the 25 and 75 mg/kg bupropion doses caused greater reductions of nicotine self-administration than either dose of bupropion given alone. However, higher dose varenicline did not have this effect. Lower dose bupropion did not augment varenicline effects. Only the high bupropion dose significantly enhanced the varenicline effect. Likewise, combinding 1 mg/kg varenicline with 75 mg/kg bupropion reduced self-administration to a greater extent than either dose alone. These results demonstrate that combination therapy with varenicline and bupropion may be more beneficial than monotherapy with either drug alone.
Keywords: Varenicline, Bupropion, Interactions, Nicotine, Self-administration
1. Introduction
Tobacco use remains the single largest preventable cause of disease and premature death worldwide (CDC, 2014). Current treatments to promote tobacco cessation are only modestly effective. There is much room for improvement. There are currently two pharmacological therapies approved by the Food and Drug Administration (FDA) for tobacco addiction that do not contain nicotine: bupropion and varenicline (FDA, 2012). Bupropion, a norepinephrine/dopamine reuptake inhibitor (NDRI) with nicotinic acetylcholine receptor (nAChR) inhibitory activity (Lukas et al. , 2010), was originally developed as an atypical antidepressant medication, but was later approved by the FDA for use as a smoking cessation aid in 1997. Varenicline is a partial agonist at α4β2*, α6β2* and α3β4 nAChRs, and a full agonist at α7 nAChRs (Bordia et al. , 2012, Mihalak et al. , 2006, Rollema et al. , 2007); in 2006, varenicline became the first non-nicotine therapeutic to be approved by the FDA specifically to treat tobacco addiction. Both of these drug treatments have been shown to reduce cravings and tobacco use in human subjects, and both also reduce nicotine self-administration in rodent models of nicotine addiction (Le Foll et al. , 2012, O'Connor et al. , 2010, Rauhut et al. , 2005, Rauhut et al. , 2003, Reus et al. , 2007). However, although the initial abstinence rates for each treatment are high, the rates of abstinence after one year of treatment were found to be only around 15% for bupropion and 23% for varenicline (Jorenby et al. , 2006). While these numbers were shown to be significantly better than placebo treatment, there is a clear need to develop better treatment strategies for tobacco addiction.
There has recently been increased interest in the idea of employing varenicline and bupropion as a combination therapy for smoking cessation. It has previously been shown that combination therapy with bupropion and the nicotine patch produces more favorable outcomes than the nicotine patch alone (Jorenby et al. , 1999), and that augmenting nicotine replacement therapy (NRT) with bupropion reduces failure rates for smokers who do not decrease smoking by more than 50% in the two weeks preceding their target quit date (Rose and Behm, 2013). Similar results have been found regarding varenicline and NRT (Koegelenberg et al. , 2014). The initial efficacy results for varenicline/bupropion combination therapy in humans have been promising for shorter-term abstinence rates, if somewhat mixed for prolonged abstinence at 52 weeks (Ebbert et al. , 2009, Ebbert et al. , 2014, Rose and Behm, 2014). In addition, these studies have shown that combination therapy with varenicline and bupropion resulted in a reduction in post-cessation weight gain among study participants; weight gain being a commonly reported reason for the continuance of tobacco use (Veldheer et al. , 2014).
To date, combination treatment with varenicline and bupropion has not been evaluated in preclinical animal models of nicotine addiction. Animal models can be helpful in clearly determining optimal dose combinations in a relatively economical way. The different mechanisms of action of each drug make them ideal candidates for use as a combination therapy for tobacco addiction, both to reduce craving for nicotine as well as to alleviate the somatic and affective symptoms of tobacco withdrawal. Indeed, both drugs have previously been shown, when administered individually, to reduce nicotine self-administration in rats and reduce withdrawal symptoms associated with nicotine (Cryan et al. , 2003, Igari et al. , 2014, Malin et al. , 2006, Paterson et al. , 2007). It is currently unknown whether the effects of a combination of varenicline and bupropion would be additive, synergistic, or time-course dependent and what the optimal dose combinations of these drugs would be. Previously we found that the nicotinic partial agonist sazetidine-A has a more prominent effect reducing nicotine self-administration later in the session {Johnson et al., 2012). In contrast, we found that the monoamine uptake inhibitor amitifadine had greater efficacy during the beginning of the test session {Levin et al., 2014). Therefore, we hypothesized that the nicotinic partial agonist varenicline would decrease nicotine self-administration preferentially during the later part of the session while the monoaminergic reuptake inhibitor bupripion would preferentially decrease nicotine self-administration during the initial part of the session. Nonetheless, it is a possibility that, given in combination, each drug may produce efficacious results at lower doses than would be needed if each drug were given individually. It remains to be seen whether this is indeed the case the rodent model.
This study was conducted to determine the interactive effects of combination treatment with varenicline and bupropion on nicotine self-administration behavior in rats. Each drug was administered both individually and in a series of combinations before self-administration sessions began to evaluate these effects. It was hypothesized that administration of higher doses of each compound would reduce nicotine self-administration in the rats, while lower doses given in combination would augment this reduction. It was also hypothesized that the effects of each drug would be time-course dependent throughout each session. The doses chosen for each drug were determined based on the extant literature and include doses that have been reported to have no undesirable off-target effects, such as suppression of food self-administration (George et al. , 2011, Liu et al. , 2008, O'Connor et al., 2010, Rauhut et al., 2005, Rauhut et al., 2003, Rollema et al., 2007). Alsoincluded in the study were sub-threshold doses that fall below those which have typically been observed to reduce nicotine self-administration. The results of this study could inform further research into the viability of combination therapy with varenicline and bupropion for smoking cessation treatments.
2. Materials and Methods
2.1. Subjects
Young-adult female Sprague-Dawley rats (Charles River Labs, Raleigh, NC, USA) were used in the study. At the time of catherization surgery, the rats were 60 days old and had an average weight of 173 grams. The rats were singly housed at Duke University in a vivarium adjacent to the testing facility. Rats were housed singly to prevent catheter harness damage occurring by cage-mates. The animals were housed in standard laboratory conditions and kept on a reverse 12:12 hr light/dark cycle. A total of 13 animals were used in the study. All testing was performed during the animals’ “active” (dark) phase of the cycle. While in their homecage environment rats were allowed unlimited access to fresh water and once behavioral testing began were kept on a restricted diet of standard rat chow so that each rat's body weight was approximately 85% of ad libitum feeding levels. All testing procedures in this study were conducted according to AAALAC guidelines and approved by the Duke University Animal Care and Use Committee.
2.2 Drugs
Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Varenicline tartrate was purchased from Abcam Inc. (Cambridge, MA, USA), and bupropion HCl was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All compounds were dissolved in 0.9% sterile saline (Hospira Inc, Lake Forest, IL, USA). For combined drug treatments of varenicline and bupropion, both compounds were dissolved together in the same sterile saline solution. Doses for each solution were injected subcutaneously (s.c.) in a volume of 1 ml/kg of body weight.
2.3 Surgical Procedures
Catheters were surgically implanted into the right jugular veins of each animal in the manner as previously described (Hall et al. , 2014). Briefly, animals were anesthetized with a combination of ketamine (60 mg/kg i.p.) and dexmedetomidine (0.15 mg/kg i.p.) and the jugular vein exposed via blunt dissection using aseptic technique. The catheters (SAI Infusion Technologies, Libertyville, IL, USA) were then implanted in the vein and the opposing end routed subcutaneously around the animal's back to emerge between the scapulae where they were attached to an infusion harness. Surgical wounds were treated with the topical anesthetic bupivacaine, and each animal was administered ketoprofen (5.0 mg/kg, s.c.) for postoperative pain. Catheters were flushed daily after each self-administration session with a lock solution that contained heparinized saline, and the antibiotic gentamicin (8mg/ml, Butler Schein Animal Health, Dublin, OH, USA).
2.4 Behavioral Procedures
All behavioral procedures were conducted in operant chambers (Med Associates, St. Albans, VT, USA) that measured 30.5 X 24.1 X 21.0 cm. Each operant chamber contained two response levers, two cue-lights (one placed above each response lever), a single house light, a tone generator, and a food trough. Animals were initially trained to press a lever to receive a 45 mg food pellet reward via FR1 response. The FR1 schedule was used to facilitate direct comparison to our previous studies with a wide variety of drug treatment some of which increase and others of which decrease nicotine self-administration. This schedule provides ample opportunity to see effects in both directions. An illuminated cue-light above one of the two levers in the operant chamber indicated the “active” lever. Criteria for completing the operant response training were defined as three consecutive 30 min sessions earning ≥ 50 pellets. Once the training criteria were met, rats underwent catheterization surgery (see above). After recovery from surgery, nicotine self-administration sessions were begun. Each self-administration session lasted 60 min. During self-administration sessions, a response on an active lever resulted in the delivery of a 50 μl infusion of nicotine (0.03 mg/kg, based on freebase weight) and the activation of the tone generator for 0.5 sec. Responses on the inactive lever had no consequence in the operant program to deliver nicotine and proceed through the session. Each infusion of nicotine was followed by a 20 sec timeout period wherein the cue-light above the active lever was extinguished and lever responses were recorded but no nicotine infusion was delivered. All behavioral sessions were programmed and recorded using MED-PC software (Med Associates, St. Albans, VT, USA).
After 10 baseline sessions of nicotine self-administration, sessions preceded by acute treatment with doses of varenicline and bupropion were begun. Drug solutions were injected (s.c.) 15 min before the start of each nicotine self-administration session. Doses of each compound were given both individually and in combination (Table 1). The order of the doses for each compound treatment was randomized for each animal, and the complete order of doses for each animal was given once. In the case of combined treatment, both varenicline and bupropion were dissolved together in solution, so that animals only received one injection of drug solution before each self-administration session. There was at least a day between consecutive injections. There were not additional training days between drug doses. Upon completion of nicotine self-administration sessions preceded by drug pretreatment, all animals were tested to ensure catheter patency using 0.3 ml of a solution of methohexital at a concentration of 5.0 mg/ml.
Table 1.
Doses of varenicline and bupropion used in nicotine self-administration sessions. Doses are presented as mg/kg.
| 8.33 Bup | 25.0 Bup | 75.0 Bup | |
|---|---|---|---|
| 0.3 Var | 0.3 Var+8.33 Bup | 0.3 Var+25.0 Bup | 0.3 Var+75.0 Bup |
| 1.0 Var | 1.0 Var+8.33 Bup | 1.0 Var+25.0 Bup | 1.0 Var+75.0 Bup |
| 3.0 Var | 3.0 Var+8.33 Bup | 1.0 Var+25.0 Bup | 3.0 Var+75.0 Bup |
Var = varenicline; Bup = bupropion
2.5 Statistical Analysis
Nicotine self-administration (infusions per session) data were evaluated for statistical significance by analysis of variance (ANOVA). Within subjects factors were varenicline dose, bupropion dose and 15-min block within each 1-hour session. The drug doses were given in a counterbalanced order among the rats. Possible differential bupropion and varencline effects in high and low responding rats was assessed by having a between subjects factor dividing the rats in to high and low responders for nicotine based on a median split based on the pre-drug baseline performance.
3. Results
3.1. Effects of combination treatment with varenicline and bupropion on nicotine self-administration
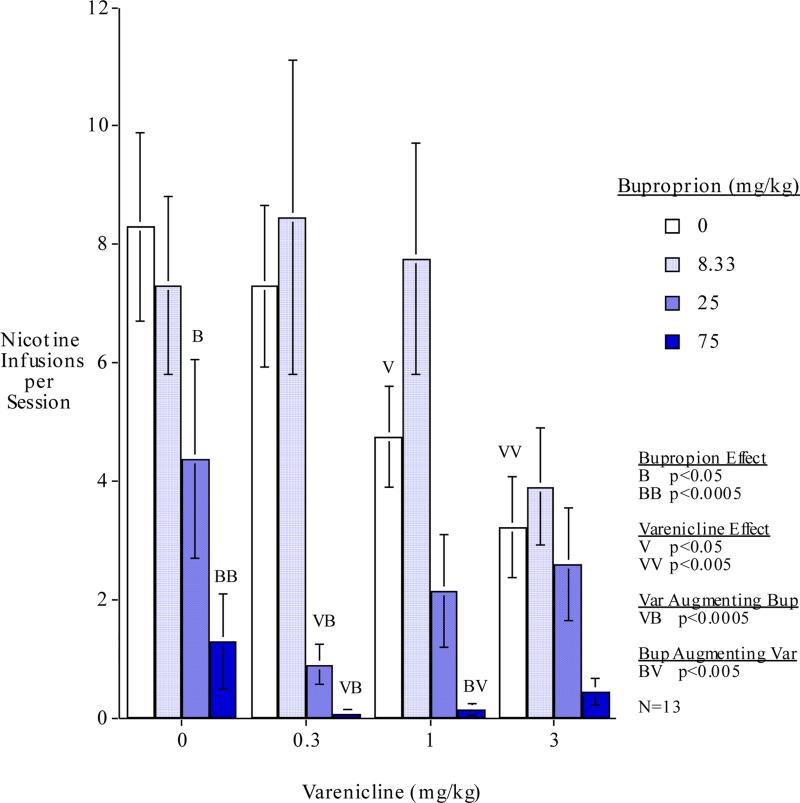
The main effects of varenicline (F(3,33)=3.73, p<0.025) and bupropion (F(3,33)=21.64, p<0.0005) were significant. The varenicline x bupropion interaction (F(9,99)=1.98, p<0.05) prompted follow-up simple main effects tests of each drug dose alone and compared with dose combinations. These comparisons showed that the when given alone varenicline at doses of 1 (F(1,99)=4.50, p<0.05) and 3 mg/kg (F(1,99)=9.67, p<0.005) caused significant decreases in nicotine self-administration compared with vehicle control. This corresponded to 42.6% and 61.2% decreases in nicotine self-administration respectively. The threshold for effect of varenicline was detected inasmuch as the lowest varenicline dose tested 0.3 mg/kg did not result in any significant effect on nicotine self-administration. Bupropion when given alone at doses of 25 (F(1,99)=5.71, p<0.05) and 75 mg/kg (F(1,99)=18.14, p<0.0005) caused significant decreases in nicotine self-administration compared with vehicle control. This corresponded to 47.2% and 84.3% decreases in nicotine self-administration respectively. The threshold for effect of bupropion was detected inasmuch as the lowest dose tested 8.33 mg/kg did not produce any significant effect on nicotine self-administration. There were no significant effects observed of drug treatments on inactive lever responding from our chosen dosages.
Over the dose-effect ranges of both drugs there were combinations that showed significant mutually augmenting effects in reducing nicotine self-administration. Low dose varenicline significantly augmented bupropion effectiveness whereas higher varenicline doses did not. In contrast high dose bupropion augmented varenicline effectiveness whereas lower dose did not. As shown in Figure 1, the low 0.3 mg/kg varenicline dose significantly augmented the reduction of nicotine self-administration by the 25 mg/kg (F(1,99)=16.29, p<0.0005) and 75 mg/kg (F(1,99)=20.84, p<0.0005) bupropion doses. Interestingly, the higher varenicline doses did not significantly augment bupropion's effects. While interactions with the 75 mg/kg bupropion dose may have been limited by a floor effect, there was actually a trend to diminished effect when combining the higher doses of varenicline with the 25 mg/kg bupropion dose (Fig. 1). The high 75 mg/kg bupropion dose significantly augmented the effectiveness of 1.0 mg/kg of varenicline (F(1,99)=8.52, p<0.005). There was a trend toward 75.0 mg/kg of bupropion also augmenting the effectiveness of 3.0 mg/kg of varenicline but this was not quite significant (p<0.09). However, the lower bupropion doses did not augment varenicline's effects on nicotine self-administration. In fact, 8.33 mg/kg of bupropion given together with the 1 mg/kg varenicline dose, nearly significantly (p<0.06) reversed the varenicline effect (Fig. 1).
Figure 1.
. Bupropion - varenicline interactions: total nicotine self-administration during each 1-hour session (mean±sem) n=13
3.2. Treatment effects during each 15 min time block
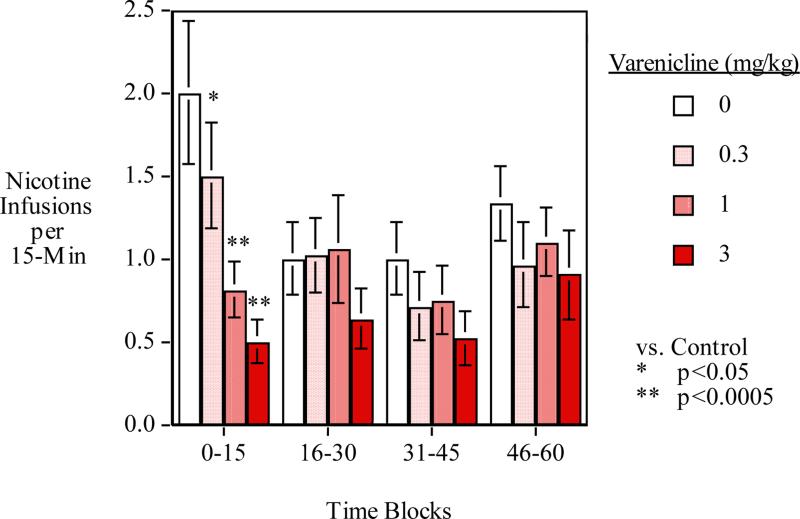
Figure 2 shows the breakdown of each 15-min time block averaged across sessions. The main effect of 15-min time blocks within the session was significant (F(3,33)=8.27, p<0.025). After higher levels of responding for nicotine during the initial 15-min period, levels dropped during the second and third 15-min blocks. Responding rose again during the fourth 15-min block of the test session. There was a significant interaction of varenicline x time-block (F(9,99)=2.75)=0.01).
Figure 2.
Varenicline effects during each 15-min time period during the 1-hour session averaged over the bupropion conditions (mean±sem)
3.3. Treatment effects in high vs. low responding rats
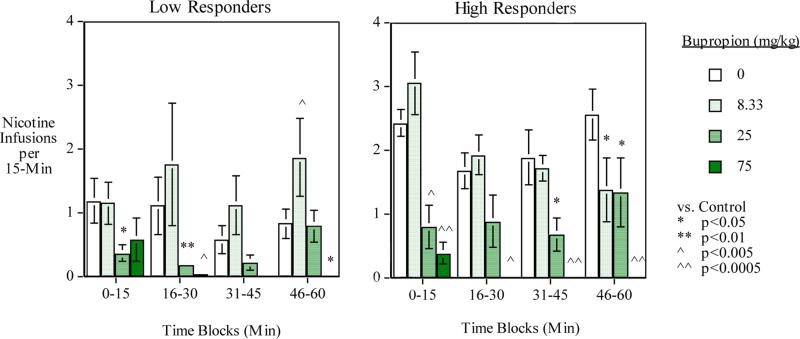
The main effect of low vs. high baseline responders was significant (F(1,11)=5.14, p<0.05). As expected the high baseline performers self-administered more nicotine during the drug test phase than the low baseline responders. There was a significant three-way interaction of bupropion x time-block x baseline level of self-administration (F(9,99)=2.24, p<0.05). To follow up this interaction tests of the simple main effects of drug treatment actions during each time block were tested. As shown in Figure 3, the tests of the simple main effects showed differential bupropion effects across the session for low and high level nicotine users. The high bupropion dose (75 mg/kg) produced substantial decreases in nicotine self-administration in both high and low nicotine self-administering groups. More modest lowering of nicotine self-administration was seen in both groups with the middle (25 mg/kg) bupropion dose. Interestingly, the low bupropion dose (8.33 mg/kg) caused a significant decrease in nicotine self-administration in the high nicotine user group (N=6) (F(1,45)=4.83, p<0.05), but caused a significant increase in nicotine self-administration in the low nicotine user group (N=7) (F(1,54)=4.83, p<0.005). No significant interaction of varenicline effects in low vs. high responding rats was seen.
Figure 3.
Bupropion effects during each 15-min time period during the 1-hour session averaged over the varenicline conditions: Low (N=7) and high (N=6) baseline nicotine self-administering groups (mean±sem)
4. Discussion
The results of this study provide evidence that the rat self-administration model is responsive to drug treatments that have been shown to be effective in aiding smoking cessation. As expected, treatment with higher doses of varenicline and bupropion significantly reduced nicotine self-administration compared to vehicle treatment when each compound was administered individually before testing sessions. Similar findings have been reported in previous preclinical studies (O'Connor et al., 2010, Rauhut et al., 2003). Neither drug, when given alone at the lowest doses tested, had any significant effect on nicotine self-administration in this study. Interestingly, our lowest chosen dose of varenicline (0.3 mg/kg) has been previously reported to reduce nicotine self-administration (O'Connor et al., 2010). However, methodological differences between O'Connor et al. and the current study likely account for this disparity; including different schedules of reinforcement (FR1 vs. FR3) as well as different doses of nicotine per infusion (0.03 vs. 0.015 mg/kg). O'Connor et al. also observed that repeated administration of 0.3 mg/kg increased the effect at this dose. In our study each animal was given this dose only once throughout testing sessions. Nonetheless, the combined evidence from our study and O'Connor et al. shows that chronic administration of low dose varenicline could be an efficacious strategy, particularly when coupled with bupropion treatment, which was the important finding in our study.
The novel findings in our study are that combination treatment with varenicline and bupropion reduce nicotine self-administration more effectively than single treatment with either drug, and that lower but not higher doses of varenicline augment the effects of higher doses of bupropion and higher but not lower doses of bupropion augment the effectiveness of varenicline. There were also significant findings regarding the time-course of effects of each drug. Varenicline was shown to be largely effective early, in the first 15 minutes of the session (Fig. 2), while bupropion's effects were spread across the entire session (Fig. 3).
The interactions of bupropion and varenicline provide useful information for helping to guide human studies using these treatments in combination. Our study shows that varenicline and bupropion do augment each other's effects, but they appear to do so most effectively at different points on their dose effect functions. Interestingly, it was the low dose of varenicline (0.3 mg/kg), a dose that was sub-threshold by itself, that significantly augmented the effectiveness of bupropion. Higher varenicline doses were not significantly effective in augmenting bupropion efficacy in reducing nicotine self-administration. In contrast it was the high dose of bupropion that significantly augmented the varenicline efficacy. Lower dose bupropion was not effective and even showed a trend for reversing the varenicline effect of reducing nicotine self-administration. While this near reversal effect of bupropion at the lowest dose may seem puzzling, it has been shown previously that lower doses of bupropion can significantly increase, rather than decrease, nicotine self-administration (Rauhut et al., 2003). It was speculated that, among other possibilities, the increase Rauhut et al. observed could have been due to attenuation of nicotine's reinforcing effects. Taken with the results found in this study, it may be most efficacious to use doses falling in the intermediate range of the response curve of both drugs.
The largest reductions in nicotine intake due to varenicline treatment, averaged across bupropion conditions, were observed within the first 15 minutes of the 1 hr sessions. Under control conditions, the rats displayed a robust intake of nicotine in the first 15 minutes of each session, while under drug treatment, there was a dose-dependent reduction of intake compared with control. In subsequent 15 min blocks the control condition intake tapered off to levels comparable to drug treatment conditions. While not an initial hypothesis of this study, these results may be taken to suggest that treatment with varenicline disrupts processing of environmental stimuli associated with nicotine use. All self-administration sessions in our study began with the illumination of the cue light (signaling an active lever) as well as three consecutive activations of the tone generator. It would appear that under vehicle treatment the animals showed a large reaction to the signal of the availability of nicotine, whereas during drug treatments they did not. However, the results of the few preclinical studies that have examined varenicline's effects on nicotine-associated environmental stimuli have been somewhat equivocal. Using an identical dose range of varenicline as was used in our study, Le Foll et al. found that 1.0 and 3.0 mg/kg of varenicline significantly reduced cue-induced reinstatement of nicotine seeking compared to control treatment, while 0.3 mg/kg increased the behavior (Le Foll et al., 2012). It should be noted that Le Foll and colleagues were examining the effects of pretreatment time differences on varenicline's effects in this study (120 min pretreatment time vs. previously reported times of 15-30 min) which may have affected the outcomes. Nonetheless, the study demonstrated that higher doses of varenicline can have long lasting effects on nicotine seeking. Using a slightly different dose range of varenicline (0.5, 1.5, & 2.5 mg/kg), Wouda et al. found that 0.5 mg/kg significantly increased cue-induced reinstatement of nicotine seeking, but found no effect at either 1.5 or 2.5 mg/kg (Wouda et al. , 2011). A more comprehensive study of the effects of varenicline on nicotine seeking was conducted by O'Connor et al, examining the drug's effects on nicotine cue, prime and cue+prime-induced reinstatement. Using our chosen dose range of varenicline (0.3-3.0 mg/kg), O'Connor et al. showed that in the absence of a nicotine prime, the drug had no effect on reinstatement behavior to nicotine, but did reduce both prime and cue+prime-induced reinstatement. While there are likely explanations for these different findings (precise methodology, dose range of varenicline, etc.), it is clear that more work should be done to better define the effects of varenicline on nicotine-associated environmental cues and stimuli.
It should be emphasized that, despite the evidence presented above, our study did not examine reinstatement behavior specifically, and that our 15min block results were averaged across all combinations of varenicline and bupropion. Bupropion's effects at higher doses were shown to be more spread out across each 15 min block. Due to the limited current knowledge regarding bupropion and nicotine self-administration, it is difficult to speculate on the exact effect the drug is exerting on the behavior. As stated previously, biphasic patterns on nicotine intake have been observed with bupropion depending on the dose administered, and there is much speculation regarding the mechanism behind these differential effects. However, it has been suggested that bupropion's effects on nicotine addiction lie outside the primary or conditioned reinforcing effects of nicotine (Liu et al., 2008, Paterson et al., 2007, Paterson et al. , 2008, Stairs et al. , 2007).
In summary, the results from this study show that combination treatment with varenicline and bupropion reduces nicotine self-administration in rats more effectively than monotherapy with either drug and that the interactions are dose-specific. Our data suggest that using doses that fall in the intermediate range of the dose-response curve for each drug may be the most efficacious strategy for cessation treatment, which may also help to avoid undesirable off-target side effects. While future studies are needed to demonstrate the efficacy of chronic treatment with a combination of varenicline and bupropion, the results from our study give promise for the success of currently ongoing studies in humans exploring this novel treatment strategy.
Highlights.
Higher doses varenicline (3 mg/kg) and bupropion (75 mg/kg) each reduced nicotine self-administration.
Lower dose varenicline which does not by itself reduce nicotine self-administration, significantly augmented bupropion effects.
The higher dose varenicline did not augment bupropion effects.
Only the high bupropion dose significantly enhanced the varenicline effect.
Combination therapy with varenicline and bupropion may be more beneficial than monotherapy with either drug alone.
Acknowledgement
This research was supported by P50 grant DA027840 from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at alpha6beta2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342:327–34. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Department of Health and Human Services [12/01/14];Smoking and Tobacco Use—Fact Sheet: Health Effects of Cigarette Smoking. 2014 http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm#toll.
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2009;11:234–9. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA : the journal of the American Medical Association. 2014;311:155–63. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA [12/01/2014];FDA 101: Smoking cessation products. 2012 http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm198176.htm.
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology. 2011;213:715–22. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacology, Biochemistry, and Behavior. 2014 doi: 10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology. 2014;39:455–65. doi: 10.1038/npp.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA : The Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal of Medicine. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O'Brien JA, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA : The Journal of the American Medical Association. 2014;312:155–61. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012;15:1265–74. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Wells C, Johnson JE, Rezvani AH, Bymaster FP, Rose JE. Amitifadine, a triple monoamine reuptake inhibitor, reduces nicotine self-administration in female rats. European Journal of Pharmacology. 2014 doi: 10.1016/j.ejphar.2015.06.041. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology. 2008;196:365–75. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Muresan AZ, Damaj MI, Blough BE, Huang X, Navarro HA, et al. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: aids to smoking cessation. Journal of Medicinal Chemistry. 2010;53:4731–48. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology. 2006;184:494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208:365–76. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. The European Journal of Neuroscience. 2007;25:3099–108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine & Tobacco Research. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine & Tobacco Research. 2005;7:901–7. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reus VI, Obach RS, Coe JW, Faessel H, Rollema H, Watsky E, et al. Varenicline: new treatment with efficacy in smoking cessation. Drugs of Today. 2007;43:65–75. doi: 10.1358/dot.2007.43.2.1069956. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. The American Journal of Psychiatry. 2013;170:860–7. doi: 10.1176/appi.ajp.2013.12070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination Treatment With Varenicline and Bupropion in an Adaptive Smoking Cessation Paradigm. The American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13050595. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Wei X, Boustany C, Hojahmat M, Cassis LA, et al. Effects of nornicotine enantiomers on intravenous S(-)-nicotine self-administration and cardiovascular function in rats. Psychopharmacology. 2007;190:145–55. doi: 10.1007/s00213-006-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldheer S, Yingst J, Foulds G, Hrabovsky S, Berg A, Sciamanna C, et al. Once bitten, twice shy: concern about gaining weight after smoking cessation and its association with seeking treatment. International Journal of Clinical Practice. 2014;68:388–95. doi: 10.1111/ijcp.12332. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]