Abstract
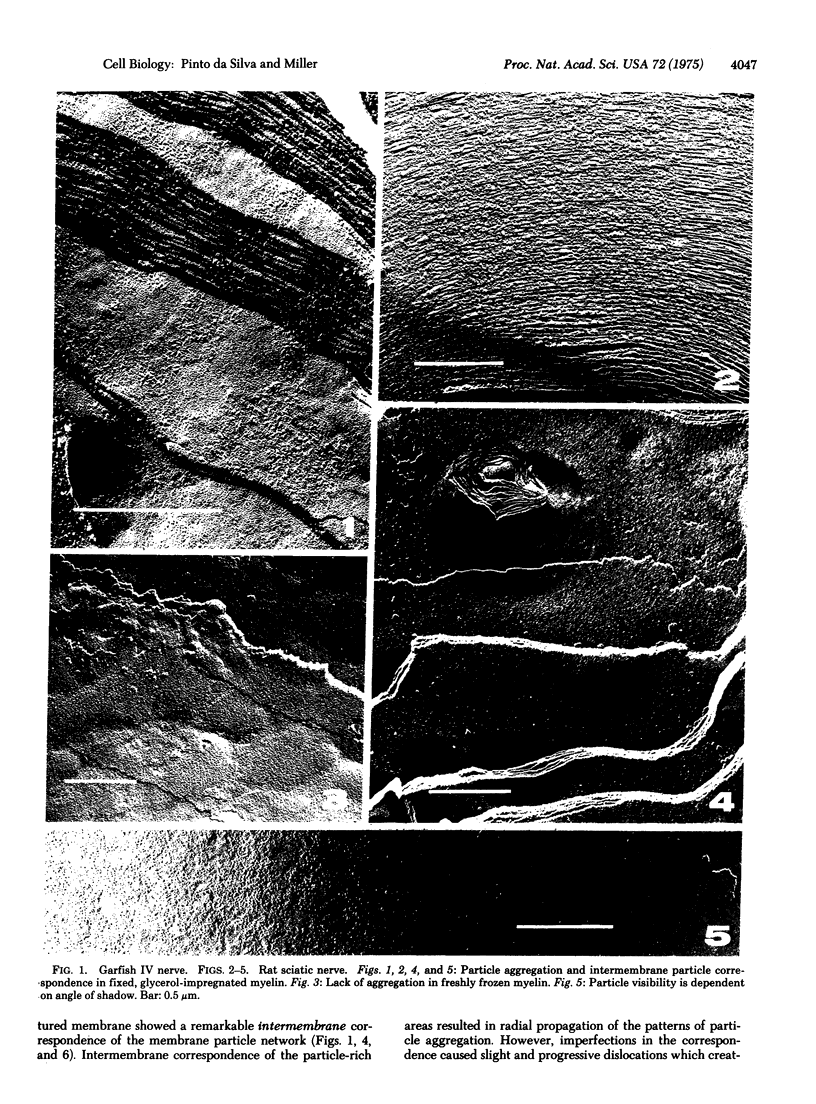
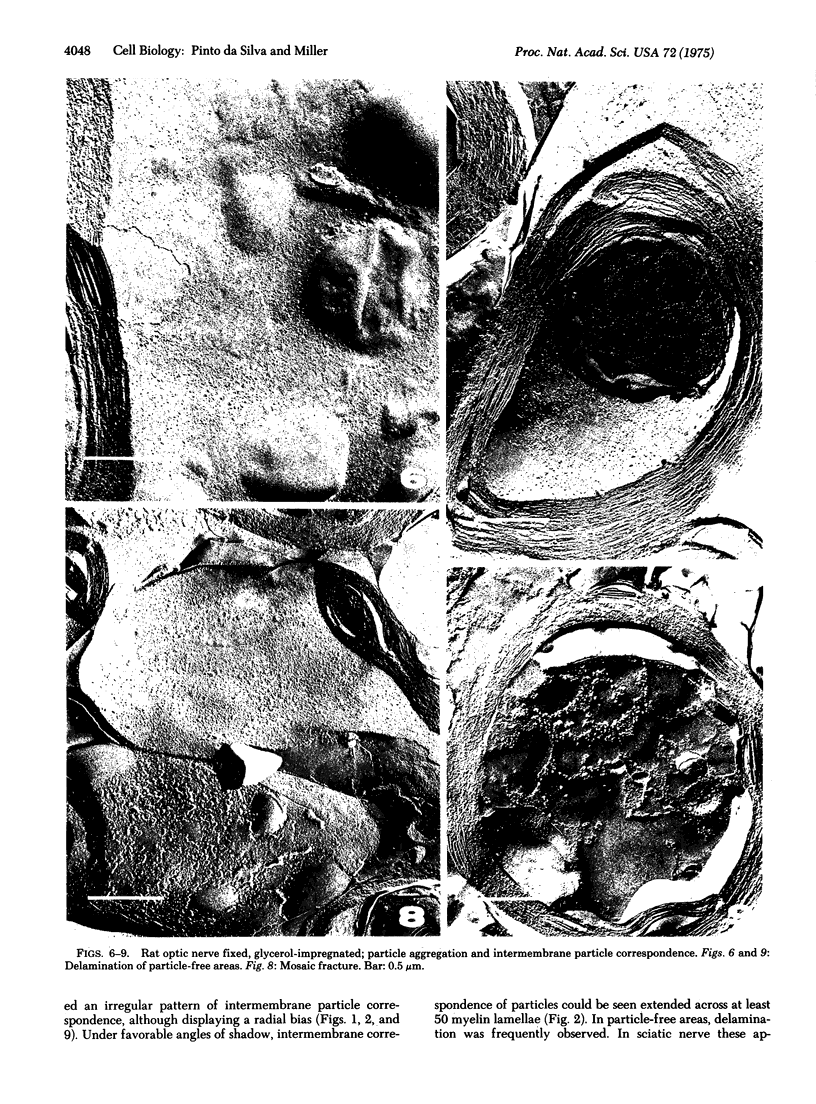
Freeze-fracture electron microscopy reveals constant and widespread presence of membrane particles on the fracture faces of frozen myelin. In unfixed myelin frozen shortly after dissection the distribution of the particles is uniform. In glutaraldehyde-fixed and/or glycerol-impregnated myelin the particles frequently occupy a network interspersed with circumscribed particle-free areas of variable dimension. In these membranes the network of particles is propagated radially across many lamellae. Particle-rich areas are closely apposed and contrast with frequent delamination of adjacent particle-free regions. We propose that, as in other plasma membranes, the particles of myelin represent protein-containing structures intercalated across the hydrophobic matrix of a membrane with bilayer organization. Our results indicate that these structures contain sites which mutually interact at the surface of apposed membranes and may be important in maintaining the organizational integrity of myelin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B., Hallpike J. F., Turner D. R. Histochemistry of myelin. XII. Anionic straining of myelin basic proteins for histology, electrophoresis and electron microscopy. J Neurochem. 1971 Mar;18(3):389–394. doi: 10.1111/j.1471-4159.1971.tb11966.x. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen myelin. Exp Cell Res. 1967 Mar;45(3):703–707. doi: 10.1016/0014-4827(67)90175-9. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R. Properties, structure and possible neuroreceptor role of the encephalitogenic protein of human brain. Nature. 1971 Jan 1;229(5279):25–28. doi: 10.1038/229025a0. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Kirschner D. A. Myelin membrane structure at 10 A resolution. Nat New Biol. 1971 May 12;231(19):46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- D'Monte B., Mela P., Marks N. Metabolic instability of myelin protein and proteolipid fractions. Eur J Biochem. 1971 Nov 11;23(2):355–365. doi: 10.1111/j.1432-1033.1971.tb01629.x. [DOI] [PubMed] [Google Scholar]
- Da Silva P. P., Martinez-Palomo A. Induced redistribution of membrane particles, anionic sites and con A receptors in Entamoeba histolytica. Nature. 1974 May 10;249(453):170–171. doi: 10.1038/249170a0. [DOI] [PubMed] [Google Scholar]
- Deamer D. W. Isolation and characterization of a lysolecithin-adenosine triphosphatase complex from lobster muscle microsomes. J Biol Chem. 1973 Aug 10;248(15):5477–5485. [PubMed] [Google Scholar]
- Deamer D. W., Leonard R., Tardieu A., Branton D. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta. 1970;219(1):47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Dermietzel R. Junctions in the central nervous system of the cat. I. Membrane fusion in central myelin. Cell Tissue Res. 1974 May 8;148(4):565–576. doi: 10.1007/BF00221940. [DOI] [PubMed] [Google Scholar]
- Easton D. M. Garfish olfactory nerve: easily accessible source of numerous long, homogeneous, nonmyelinated axons. Science. 1971 May 28;172(3986):952–955. doi: 10.1126/science.172.3986.952. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Glycophorin in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4653–4657. doi: 10.1073/pnas.71.12.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONJIN R., KOSAKA T., TAKANO I., HIRAMATSU K. ELECTRON MICROSCOPY OF NERVE FIBERS VII. ON THE ELECTRON DENSE RADIAL COMPONENT IN THE LAMINATED MYELIN SHEATH. Okajimas Folia Anat Jpn. 1963 Jul;39:39–53. doi: 10.2535/ofaj1936.39.3_39. [DOI] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M. A structural analysis of the myelin sheath in the central nervous system. J Cell Biol. 1967 Aug;34(2):555–567. doi: 10.1083/jcb.34.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston R. B., Pfenniger K., Moor H., Akert K. Specialized paranodal and interparanodal glial-axonal junctions in the peripheral and central nervous system: a freeze-etching study. Brain Res. 1973 Aug 17;58(1):1–24. doi: 10.1016/0006-8993(73)90820-2. [DOI] [PubMed] [Google Scholar]
- London Y., Demel R. A., Geurts van Kessel W. S., Vossenberg F. G., van Deenen L. L. The protection of A1 myelin basic protein against the action of proteolytic enzymes after interaction of the protein with lipids at the air-water interface. Biochim Biophys Acta. 1973 Jul 18;311(4):520–530. doi: 10.1016/0005-2736(73)90127-2. [DOI] [PubMed] [Google Scholar]
- PETERS A. FURTHER OBSERVATIONS ON THE STRUCTURE OF MYELIN SHEATHS IN THE CENTRAL NERVOUS SYSTEM. J Cell Biol. 1964 Feb;20:281–296. doi: 10.1083/jcb.20.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Fudenberg H. H. Anionic sites on the membrane intercalated particles of human erythrocyte ghost membranes. Freeze-etch localization. Exp Cell Res. 1973 Sep;81(1):127–138. doi: 10.1016/0014-4827(73)90119-5. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Gilula N. B. Gap junctions in normal and transformed fibroblasts in culture. Exp Cell Res. 1972;71(2):393–401. doi: 10.1016/0014-4827(72)90309-6. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Gulik-Krzywicki T., Sardet C. Association of the membrane-penetrating polypeptide segment of the human erythrocyte MN-glycoprotein with phospholipid bilayers. I. Formation of freeze-etch intramembranous particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3294–3298. doi: 10.1073/pnas.71.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. P., Martínez-Palomo A., Gonzalez-Robles A. Membrane structure and surface coat of Entamoeba histolytica. Topochemistry and dynamics of the cell surface: cap formation and microexudate. J Cell Biol. 1975 Mar;64(3):538–550. doi: 10.1083/jcb.64.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. P., Nicolson G. L. Freeze-etch localization of concanavalin A receptors to the membrane intercalated particles of human erythrocyte ghost membranes. Biochim Biophys Acta. 1974 Sep 23;363(3):311–319. doi: 10.1016/0005-2736(74)90071-6. [DOI] [PubMed] [Google Scholar]
- Singer M., Bryant S. V. Movements in the myelin Schwann sheath of the vertebrate axon. Nature. 1969 Mar 22;221(5186):1148–1150. doi: 10.1038/2211148a0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Eng L. F. The turnover of the lipid components of myelin. J Am Oil Chem Soc. 1965 Dec;42(12):1013–1018. doi: 10.1007/BF02636894. [DOI] [PubMed] [Google Scholar]
- Smith M. E. The turnover of myelin in the adult rat. Biochim Biophys Acta. 1968 Oct 22;164(2):285–293. doi: 10.1016/0005-2760(68)90154-9. [DOI] [PubMed] [Google Scholar]
- TEWARI H. B., BOURNE G. H. Neurokeratin network of the peripheral nerve fibre myelin sheath as a centre of metabolic activity. Nature. 1960 May 21;186:645–646. doi: 10.1038/186645a0. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER H., COLLINS G. H. COMPARISON OF OSMIUM TETROXIDE AND GLUTARALDEHYDE PERFUSION FIXATION FOR THE ELECTRON MICROSCOPIC STUDY OF THE NORMAL RAT PERIPHERAL NERVOUS SYSTEM. J Neuropathol Exp Neurol. 1964 Jan;23:109–126. doi: 10.1093/jnen/23.1.109. [DOI] [PubMed] [Google Scholar]