Abstract
Male Syrian hamsters (Mesocricetus auratus) treated with anabolic/androgenic steroids (AAS) during adolescence (P27–P56) display highly escalated and mature forms of offensive aggression correlated with increased γ-aminobutyric acid (GABA) afferent development as well as decreased GABAA receptors in the latero-anterior hypothalamus (LAH) – an area of convergence for developmental and neuroplastic changes that underlie offensive aggressive behaviors in hamsters. This study investigated whether microinfusion of a GABAA receptor agonist (muscimol; 0.01 – 1.0 pM) or antagonist (bicuculline; 0.04 – 4.0 pM) directly into the LAH modulate adolescent AAS-induced offensive aggression. Activation of LAH GABAA receptors enhanced adolescent AAS-induced offensive aggression, beginning at the 0.1pM dose, when compared with AAS-treated animals injected with saline into the LAH. Importantly, GABAA receptor agonism within the LAH significantly increased the frequency of belly/rear attacks, while simultaneously decreasing the frequency of frontal attacks. These data identify a neuroanatomical locus where GABAA receptor activation functions to enhance aggression in adolescent AAS-treated animals, while also promoting the display of mature forms of aggression and suppressing juvenile play behaviors.
INTRODUCTION
For over a decade we have used pubertal male Syrian hamsters (Mesocricetus auratus) as an adolescent animal model to investigate the effects of adolescent AAS exposure on the behavioral neurobiology of offensive aggression (DeLeon et al., 2002; Harrison et al., 2000a; Jackson et al., 2005; Knyshevski et al., 2005a,b; Ricci et al., 2004, 2007; see Melloni and Ricci, 2010) for a review. Behavioral data from these studies show that adolescent hamsters repeatedly exposed to moderate doses of AAS (5.0 mg/kg/day) display a mature and significantly escalated aggressive phenotype in the absence of prior social interactions and established dominance cues, suggesting that this treatment regimen impacts the temporal development of aggression and the select brain systems implicated in the complex control of this behavior. Neurobiological data from these studies identify the anterior hypothalamus (AH) as a point of convergence for developmental and neuroplastic changes that align with the mature and highly aggressive phenotype. In hamsters, the AH exists at the center of a neural network of reciprocal connections between the bed nucleus of the stria terminalis, lateral septum, medial amygdala, and ventrolateral hypothalamus that regulate offensive aggression (Delville et al., 2000). Recently we showed that adolescent hamsters stimulated to respond aggressively following AAS administration display significant alterations in the development and function of several neurotransmitter systems implicated in the control of aggressive behavior, i.e., the vasopressin (AVP) (Carrillo et al., 2011; Grimes et al., 2006, 2007; Harrison et al., 2000b; Melloni and Ricci, 2010), serotonin (5HT) (Grimes and Melloni, 2002, 2005; Ricci et al., 2006) and dopamine (DA) neural systems (Melloni and Ricci, 2010; Ricci et al., 2009; Schwartzer et al., 2009; Schwartzer and Melloni, 2010a, 2010b). Notably, these alterations were each observed in a ventrolateral subregion of the AH designated the latero-anterior hypothalamus (LAH) (DeLeon et al., 2002; Grimes and Melloni, 2005; Harrison et al., 2000b; Ricci et al., 2006). Behavioral pharmacology studies employing receptor agonists and antagonists for the AVP, 5HT, and DA neural systems show that increased aggressive behavior in adolescent AAS-treated hamsters is modulated directly by the activity of these systems within the LAH (Carrillo et al., 2011; Harrison et al., 2000b; Schwartzer and Melloni, 2010a, 2010b). Together, these data suggest that the LAH is an important point of convergence for neurodevelopmental and neuroplastic changes underlying adolescent AAS-induced offensive aggression, supporting the notion that the aggression-stimulating properties of adolescent AAS exposure are modulated by LAH neural signaling through select neurochemical systems.
The neurotransmitter γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system (CNS). Similar to AVP, 5HT, and DA, GABA has been implicated in aggressive behavior in various species and behavioral models, yet its role appears complex, both suppressing (Clement et al., 1987; Earley and Leonard, 1977; Guillot and Chapouthier, 1996, 1998; Haug et al., 1984; Krsiak et al., 1981; Poshivalov, 1981; Potegal et al., 1982; Puglisi-Allegra et al., 1981) and facilitating (Christmas and Maxwell, 1970; Cole and Wolf, 1970; Depaulis and Vergnes, 1985; DiMascio, 1973; Rodgers and Waters, 1985; Stork et al., 2000; Weerts et al., 1993) aggression. Across the neuraxis, GABA has been localized to both neurons and axon terminals (Bowers et al., 1998; Chen et al., 1998; Feldblum et al., 1993; Fenelon et al., 1995; Sur et al., 1999; Tappaz and Brownstein, 1977; Tappaz et al., 1977), including those that comprise the reciprocal hypothalamic neural circuit regulating aggressive behavior in hamsters; in particular the AH and LAH (Grimes et al., 2003; Schwartzer et al., 2009). In the CNS, GABA has been shown to exert its inhibitory action mainly through post-synaptic GABAA receptors, and aggressive behavior has been shown to be mediated by alterations in GABAA receptor activity (de Almeida et al., 2005; Jorge et al., 2002; Miczek et al., 2003). Recently, we localized a dense concentration of GABAA receptors aligned across the LAH (Schwartzer et al., 2009), so it is possible that GABA activity alters the activity of LAH-GABAA receptor-containing neurons that normally function to modulate aggression.
Previously, we showed that adolescent AAS exposure increases GABA-containing afferent terminals across the AH in hamsters (Grimes et al., 2003), suggesting that AH GABA activity is increased in aggressive, adolescent AAS-treated animals. This increase was not localized specifically to the LAH as we observed previously with AVP, 5HT, and DA afferents, suggesting that AH GABA neurons provide a diffuse inhibitory influence over neurons throughout the AH, and that this inhibitory influence is increased by adolescent AAS exposure. Conversely, an increase in GABA-containing neuronal somata were found exclusively within the LAH brain region after AAS exposure (Ricci et al., 2009), suggesting that the source of increased GABA afferents within the AH originate from GABA neurons in this brain region, and that adolescent AAS-induced increases in LAH GABA activity contribute to the mature and highly escalated adolescent AAS-induced aggressive phenotype. In contrast, while adolescent AAS exposure increases GABA production within the LAH and afferent development across the AH, subsequent studies from our laboratory have shown that aggressive, adolescent AAS-treated hamsters have fewer GABAAα1 subunit-containing receptors in the LAH (Schwartzer et al., 2009), bringing into question whether the development of the mature, highly aggressive phenotype in AAS-treated animals is due to an increase in the overall activity of GABA within the LAH. To examine this question, animals were treated with AAS throughout adolescent development and tested for aggressive behavior following direct pharmacological manipulations of LAH GABA signaling to determine whether alterations in GABA signaling through GABAA receptors in the LAH modulate adolescent AAS-induced offensive aggression.
METHODS
Subjects
Male Syrian hamsters (Mesocricetus auratus) (N=105) were obtained from Charles River Laboratories (Wilmington, MA) individually housed in polycarbonate cages, and maintained at ambient room temperature (22–24°C, with 55% relative humidity) on a reverse light-dark cycle (14L:10D; lights off at 08:00h) as previously described (Grimes and Melloni, 2002). Food and water were freely available. For aggression testing, stimulus (intruder) males of equal size and weight to the experimental animals were obtained from Charles River Laboratories one week prior to the behavioral test, group-housed (five animals per cage) in large polycarbonate cages, and maintained as above to acclimate to the animal facility. All studies employing live animals were pre-approved by the Northeastern University Institutional Animal Care and Use Committee and all methods used were consistent with guidelines provided by the National Institutes of Health for the scientific treatment of animals.
Experimental Treatment
On postnatal day (P) 27 hamsters (n=90) received daily subcutaneous (s.c.) injections (0.1–0.2ml) of an AAS mixture consisting of 2 mg/kg testosterone cypionate, 2 mg/kg nandrolone decanoate, and 1 mg/kg boldenone undecylenate dissolved in sesame oil, for 30 consecutive days during adolescent development (P27–P56). As a control, a separate set of animals received s.c. injections of an equivalent volume of sesame oil (vehicle) alone (n=15). This treatment regimen, designed to mimic a chronic AAS use regimen (Pope and Katz, 1988, 1994), has been shown repeatedly to produce highly aggressive animals in greater than 90% of the AAS treatment pool (Ricci et al., 2013).
Surgical Procedure
One week prior to aggression testing (P50), animals (N=105) were anesthetized with isoflurane (1–4%, inhalation) and placed into a stereotaxic device for unilateral implantation of a 26 gauge guide cannula aimed at the LAH. An incision was made to expose the dorsal surface of the skull to reveal the position of lambda and bregma landmarks. A small hole was drilled into the skull at the coordinate position necessary to gain access to the latero-anterior hypothalamus, i.e., 0.7mm posterior to bregma, 0.6mm lateral to the midsagittal suture, 6.8mm ventral from dura. The cannula was placed in the brain angled at 8 degrees and anchored to the skull using dental screws and acrylic and head-wound then sutured closed.
Microinjections
A 2-μl Hamilton syringe was connected to a 33-gauge stainless injection needle via polyethelene tubing. Injection of drugs into the LAH was performed by lowering the injection needle through the guide cannula, delivering a final volume of 0.5μl of drug over 3min, and leaving the cannula in position for an additional minute to allow for drug diffusion away from the injector tip. The internal-injector cannula protruded 1mm beyond the guide cannula toward the LAH (Figure 1). Based on the volume selected and the diffusion rate of microinjected substances (Routtenberg, 1972) together with preliminary studies performed in our laboratory with microinjected dyes (data not shown), the spread of drug beyond the LAH of animals with correctly verified cannula placements is limited. Importantly, while it is difficult to restrict the spread of drug to the LAH and limit diffusion dorsal of the injection site (i.e. to the medial dorsal AH), the sparse distribution of GABA receptors in the dorsal medial regions of the LAH (Ricci et al., 2009) would suggest that receptor agonism and antagonism effects were produced within the LAH.
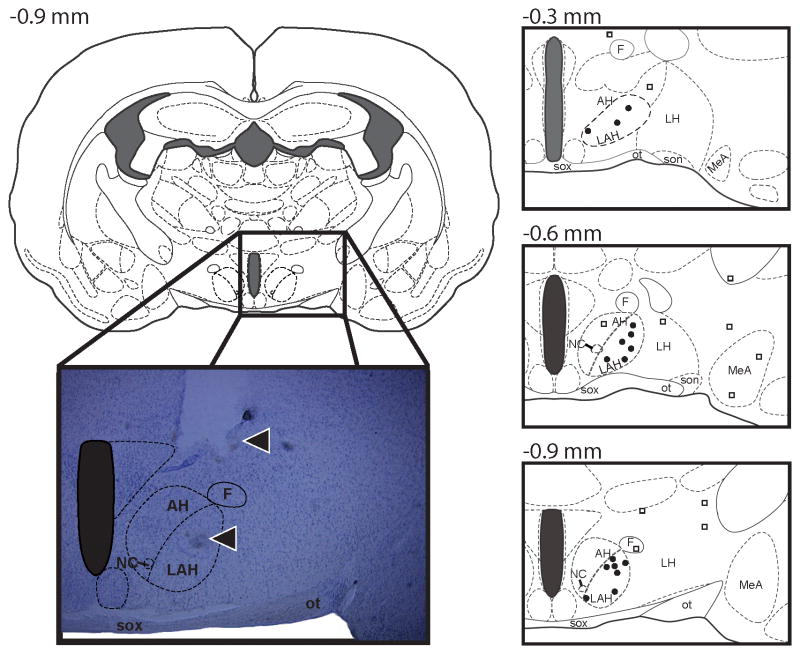
Figure 1.
A series of three atlas schematics at various coronal positions (Morin and Wood, 2001) and a representative photomicrograph of a coronal section of the Syrian hamster brain indicating the sites of central administration of muscimol into the latero-anterior hypothalamus (LAH). The symbol (●) undicates an injection “hit” within the LAH, while the symbol (□) indicates an injection “miss” outside of the LAH target zone. Note in the photomicrograph (from the dorsal to ventral axis) the track of the guide cannula and injection needle (arrows). AH, anterior hypothalamus; F, fornix; LAH, lateral anterior hypothalamus; LH, lateral hypothalamus; MeA, medial amygdala; NC, nucleus circularis; SOX, optic chiasm, ot, optic tract.
Behavioral Testing
After the microinjection, animals were returned to their home cage for ten minutes before undergoing behavioral testing. Hamsters were tested for offensive aggression using the resident-intruder paradigm, a well-characterized and ethologically valid model of offensive aggression in Syrian hamsters (Floody and Pfaff, 1974; Lerwill and Makings, 1971). Briefly, a novel intruder of similar size and weight was introduced into the home cage of the experimental animal (resident) and the resident was scored for general measures of offensive aggression (i.e., the frequency of attacks and bites) towards intruders, followed by a more detailed analysis of specific and targeted aggressive responses including frontal and rump/belly attacks, as previously described (Grimes et al., 2003; Ricci et al., 2006; Wommack and Delville, 2003) and flank marking, i.e., a measure of social communication that is part of the larger ethogram of offensive aggression in hamsters (Floody and Pfaff, 1977; Lerwill and Makings, 1971). An attack was scored each time the resident animal would pursue and then either [1] lunge toward and/or [2] confine the intruder by upright and sideways threat; each was generally followed by a direct attempt to bite the intruder’s dorsal rump and/or ventral target area(s). Latency to attack was defined as the period of time between the beginning of the behavioral test and the first attack the residents made toward an intruder. In the case of no attacks, latencies to attack were assigned the maximum latency (i.e., 600s). Each aggression test lasted for 10 minutes and was videotaped and scored manually by two observers unaware of the hamsters’ experimental treatment. Inter-rater reliability was set at 95%. No intruder was used for more than one behavioral test, and all subjects were tested during the first 4 hours of the dark cycle under dim red illumination, to control for circadian influences on behavioral responding. In addition to aggressive behaviors, residents were tested for an array of social, comfort, and motor behaviors, to control for nonspecific effects of GABA agonists and antagonists. Social interest was measured by determining physical contact time, i.e., the duration of time which experimental residents initiated and maintained contact with the intruder. Self-grooming by experimental residents during the 10-minute agonistic encounter was used as a measure of comfort behavior, while changes in locomotor activity (i.e., line crosses) and escape-attempts (i.e., wall climbing) were used to measure possible changes in motor activity due to GABA receptor agonism/antagonism.
Experimental Design
Syrian hamsters (P27) received daily s.c. injections of an AAS cocktail for 30 days of adolescence (P27–P56). One day following the last injection (P57), AAS-treated hamsters were randomly assigned to treatment groups (n≥15/group, 7 groups) and tested for offensive aggression following an injection into the LAH of saline (0 pM) or one of three doses of the GABAA receptor agonist muscimol (0.01, 0.1, or 1.0 pM) or the GABAA receptor antagonist bicuculine (0.04, 0.4, or 4.0 pM). Muscimol and bicuculine were selected on the basis of their affinities for the GABAA receptor [muscimol; Ki = 0.1 uM, bicuculine; Ki = 14.3 uM], and the dose ranges were selected on the basis of previous effective doses in rats and mice (Clement et al., 1987; Earley and Leonard, 1977; Guillot and Chapouthier, 1996, 1998; Haug et al., 1984; Krsiak et al., 1981; Poshivalov, 1981; Potegal et al., 1982; Puglisi-Allegra et al., 1981).
Histology
To verify cannula placement, animals were anesthetized with isoflurane on the day following behavioral testing and transcardially perfused with 4% paraformaldehyde. Brains were removed, postfixed for 90min in perfusion fixative, and cryoprotected overnight in 30% sucrose at 41°C. Brains were cut at 40 mm on a freezing microtome in serial, coronal sections and mounted on gelatin-coated slides. Sections were stained with cresyl violet, dehydrated through a series of alcohols, cleared with xylene, and coverslipped with Cytoseal (Stephens Scientific, Kalamazoo, Michigan, USA). Only animals with correctly placed cannula tips into the AH were included in the statistical analysis (Figure 1).
Drugs
Testosterone cypionate, nandrolone decanoate, and boldenone undecylenate were purchased from Steraloids Inc. (Newport, RI) and prepared in sesame oil. Muscimol and bicuculine were purchased from Sigma Aldrich (St. Louis, MO) and dissolved in 0.9% (wt/vol) normal saline.
Statistical Analysis
To determine whether stereotaxic surgery altered aggression compared to surgically naïve controls, AAS-treated animals injected with saline on the test day were collapsed across experiments and compared to surgically naïve AAS-treated hamsters using Student’s t-test. For microinjection studies, behavioral measures were compared between treatment groups using one-way ANOVA, followed by Fisher’s protected least significant difference post hoc test (two-tailed) when applicable. Aggression scores of animals with cannulae placed outside the AH were compared to AAS-treated hamsters injected with saline using one-way ANOVA. The alpha level was set at 0.05 for all analyses.
RESULTS
Histological verification
In this experiment, 65 animals with correct unilateral cannula placements in the LAH were included in the behavioral analysis (Muscimol; 0.0 pM: n=13, 0.01 pM: n=8, 0.1 pM: n=8, 1.0pM : n=8; Bicuculine; 0.04 mM: n=10, 0.4mM : n=9, 4.0 mM: n=9). Some animals were injected outside the LAH (Muscimol; 0.0pM : n=2, 0.01pM : n=4, 0.1pM : n=4, 1.0pM: n=4; Bicuculine; 0.04mM: n=2, 0.4mM: n=3, 4.0mM: n=3) with cannulae placed laterally in either the rostral or caudal direction to the LAH (Figure 1). Treatment with muscimol or bicuculine outside the AH had no significant effect on adolescent AAS-induced aggression t(12)=0.42, NS.
GABA Effects on Behavior
GABA Effects on Offensive Aggression
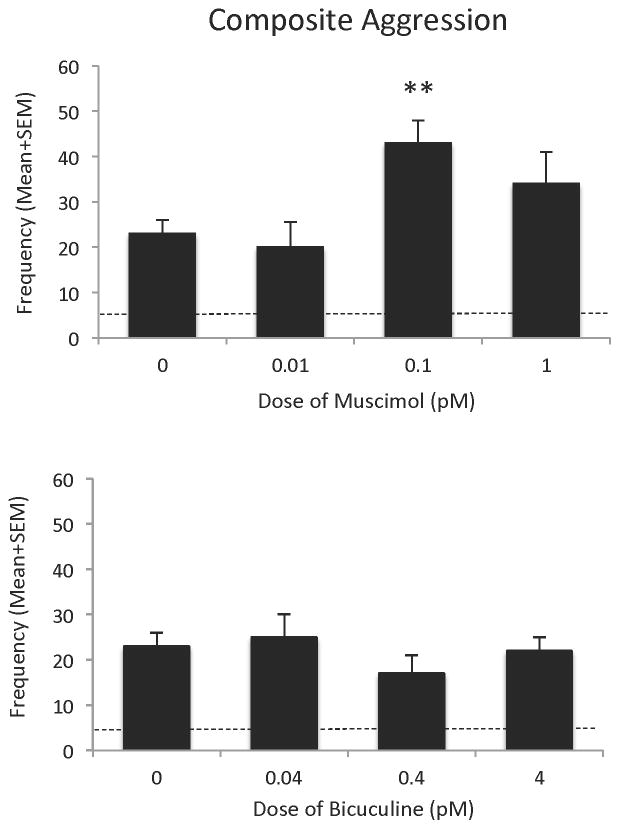
Microinjection of the GABAA receptor agonist muscimol into the LAH produced a significant overall effect on offensive aggression, [F(3,35)=4.66, p<0.01], with an effective dose of 0.1 pM. At this dose, muscimol treatment significantly increased composite aggression [t(19)=3.85, p<0.01], when compared with aggressive, adolescent AAS-treated hamsters injected with saline into the LAH. Specifically, infusion of muscimol into the LAH produced a 2-fold increase in the frequency of aggressive behaviors during the 10min test period (Figure 2 – upper panel). Lower (0.01 pM) and higher (1.0 pM) doses of muscimol, however, had no significant effect on composite aggression scores: 0.01pM [t(19)=0.34, NS] and 1.0pM [t(19)=1.38, NS]. In comparison, microinjection of the GABAA receptor antagonist bicuculine into the LAH of aggressive, adolescent AAS-treated hamsters. at all doses applied had no significant effect on offensive aggression, [F(3,25)=0.82, NS] compared to AAS-treated hamsters injected with saline into the LAH (Figure 2 – lower panel).
Figure 2.
Composite aggression score of anabolic/androgenic steroid (AAS)-treated animals after injection of muscimol (0.01 pM –1.0 pM) or bicuculine (0.04 pM –4.0 pM) in the latero-anterior hypothalamus, compared with AAS-treated hamsters injected with saline (0.0 pM) into the latero-anterior hypothalamus on the test day. Bars denote SEM. Dashed lines indicate the mean behavior of sesame oil control animals. **p<0.01
The GABAA receptor agonist muscimol produced an overall effect on several components of the aggressive response of adolescent AAS-treated hamsters. In particular, muscimol significantly increased the frequency of attack behavior [F(3,35)=5.11, p<0.001] in AAS-treated animals, compared to hamsters injected with saline into the LAH, with the effective dose from ranging from 0.1 pM [t(19)=3.6, p<0.001] to 1.0 pM [t(19)=2.21, p<0.05]. In fact, infusion of these two doses of muscimol into the LAH of aggressive, adolescent AAS-treated animals produced a 2–2.5 fold increase in the frequency of attacks observed during the 10min test (Figure 3 – left panel). No similar effects were observed when aggressive, adolescent AAS-treated animals were treated with lower (0.01 pM) doses of muscimol [t(19)=0.26, NS]. Similarly, microinjection of the GABAA receptor agonist muscimol into the LAH of aggressive, adolescent AAS-treated hamsters produced a trend towards an increase in the frequency of bites [F(3,35)=2.18, p=0.07] during the 10 minute test period. In particular, compared to littermate controls injected with saline into the LAH, AAS-treated hamsters administered muscimol showed a significant increase in the frequency of bites during the test, specifically at an effective dose of 0.1 pM [t(19)=2.75, p<0.01]. At this dose adolescent AAS-treated animals displayed a nearly 3-fold increase in the frequency of bites observed during the 10 minute test (Figure 3 – right panel). No similar effects were observed when aggressive, adolescent AAS-treated animals were treated with lower (0.01 pM) or higher (1.0 pM) doses of muscimol [t(19)=0.26 and t(19)=1.13, respectively, NS].
Figure 3.
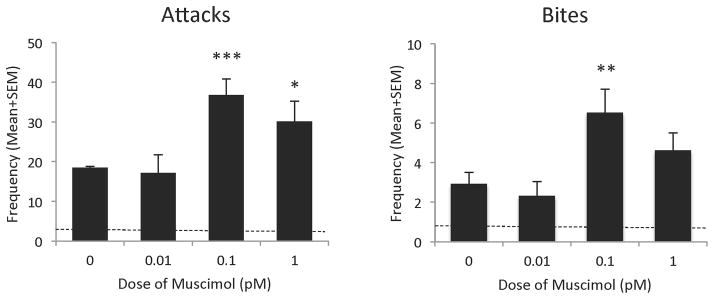
Effects of injection of muscimol (0.01 pM –1.0 pM) in the latero-anterior hypothalamus on the frequency of offensive aggression (attacks and bites) in anabolic/androgenic steroid (AAS)-treated animals, compared with AAS-treated hamsters injected with saline (0.0 pM) into the latero-anterior hypothalamus on the test day. Bars denote SEM. Dashed lines indicate mean behavior of sesame oil control animals *p<0.05, **p<0.01
A more detailed examination of effects of muscimol on the aggressive phenotype showed that the GABAA receptor agonist produced a significant overall effect on select targeted offensive responses, namely belly/rear attacks [F(3,35)=6.64, p<0.001] and frontal attacks [F(3,35)=3.79, p<0.01]. In particular, similar to the effect observed for general attacks alone, adolescent AAS-treated animals administered muscimol significantly increased the frequency of attacks targeted to the belly/rear region of intruders, compared to hamsters injected with saline into the LAH, with the effective dose ranging from 0.1 pM [t(19)=4.9, p<0.001] to 1.0 pM [t(19)=2.84, p<0.01]. Infusion of these doses of muscimol into the LAH of aggressive, adolescent AAS-treated animals produced a 1.5–2 fold increase in the frequency of belly/rear attacks observed during the 10 minute test (Figure 4 – left panel). No similar effects were observed when aggressive, adolescent AAS-treated animals were treated with lower (0.01 pM) doses of muscimol [t(19)=1.34, NS]. Interestingly, muscimol significantly decreased the frequency of frontal attacks in adolescent AAS-treated animals, compared to hamsters injected with saline into the LAH, with an effect only observed at the low (0.01 pM) dose [t(19)=2.85, p<0.01]. Here adolescent AAS-treated animals showed a nearly 5-fold decrease in the frequency of frontal attacks observed during the 10 minute test (Figure 4 – right panel), reducing the frequency of frontal attacks to that of non-aggressive, sesame oil-treated control animals (dashed line in Figure 4 – right panel). No similar effects were observed when aggressive, adolescent AAS-treated animals were treated with higher (0.1 pM and 1.0 pM) doses of muscimol [t(19)=1.34 and t(19)=0.05, respectively, NS]. And lastly, microinjection of the GABA A receptor agonist muscimol into the LAH of aggressive, adolescent AAS-treated hamsters, at all doses applied, showed no significant overall effect on flank marking, [F(3,35)=1.28, NS], compared to AAS-treated hamsters injected with saline into the LAH (Table 1).
Figure 4.
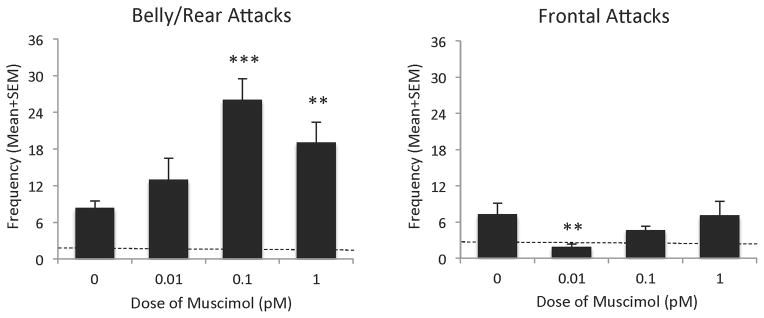
Effects of injection of muscimol (0.01 pM –1.0 pM) into the latero-anterior hypothalamus on the frequency of individual, targeted aggressive behaviors (i.e. belly/rear attacks and frontal attacks) of adolescent anabolic/androgenic steroid (AAS)-treated hamsters, compared with AAS-treated hamsters injected with saline (0.0 pM) into the latero-anterior hypothalamus on the test day. Bars denote SEM. Dashed lines indicate mean behavior of sesame oil control animals **p<0.01, ***p<0.001
Table 1.
Effects of injection of muscimol (0.01 pM –1.0 pM) into the latero-anterior hypothalamus on general measures of behavioral activation, compared with AAS-treated hamsters injected with saline (0.0 pM) into the latero-anterior hypothalamus on test day.
| BEHAVIOR | Dose Muscimol (pM)
|
||||
|---|---|---|---|---|---|
| 0 | 0.01 | 0.1 | 1 | Probability | |
| Social Behavior (±SEM) | |||||
| Flank Marking | 2.5 ± 0.9 | 1.25 ± 0.4 | 0.9 ± 0.4 | 1.1 ± 0.5 | NS |
| Contact Time (sec) | 462.5 ± 25.2 | 444.6 ± 28.5 | 460.8 ± 23.8 | 440.6 ± 21.6 | NS |
| Comfort Behavior (±SEM) | |||||
| Self Grooming | 7.1 ± 1.3 | 4.1 ± 0.9* | 3.1 ± 0.7** | 4.5 ± 0.8 | < 0.05 |
| Locomotion Behavior (±SEM) | |||||
| Line Crosses | 43.5 ± 4.8 | 39.1 ± 2.9 | 34.1 ± 2.6* | 39 ± 5.1 | < 0.05 |
| Wall Climbing | 12.5 ± 2.1 | 13.9 ± 0.5 | 9.1 ± 2.1 | 8.6 ± 2.3 | NS |
Data represents mean values with standard error of the mean.
NS, not significant
Differs from adolescent AAS-treated controls (0.0 pM dose), p<0.05
Differs from adolescent AAS-treated controls (0.0 pM dose), p<0.01
GABA Effects on Behavioral Activation
To assess any nonspecific effects of LAH injections of muscimol, animals were scored for non-aggressive behaviors during the 10 minute resident–intruder test. Interestingly, GABAA receptor agonism in the LAH produced a significant main effect on grooming behavior [F(3,35)=2.45, p<0.05] and locomotion [F(3,35)=2.55, p<0.05]. Post-hoc analysis revealed that while the highest (i.e., 1.0 pM) dose had no effect on grooming behavior [t(18)=1.6, NS], both lower doses (0.01 pM and 0.1 pM) produced a significant decrease in the frequency of grooming, when compared with AAS-treated animals injected with saline on test day: [0.01 pM: t(19)=2.0, p<0.05; 0.1 pM: t(19)=2.79, p<0.01]. In fact, animals treated with the 0.01 pM dose of muscimol showed a 2-fold decrease in grooming frequency when compared with saline-treated AAS controls (Table 1). Similarly, application of 0.1 pM muscimol to the LAH produced a significant decrease in the frequency of line crosses [t(19)=2.1, p<0.05], although this reduction was very small (<20%) (Table 1). At all doses, muscimol failed to produce a significant effect on the contact time between conspecifics [F(3,35)=1.19, NS] and wall climbing [F(3,35)=0.46, NS] (Table 1).
DISCUSSION
Pharmacological manipulation of GABA neural signaling modulates aggression in various species and animal models (Christmas and Maxwell, 1970; Clement et al., 1987; Cole and Wolf, 1970; Depaulis and Vergnes, 1985; DiMascio, 1973; Earley and Leonard, 1977; Guillot and Chapouthier, 1996, 1998; Haug et al., 1984; Krsiak et al., 1981; Poshivalov, 1981; Potegal et al., 1982; Puglisi-Allegra et al., 1981; Rodgers and Waters, 1985; Stork et al., 2000; Weerts et al., 1993), particularly through alterations in GABAA receptor signaling (de Almeida et al., 2005; Jorge et al., 2002; Miczek et al., 2003). Specifically, activation and blockade of GABAA receptor activity with muscimol and bicuculline, respectively, have been shown to modulate aggressive behavior (Hansen and Ferreira, 1986; Potegal et al., 1983; Puglisi-Allegra et al., 1981). Together, these studies show a link between GABAA receptor activity and the control of aggressive behavior. However, no research to date has explored the effects of these GABA-ergic compounds on adolescent AAS-induced offensive aggression. Moreover, the majority of previous studies applied ‘systemic’ drug delivery techniques and reported concomitant alterations in general behavioral activation. Thus, this study used local microinfusion techniques to determine whether GABAA receptor activity in the center of the neural circuit controlling aggression, the LAH, would effectively modulate adolescent AAS-induced offensive aggression. Our findings show a dose-dependent facilitation of adolescent AAS-induced aggressive responding by pharmacological agonism of GABAA receptors in the LAH, with the effective dose beginning at 0.1pM of muscimol. Conversely, pharmacological antagonism of LAH GABAA receptor activity had no significant effect on aggressive responding in adolescent AAS-treated animals. These findings are unique not only because they show specificity of the GABAA receptor agonist to alter adolescent AAS-induced offensive aggression, but also because they show a site-specific brain locus, where GABAA receptor signaling acts to alter aggressive responding in adolescent AAS-treated animals. These findings are in agreement with earlier studies showing the LAH to be a specific brain region modulating control of aggression (Melloni and Ricci, 2010).
Adolescence marks a critical developmental period during which pharmacological treatment can produce differential behavioral responses compared with adulthood. For example, adolescent rats show an increase in responsiveness to catecholaminergic antagonists but decreased sensitivity to catecholaminergic agonists when compared with younger or older rats (Spear and Brake, 1983). In the Syrian hamster, adolescent animals transition from juvenile displays of play fighting to the development of mature adult aggression behaviors (Wommack and Delville, 2003). In particular, during peri-pubescent development hamsters change the focus of their attacks from the face and cheeks (i.e., frontal attacks) to the lower belly and rump regions (i.e., belly/rear attacks) (Wommack and Delville, 2003). This behavioral change is gradual from postnatal day 28 (when frontal attacks dominate the behavioral response) to postnatal day 70 (when belly/rear attacks dominate). However, in the current study, our animals are tested for adolescent AAS-induced offensive aggression on postnatal day 57, i.e., a time at which the behavioral response pattern comprises nearly equal parts frontal:belly/rear attacks (Wommack and Delville, 2003). In our animal model of adolescent AAS-induced offensive aggression, the majority of AAS-treated adolescents target their offensive responses to the flank and rear regions of intruders (Ricci et al., 2013; Schwartzer and Melloni, 2010a; Schwartzer and Melloni, 2010b), i.e., a hallmark characteristic of the adult aggressive phenotype (Taravosh-Lahn and Delville, 2004; Wommack and Delville, 2003), although some frontal attack behavior is still observed. Thus AAS-treated animals display the adult form of offensive aggression in the absence of social learning, indicating that the AAS treatment regimen circumvents the learning of mature fighting behavior. In the current study, the analysis of the effects of muscimol on individual types of aggressive acts revealed a dose-dependent facilitation of specific aggressive behaviors characterized as mature forms of aggression (Pellis and Pellis, 1988; Wommack and Delville, 2003). Specifically, GABAA receptor agonism within the LAH increased the number of belly/rear attacks at the higher doses tested (i.e. 0.1pM and 1.0pM), while decreasing the number of frontal attacks at the lower dose (i.e., 0.01pM). In fact, adolescent AAS-treated hamsters administered 0.01pM muscimol into the LAH displayed a similar frequency of frontal attacks as non-aggressive, sesame oil-treated control animals (dashed line in Figure 4 – right panel). Together, these data suggest that enhanced GABA neural signaling within the LAH of aggressive, adolescent AAS-treated animals may function to simultaneously activate mature forms of offensive aggression and suppress juvenile play behaviors, promoting the display of more serious adult displays of aggression in AAS-treated animals. This notion is supported by our prior studies showing that adolescent AAS-treated animals that display a mature and highly escalated AAS-induced aggressive phenotype have more GABA-containing afferent terminals and neuronal somata within the AH/LAH (Grimes et al., 2003; Schwartzer et al., 2009). A more in-depth understanding of the development of the LAH GABA neural system and how it modulates aggression development, as well as the influence of adolescent AAS-exposure on both neurobiological and behavioral development is thus warranted.
Most research examining the role of GABA in aggression has made use of systemically applied GABA receptor agonists and antagonists to measure changes in aggressive responding. However, given the ubiquitous distribution of GABA receptors and the role of GABA in sedation, systemic drug administration has significant potential to cause concomitant alterations in behavioral activation. Our findings serve as the first behavioral measure of direct GABAA receptor activation in an aggression-specific locus of the adolescent AAS-treated brain, i.e., the LAH. Interestingly, enhanced aggression in adolescent AAS-treated animals administered GABAA receptor agonists to the LAH was observed in the presence of slightly lower locomotor and grooming activity. Given the extremely high number of aggressive acts observed on the part of AAS-treated residents administered 0.1 pM muscimol, the reduction in locomotion most likely reflects an increased number - and extended period(s) - of rest between attack bouts, while a reduction in grooming is likely the result of the resident’s focus on pursuing aggressive acts. This notion is supported by the fact that decreases in locomotion and grooming were only observed together and at the aggression-enhancing dose (i.e., 0.1 pM) of muscimol administered to the LAH and that animals with cannulae placed rostral or caudal to the LAH were no more or less aggressive than AAS-treated animals injected with saline into the LAH, nor did they show any significant changes in locomotion or grooming. These findings support the notion that GABAA receptor activation within the LAH further enhances aggressive responding in already aggressive, adolescent AAS-treated animals, implicating the LAH brain region as a central mediator of adolescent AAS-induced offensive aggression. In contrast, these behavioral effects may be a direct effect of GABAA receptor activation. Systemic administration of allosteric GABAA receptor agonists has been shown to directly decrease grooming in rats (Barros et al., 1994; Nin et al., 2012) and locomotion in mice (Krsiak and Sulcova, 1990), while injections of the GABAA receptor agonist muscimol into the raphe nucleus have been shown to increase locomotion (Shim et al., 2014). Further research on the effects of GABAA receptor agonists on AAS-treated (and non-AAS) animals is warranted to better understand the effects of these compounds on hypothalamus-related behaviors. To this end, it is important to consider potential mechanisms within the hypothamus whereby GABA neural signaling through GABAA receptors can impart aggression-enhancing effects in adolescent AAS-treated animals.
The GABA neural system is sensitive to androgens in a fashion consistent with the generation of the aggressive phenotype. For instance, in rats, GABA neurons are more active and the expression of glutamic acid decarboxylase 65 (GAD65, a biosynthetic enzyme for GABA localized within synaptic terminals) is higher in the male hypothalamus (Grattan and Selmanoff, 1993; Grattan et al., 1996a,b; Searles et al., 2000). Also, castration and androgen receptor blockade decreases hypothalamic GABA levels in rat brain (Grattan and Selmanoff, 1993, 1994a,b; Grattan et al., 1996a,b; Yoo et al., 2000), which can be restored by testosterone (Grattan and Selmanoff, 1994a. b). Studies from our laboratory showed that adolescent AAS exposure increases GABA-containing afferent terminals across the entire AH (Grimes et al., 2003) and GABA-containing neuronal somata within the LAH (Schwartzer et al., 2009), suggesting that adolescent AAS exposure increases AH/LAH GABA activity, contributing to the adolescent AAS-induced aggressive phenotype. Further, sex- and age- specific effects of androgens on GABAA receptor expression and function have been identified and AAS exposure has been shown to directly alter GABAA receptor expression and function in rats (Clark and Henderson, 2003; Henderson et al., 2006) while in mice, chronic AAS alters the expression of GABAA receptors, namely α1,2,4,5 and γ2 subunits (Pibiri et al., 2006). Recent studies from our laboratory show that aggressive, adolescent AAS-treated hamsters have fewer GABAAα1 receptors in the LAH compared to controls (Schwartzer et al., 2009). Interestingly, this reduction is identical to the adolescent AAS-induced decrease in 5HT afferent innervation we observed in several prior studies (Grimes and Melloni, 2002, 2006), leading us to hypothesize that GABAAα1 receptors are located on 5HT-containing pre-synaptic terminals in the LAH, and that the decrease in GABAAα1 receptors in the LAH of adolescent AAS-treated animals is representative of the reduction in 5HT afferent innervation to the LAH during adolescent AAS exposure (Figure 5). It is possible that adolescent AAS-induced increases in GABA-containing neurons and afferent terminals in the LAH increase the inhibitory effect of GABA on GABAAα1 receptors located on the remaining 5HT neural afferents in the LAH (Figure 5 – right panel). There is evidence that 5HT activity within the LAH normally functions to inhibit offensive aggression by suppressing the activity of AVP neurons in the LAH (Ferris et al., 1997; Melloni and Ricci, 2010). From a functional standpoint, an AAS-induced increase in GABAergic inhibition of the remaining 5HT afferent terminals in the LAH would serve to dis-inhibit downstream AVP neurons, facilitating offensive aggression in adolescent AAS-treated animals. And, further activation of the remaining GABAAα1 receptors by muscimol should enhance the suppression of 5HT release from the remainder of the 5HT afferent fibers, further augmenting the aggressive phenotype in adolescent AAS-treated animals. Conversely, inhibition of GABAAα1 receptors by bicuculine should block the GABA-induced suppression of 5HT release from remaining 5HT afferent fibers within the LAH, potentially suppressing the aggressive phenotype. However, we believe this does not occur due to the significant AAS-induced reduction in 5HT afferent innervation to the LAH; therefore there is no longer sufficient 5HT within the LAH to suppress the downstream activity of AH AVP neurons facilitating aggression. Both of these circumstances are consistent with the behavioral findings reported in this study. These patterns of GABA activity would support the notion that the role of GABA in aggression is dependent upon the nature and extent of its interaction with other neural systems modulating this behavior, and offers a neural mechanism in support of the findings that GABA facilitates aggression in adolescent AAS-treated animals. This hypothesis is currently under investigation in our laboratory. In summary, the previous histological reports of increased GABA-containing afferent terminals and neurons in the LAH of adolescent AAS-treated hamsters, coupled with the current findings that GABA agonists delivered to the LAH facilitate adolescent AAS-induced offensive aggression, implicate the LAH as an important site of convergence where adolescent AAS exposure increases GABA neural development and activity to elevate aggressive responding.
Figure 5.
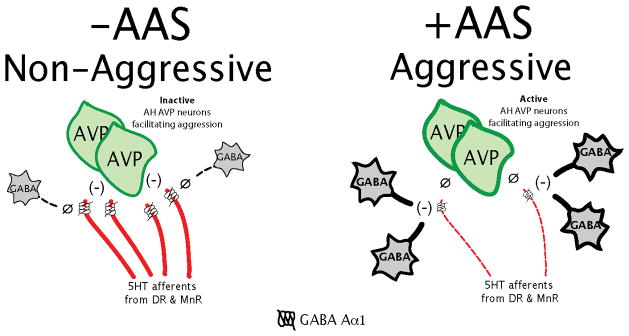
A model of the anterior hypothalamic regulation of adolescent AAS offensive aggression. -AAS (left panel: A model of the neurochemical regulation of offensive aggression based on the original model from (Ferris et al., 1997) compared to +AAS (right panel) a model of the neurochemical regulation of adolescent AAS offensive aggression based upon this same neural model. The -AAS model shows the hypothetical interaction between the serotonin (5HT), arginine vasopressin (AVP), and γ-amino-butyric acid (GABA) neural systems in the latero-anterior hypothalamus (LAH). In the model, 5HT release within the LAH normally functions to inhibit offensive aggression by suppressing (−) the activity of AH AVP neurons. Upstream of this modulation, the release of 5HT is under the inhibitory influence of GABA, acting through GABA Aα1 receptors located on 5HT afferents in the LAH. Under normal conditions (−AAS), there is insufficient GABA production, afferent innervation and release (Ø) within the LAH to inhibit the release of 5HT from the dense array of 5HT afferents that innervate the LAH brain region (red fibers). Due to the strong inhibitory (−) influence of 5HT, AH AVP neurons facilitating aggression are inactive and animals are non-aggressive. Conversely, adolescent AAS exposure (+AAS) increases GABA production, afferent innervation and release into the LAH, while suppressing the development of 5HT afferent fibers to the LAH. As a result, GABA, acting through GABA Aα1 receptors, functions to inhibit (−) the remaining 5HT afferents in the LAH; effectively suppressing the release of 5HT. Under this condition, there is insufficient 5HT release (Ø) within the LAH to suppress the activity of AH AVP neurons facilitating aggression and animals display a highly aggressive behavioral phenotype.
Acknowledgments
This work was supported by research grant (R01) DA10547 from the National Institutes on Drug Abuse (NIDA) to R.H.M.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDA.
References
- Barros HM, Tannhauser SL, Tannhauser MA, Tannhauser M. The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol Toxicol. 1994;74:339–44. doi: 10.1111/j.1600-0773.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18:5938–47. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Melloni RH. Glutamate-vasopressin interactions and the neurobiology of anabolic steroid-induced offensive aggression. Neuroscience. 2011;185:85–96. doi: 10.1016/j.neuroscience.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Chen F, Rezvani A, Jarrott B, Lawrence AJ. Distribution of GABAA receptors in the limbic system of alcohol-preferring and non-preferring rats: in situ hybridisation histochemistry and receptor autoradiography. eurochem Int. 1998;32:143–51. doi: 10.1016/s0197-0186(97)00069-7. [DOI] [PubMed] [Google Scholar]
- Christmas AJ, Maxwell DR. A comparison of the effects of some benzodiazepines and other drugs on aggressive and exploratory behaviour in mice and rats. Neuropharmacology. 1970;9:17–29. doi: 10.1016/0028-3908(70)90044-4. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–36. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Clement J, Simler S, Ciesielski L, Mandel P, Cabib S, Puglisi-Allegra S. Age-dependent changes of brain GABA levels, turnover rates and shock-induced aggressive behavior in inbred strains of mice. Pharmacol Biochem Behav. 1987;26:83–8. doi: 10.1016/0091-3057(87)90538-7. [DOI] [PubMed] [Google Scholar]
- Cole HF, Wolf HH. Laboratory evaluation of aggressive behavior of the grasshopper mouse (Onychomys) J Pharm Sci. 1970;59:969–71. doi: 10.1002/jps.2600590710. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- DeLeon KR, Grimes JM, Connor DF, Melloni RH., Jr Adolescent cocaine exposure and offensive aggression: involvement of serotonin neural signaling and innervation in male Syrian hamsters. Behav Brain Res. 2002;133:211–20. doi: 10.1016/s0166-4328(02)00004-9. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Vergnes M. Elicitation of conspecific attack or defense in the male rat by intraventricular injection of a GABA agonist or antagonist. Physiol Behav. 1985;35:447–53. doi: 10.1016/0031-9384(85)90322-1. [DOI] [PubMed] [Google Scholar]
- DiMascio A. The effects of benzodiazepines on aggression: reduced or increased? Psychopharmacologia. 1973;30:95–102. doi: 10.1007/BF00421423. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Leonard BE. The effect of testosterone and cyproterone acetate on the concentration of gamma-aminobutyric acid in brain areas of aggressive and non-aggressive mice. Pharmacol Biochem Behav. 1977;6:409–13. doi: 10.1016/0091-3057(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–43. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Steroid hormones and aggressive behavior: approaches to the study of hormone-sensitive brain mechanisms for behavior. Res Publ Assoc Res Nerv Ment Dis. 1974;52:149–85. [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91:443–64. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Regional variation in gamma-aminobutyric acid turnover: effect of castration on gamma-aminobutyric acid turnover in microdissected brain regions of the male rat. J Neurochem. 1993;60:2254–64. doi: 10.1111/j.1471-4159.1993.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Prolactin- and testosterone-induced inhibition of LH secretion after orchidectomy: role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones. J Endocrinol. 1994a;143:165–74. doi: 10.1677/joe.0.1430165. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Castration-induced decrease in the activity of medial preoptic and tuberoinfundibular GABAergic neurons is prevented by testosterone. Neuroendocrinology. 1994b;60:141–9. doi: 10.1159/000126744. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Rocca MS, Sagrillo CA, McCarthy MM, Selmanoff M. Antiandrogen microimplants into the rostral medial preoptic area decrease gamma-aminobutyric acidergic neuronal activity and increase luteinizing hormone secretion in the intact male rat. Endocrinology. 1996a;137:4167–73. doi: 10.1210/endo.137.10.8828473. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Rocca MS, Strauss KI, Sagrillo CA, Selmanoff M, McCarthy MM. GABAergic neuronal activity and mRNA levels for both forms of glutamic acid decarboxylase (GAD65 and GAD67) are reduced in the diagonal band of Broca during the afternoon of proestrus. Brain Res. 1996b;733:46–55. doi: 10.1016/0006-8993(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73:713–21. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH. Serotonin 1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behavioral Neuroscience. 2005;119:1184–94. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RHJ. Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2006;120:1242–51. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–80. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic/androgenic steroid withdrawal. Behav Neurosci. 2006;120:115–24. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with teh temporal onset of aggressive behavior during adolescent anabolic-steroid exposure in hamsters. Behav Neurosci. 2007;121:941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res. 1996;77:211–3. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Intermale aggression, GAD activity in the olfactory bulbs and Y chromosome effect in seven inbred mouse strains. Behav Brain Res. 1998;90:203–6. doi: 10.1016/s0166-4328(97)00110-1. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behav Neurosci. 1986;100:410–5. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Melloni RH., Jr Chronic low-dose cocaine treatment during adolescence facilitates aggression in hamsters. Physiol Behav. 2000a;69:555–62. doi: 10.1016/s0031-9384(00)00220-1. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH., Jr Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000b;25:317–38. doi: 10.1016/s0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Haug M, Simler S, Ciesielski L, Mandel P, Moutier R. Influence of castration and brain GABA levels in three strains of mice on aggression towards lactating intruders. Physiol Behav. 1984;32:767–70. doi: 10.1016/0031-9384(84)90192-6. [DOI] [PubMed] [Google Scholar]
- Henderson LP, Penatti CA, Jones BL, Yang P, Clark AS. Anabolic androgenic steroids and forebrain GABAergic transmission. Neuroscience. 2006;138:793–9. doi: 10.1016/j.neuroscience.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Jackson D, Burns R, Trksak G, Simeone B, DeLeon KR, Connor DF, Harrison RJ, Melloni RH., Jr Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience. 2005;133:635–46. doi: 10.1016/j.neuroscience.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABA(A) receptors change with hormonal state in the adult mouse. J Neurobiol. 2002;50:137–49. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Knyshevski I, Connor DF, Harrison RJ, Ricci LA, Melloni RH., Jr Persistent activation of select forebrain regions in aggressive, adolescent cocaine-treated hamsters. Behav Brain Res. 2005a;159:277–86. doi: 10.1016/j.bbr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Knyshevski I, Ricci LA, McCann TE, Melloni RH., Jr Serotonin type-1A receptors modulate adolescent, cocaine-induced offensive aggression in hamsters. Physiol Behav. 2005b;85:167–76. doi: 10.1016/j.physbeh.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Krsiak M, Sulcova A. Differential effects of six structurally related benzodiazepines on some ethological measures of timidity, aggression and locomotion in mice. Psychopharmacology (Berl) 1990;101:396–402. doi: 10.1007/BF02244060. [DOI] [PubMed] [Google Scholar]
- Krsiak M, Sulcova A, Tomasikova Z, Dlohozkova N, Kosar E, Masek K. Drug effects on attack defense and escape in mice. Pharmacol Biochem Behav. 1981;14(Suppl 1):47–52. [PubMed] [Google Scholar]
- Lerwill CJ, Makings P. The agonistic behavior of the golden hamster. Animal Behavior. 1971;19:714–721. [Google Scholar]
- Melloni RH, Jr, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: A hypothalamic neural model based on findings in pubertal Syrian hamsters. Horm Behav. 2010;58:177–191. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–57. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Nin MS, Couto-Pereira NS, Souza MF, Azeredo LA, Ferri MK, Dalpra WL, Gomez R, Barros HM. Anxiolytic effect of clonazepam in female rats: grooming microstructure and elevated plus maze tests. Eur J Pharmacol. 2012;684:95–101. doi: 10.1016/j.ejphar.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting in the Syrian golden hamster Mesocricetus auratus Waterhouse, and its relationship to serious fighting during postweaning development. Dev Psychobiol. 1988;21:323–37. doi: 10.1002/dev.420210404. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17:1537–41. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am J Psychiatry. 1988;145:487–90. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–82. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Poshivalov VP. GABA-ergic correlates of aggression and intraspecies sociability of mice subjected to isolation. Biull Eksp Biol Med. 1981;91:584–7. [PubMed] [Google Scholar]
- Potegal M, Perumal AS, Barkai AI, Cannova GE, Blau AD. GABA binding in the brains of aggressive and non-aggressive female hamsters. Brain Res. 1982;247:315–24. doi: 10.1016/0006-8993(82)91256-2. [DOI] [PubMed] [Google Scholar]
- Potegal M, Yoburn B, Glusman M. Disinhibition of muricide and irritability by intraseptal muscimol. Pharmacol Biochem Behav. 1983;19:663–9. doi: 10.1016/0091-3057(83)90342-8. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Simler S, Kempf E, Mandel P. Involvement of the GABAergic system on shock-induced aggressive behavior in two strains of mice. Pharmacol Biochem Behav. 1981;14(Suppl 1):13–8. doi: 10.1016/s0091-3057(81)80004-4. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type-3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure. Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Rasakham S, Grimes JM, Melloni RH. Serotonin 1A receptor activity and expression modulate adolescent anabolic/androgenic steroid induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Connor DF, Morrison R, Melloni RH., Jr Risperidone exerts potent anti-aggressive effects in a developmentally immature animal model of escalated aggression. Biol Psychiatry. 2007;62:218–25. doi: 10.1016/j.biopsych.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Schwartzer JJ, Melloni RH., Jr Alterations in the anterior hypothalamic dopamine system in aggressive adolescent AAS-treated hamsters. Horm Behav. 2009;55:348–55. doi: 10.1016/j.yhbeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Morrison TR, Melloni RH. Adolescent anabolic/androgenic steroids: Aggression and anxiety during exposure predict behavioral responding during withdrawal in Syrian hamsters (Mesocricetus auratus) Horm Behav. 2013;64:770–780. doi: 10.1016/j.yhbeh.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Waters AJ. Benzodiazepines and their antagonists: a pharmacoethological analysis with particular reference to effects on “aggression”. Neurosci Biobehav Rev. 1985;9:21–35. doi: 10.1016/0149-7634(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Intracranial chemical injection and behavior: a critical review. Behav Biol. 1972;7:601–41. doi: 10.1016/s0091-6773(72)80073-7. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH., Jr Dopamine activity in the lateral anterior hypothalamus modulates AAS-induced aggression through D2 but not D5 receptors. Behav Neurosci. 2010a;124:645–55. doi: 10.1037/a0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH., Jr Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression the Syrian hamsterr. Behav Pharm. 2010b;21:314–322. doi: 10.1097/FBP.0b013e32833b10f1. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Ricci LA, Melloni RH., Jr Interactions between the dopaminergic and GABAergic neural systems in the lateral anterior hypothalamus of aggressive AAS-treated hamsters. Behav Brain Res. 2009;203:15–22. doi: 10.1016/j.bbr.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Searles RV, Yoo MJ, He JR, Shen WB, Selmanoff M. Sex differences in GABA turnover and glutamic acid decarboxylase (GAD(65) and GAD(67)) mRNA in the rat hypothalamus. Brain Res. 2000;878:11–9. doi: 10.1016/s0006-8993(00)02648-2. [DOI] [PubMed] [Google Scholar]
- Shim I, Stratford TR, Wirtshafter D. Dopamine is differentially involved in the locomotor hyperactivity produced by manipulations of opioid, GABA and glutamate receptors in the median raphe nucleus. Behav Brain Res. 2014;261:65–70. doi: 10.1016/j.bbr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of alpha5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–70. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Tappaz ML, Brownstein MJ. Origin of glutamate-decarboxylase (GAD)-containing cells in discrete hypothalamic nuclei. Brain Res. 1977;132:95–106. doi: 10.1016/0006-8993(77)90708-9. [DOI] [PubMed] [Google Scholar]
- Tappaz ML, Brownstein MJ, Kopin IJ. Glutamate decarboxylase (GAD) and gamma-aminobutyric acid (GABA) in discrete nuclei of hypothalamus and substantia nigra. Brain Res. 1977;125:109–21. doi: 10.1016/0006-8993(77)90363-8. [DOI] [PubMed] [Google Scholar]
- Taravosh-Lahn K, Delville Y. Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Horm Behav. 2004;46:428–35. doi: 10.1016/j.yhbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Tornatzky W, Miczek KA. Prevention of the pro-aggressive effects of alcohol in rats and squirrel monkeys by benzodiazepine receptor antagonists. Psychopharmacology (Berl) 1993;111:144–52. doi: 10.1007/BF02245516. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Repeated social stress and the development of agonistic behavior: individual differences in coping responses in male golden hamsters. Physiol Behav. 2003;80:303–8. doi: 10.1016/j.physbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Yoo MJ, Searles RV, He JR, Shen WB, Grattan DR, Selmanoff M. Castration rapidly decreases hypothalamic gamma-aminobutyric acidergic neuronal activity in both male and female rats. Brain Res. 2000;878:1–10. doi: 10.1016/s0006-8993(00)02600-7. [DOI] [PubMed] [Google Scholar]