Abstract
The Rad9 gene is evolutionarily conserved from yeast to human, and plays crucial roles in genomic maintenance, DNA repair and cell cycle checkpoint controls. However, the function of this gene with respect to tumorigenesis is not well understood. A Rad9-null mutation in mice causes embryonic lethality. In this study, we created mice in which mouse Rad9, Mrad9, was deleted only in keratinocytes to permit examination of the potential function of the gene in tumor development. Mice with Mrad9+/− or Mrad9−/− keratinocytes demonstrated no overt, spontaneous morphological defects and appeared similar to wild-type controls. Painting the carcinogen 7,12-dimethylbenzanthracene (DMBA) onto the skin of the animals caused earlier onset and more frequent formation of tumors and senile skin plaques in Mrad9−/− mice, compared to Mrad9+/− and Mrad9+/+ littermates. DNA damage response genes p21, p53 and Mrad9B were expressed at higher levels in Mrad9−/− relative to Mrad9+/+ skin. Keratinocytes isolated from Mrad9−/− skin had more spontaneous and DMBA-induced DNA double strand breaks than Mrad9+/+ keratinocytes, and the levels were reduced by incubation with the antioxidant EGCG. These data suggest that Mrad9 plays an important role in maintaining genomic stability and preventing tumor development in keratinocytes.
Keywords: Rad9, conditional gene knockout, genomic stability, skin tumorigenesis, skin ageing
Introduction
Cells are constantly exposed to endogenous and exogenous conditions that cause stresses and can induce genomic DNA lesions. Eukaryotic cells have evolutionarily conserved mechanisms that monitor and coordinate cell cycle progression, through checkpoint control activities, with repair of DNA damage. Mutations in genes that play roles in cell cycle checkpoint control and DNA repair are often associated with tumorigenesis. For example, targeted deletion of p53, Brca1 or Atm cell cycle checkpoint genes enhances tumor development (1–5). Rad9 plays important roles in multiple cell cycle checkpoint controls and DNA repair (6–8). Hence, Rad9 is likely a gatekeeper gene that prevents cancer development by promoting repair of DNA damage before deleterious effects ensue.
Several recent studies showed that Rad9 was aberrantly expressed in tumors. High expression was detected in human non-small cell lung carcinomas (9) and breast tumors (10). A significantly higher frequency of a single nucleotide polymorphism (SNP) was observed at the second position of codon 239 (His/Arg heterozygous variant) in human non-small cell lung carcinomas (11). In contrast, Rad9 expression levels were found to be lower in prostate cancers in an analysis of three pairs of prostate normal/cancer tissue (12), but a much more extensive analysis found the opposite to be true (13). In fact, aberrantly high levels of Rad9 have been functionally linked to prostate cancer. Combined haploinsufficiency for mouse atm and Mrad9 causes sensitivity to the morphological transformation of mouse embryonic fibroblasts (MEFs) by ionizing radiation while haploinsufficiency of Mrad9 alone does not confer a predisposition to transformation (14). The aforementioned studies established a correlation between cancer development and abnormal Rad9 expression or polymorphism. With respect to expression, aberrantly high or low levels of the protein can predispose to carcinogenesis. However, it remains unclear whether this effect on cancer development is significant for other cancer types, or what the underlying molecular mechanism might be.
A homozygous Rad9-null mutation causes mouse embryonic lethality (15); thus this animal model cannot be used to test whether Rad9 deletion influences tumorigenesis. In this study, we constructed mice selectively deleted for Mrad9 in keratinocytes, thereby circumventing issues related to animal viability. Mice with Mrad9+/− or Mrad9−/− keratinocytes showed no overt, spontaneous, morphological defects. However, the application of DMBA to skin induced a dramatically higher rate of tumor formation in Mrad9−/− mice than in Mrad9+/+ or Mrad9+/− controls. Isolated Mrad9−/− keratinocytes demonstrated enhanced DNA lesions compared to Mrad9+/+ or Mrad9+/− keratinocytes. The Mrad9−/− cells had aberrant cell cycle distribution and an increased rate of apoptosis. These data suggest that Mrad9 is critical for maintaining genomic stability and preventing tumor development.
Materials and Methods
Mouse strains and genotyping
In order to create skin-conditional Mrad9 knockout mice, Mrad9Tar/Tar (15) 129SvEv strain animals were mated to Keratin 5-Cre transgenic mice in which Cre expression is under the control of the Keratin 5 promoter specifically activated in keratinocytes (16). Keratin 5-Cre transgenic mice were originally created using Chinese KM mice and then mated with C57BL/6 mice at least for 10 generations, so it is a hybrid of KM and C57BL/6 mice but with more C57BL/6 genetic background. Methods for DNA isolation and PCR genotyping of mouse tissues as well as isolated keratinocytes for the Mrad9-loxP loci and Cre-mediated recombination were similar to procedures described (15). To detect the presence of the targeted sequence, primers 5’-TTCGGGTGGGAGAATCAGAC-3’ (T1) and 5’-GGATCTCTCCCCATTCACCA-3’ (T2) were used (Figure 1A). To detect the first two exons of Mrad9, primers 5’-CCGGGTGAACCAATAAGGAA-3’ (D1) and 5’-AAGGAAGCAGGCATAGGCAG-3’ (D2) were used. PCR conditions were 95°C for 5 min to initially denature DNA, then 35 cycles at 95°C for 30 sec, 55°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 5 min. The final reaction mix (25 µl) contained 1× Easy Taq SuperMix (TransGen Biotech Co, Beijing), 1 µl genomic DNA and 10 pmol of each primer. All of the animal handling for these protocols and in the following procedures was performed following methods approved by the Institutional Animal Care and Use Committee at the Institute of Biophysics, Chinese Academy of Sciences.
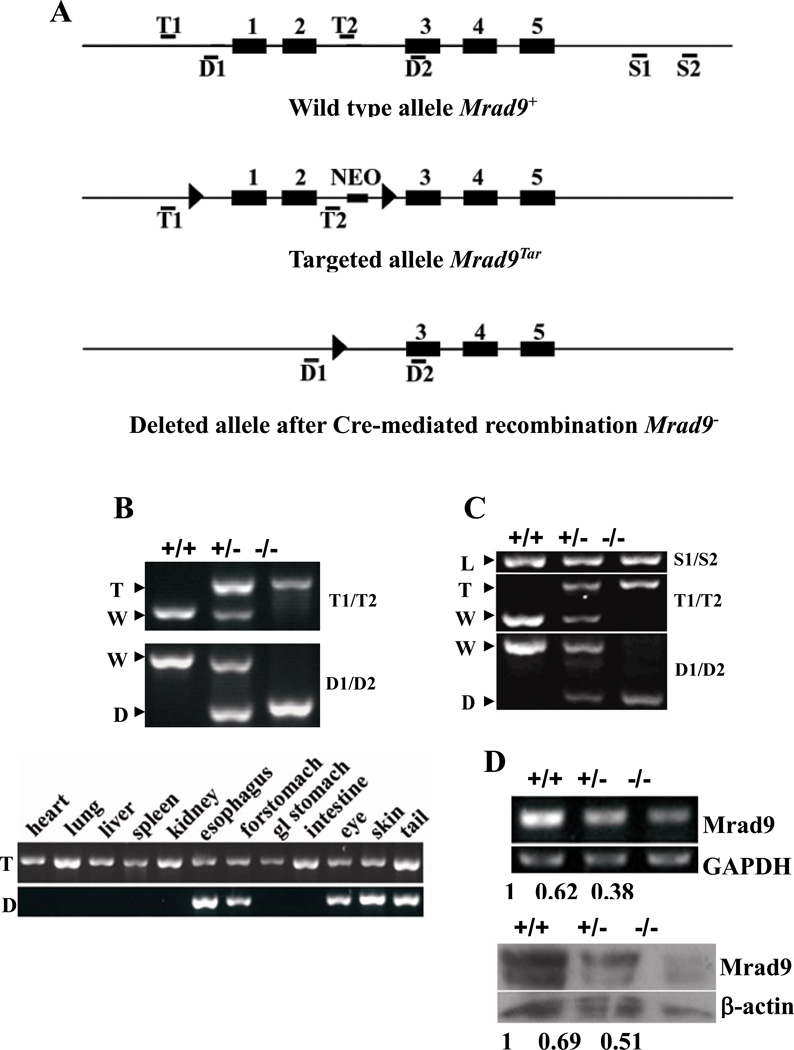
Figure 1. Mrad9 deletion in keratinocytes.
(A) Maps of original, targeted and deleted Mrad9 genomic DNA fragments. Black boxes represent exons, and thin lines represent introns as well as DNA sequences surrounding Mrad9. Locations of primer pairs for detecting the targeting (T1/T2), deletion (D1/D2) of the first two exons and DNA loading control (S1, S2) are marked. (B) PCR genotyping of Mrad9 deletion in mouse tissues. The top panel shows results using primers T1/T2 and D1/D2, and mouse tail DNA as template. The bottom panel is the genotyping results using various mouse tissues. The deleted signature DNA band was only observed in tissues bearing keratinocytes. Gland stomach is represented by gl stomach. (C) PCR-genotyping on skin keratinocytes incubated for three days after isolation. The PCR was semi-quantitative and revealed the Cre efficiency to delete Mrad9. A PCR result using S1/S2 primers is given on the top as the DNA loading control. (D) Semi-quantitative RT-PCR and Western blotting analyses for Mrad9 mRNA and protein levels, respectively, of one-day old mouse pups. Quantitative comparisons to the wild type are listed below the representative experimental results. +/+, +/− and −/− represent Mrad9+/+, Mrad9+/− and Mrad9−/− genotypes, respectively. W, T, D and L on the left of PCR panels indicate signature bands of wild type, targeted, deleted and loading control of Mrad9, respectively.
Semi-quantitative genomic PCR
To examine Mrad9-deletion efficiency in mouse keratinocytes, one sequence downstream of the targeted site was used as template for a DNA loading control. The DNA fragment was generated by PCR using primers 5’-TTCTGTCCTTTCCCCTTGCA-3’ and 5’-GGAGGAAAGCAACAAGTCCT-3’. PCR conditions were the same as described above for genotyping except 60°C was used as the annealing temperature and 28 cycles were run. Genotyping for the successful targeting and deletion of Mrad9 was carried out with the same method described above.
RT-PCR
Total RNA was prepared from epidermis of newborn mice using the RNeasy Mini kit (QIAGEN), as described by the manufacturer. For RT-PCR, 2 µg total RNA were reverse-transcribed in a 25 µl reaction volume to form cDNA using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). PCR amplification of Mrad9 was performed using 1 µl of the cDNA generated above and the primer pair: 5’-CTCTATCTGGAACCCTTGAAGGACG-3’ and 5’-CGCAATAAGTGAGGGCATGAGG-3’. Results were normalized to the amount of a PCR-amplified GAPDH fragment using 1 µl of cDNA and primers 5’- GCAAAGTGGAGATTGTTGCC-3’ and 5’- CCGTATTCATTGTCATACCA -3’. For Mrad9 PCR amplification, there was an initial DNA template denaturation at 95°C for 3 min, then 30 cycles of 95°C for 30 sec, 60°C for 1 min, 72°C for 30 sec, and a final extension at 72°C for 3 min. GAPDH PCR conditions were the same as for Mrad9 except the procedure lasted only 20 cycles.
Quantitative real-time RT-PCR
Total RNA and cDNA from mouse epidermis were isolated as described above. Real-time PCR was performed using the LightCycler system with FastStart DNA Master SYBR Green I to label amplified DNA (Roche). A standard curve method of quantification was used to calculate the expression of target genes relative to the housekeeping gene beta-actin. Experiments were performed three times. The following primer pairs were used for the PCR reactions: Mrad9B: 5’-CCCAAAAGACTATTTCCCAAG-3’ and 5’-TGTTCACAAGATACAGCTCCAA-3’; p21: 5’-GGAACATCTCAGGGCCGAAAA-3’ and 5’-GAGAGGGCAGGCAGCGTAT-3’; p53: 5’-CAGCACATGACGGAGGTCG-3’ and 5’-CTTCCAGATACTCGGGATACAA-3’; beta actin:5’-GTAAAGACCTCTATGCCAACA-3’ and 5’-GGACTCATCGTACTCCTGCT-3’. PCR procedures for the these genes were template denaturation at 94°C for 5 min, then 40 cycles of 94°C for 15 sec, 60°C for 20 sec, 72.0 C for 13 sec, and a final extension at 72°C for 3 min.
Western blotting
For preparing protein from epidermis, full-thickness skin removed from newborn mice was treated with 0.25% trypsin overnight at 4°C. The epidermis was peeled off from the dermis and dispersed in lysis buffer. To prepare cell lysate, keratinocytes incubated for 3 days were either left untreated or treated for 24 h with 0.15µg/ml DMBA (Sigma). Then the cell lysate was prepared in 1×SDS-sample buffer, from a final concentration of 104 cells/µl. Fifty µg protein was resolved on a 10% SDS-PAGE gel, and proteins were transferred to a polyvinylidene difluoride membrane. The membrane was probed consecutively with primary and peroxidase-conjugated secondary antibodies, and the signal was detected using the SuperSignal West Pico Chemiluminescence Substrate system (Prod #34077, Pierce). Primary and secondary antibodies used in this study are mouse anti-phospho-H2AX (Upstate), mouse anti-tubulin (Sigma), mouse anti-p21 (Santa Cruz), mouse anti-p53 (Oncogene), mouse anti-RAD9 (BD), rabbit anti-actin (Sigma), rabbit anti-RAD9B (prepared by Dr. Lieberman’s laboratory), peroxidase-conjugated anti-mouse IgG (A9044, Sigma), and peroxidase-conjugated anti-rabbit IgG (A9169, Sigma).
DMBA-induced skin tumor formation
A published method, with minor modifications, was used to induce skin tumors in mice by the application of DMBA (17). The backs of mice (7–8 weeks old) were shaved 2 days before tumor induction. Then, one side of the shaved dorsal skin was painted with 2 µg 7,12-dimethylbenzanthracene (DMBA) (Sigma) in 0.1 ml acetone twice a week (Wednesday and Sunday); the other side was treated with only acetone as a control. Scoring for tumors was performed once a week. Positive tumor formation was determined using the following criteria: tumors 1 mm or larger in diameter were maintained for two weeks. After positive identification of tumor formation, DMBA treatment was stopped. The longest painting was 25 weeks, then the mice were observed for tumor formation for 5 more weeks. All mice were also examined for the appearance of DMBA-induced pigment deposition spots. Criteria for the occurrence of pigment spots were detection of 10 or more spots, 1 mm or larger in diameter, on one-side of the dorsal skin. In contrast, mice were considered pigmentation negative when no spots appeared or spots were smaller than 0.5 mm in diameter and fewer than 8 appeared. Overall, 70% of the negative mice contained no pigmentation spots.
Histological analysis and immunohistochemistry
Dorsal skin samples and tumors were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, embedded in paraffin and sectioned as 8 µm slices. The sectioned tissues on slides were stained with hematoxylin and eosin (HE) (18, 19) or Van Gieson's Solution (20). Immunohistochemical staining was carried out using a kit (ImmunoCruz Staining Systems, Beijing Zhongshan Golden Bridge Biotechnology, Co, Beijing, China). The endogenous peroxidase activity in the specimens was blocked by treatment with 0.3% H2O2 and samples were then rinsed with PBS. The specimens were probed consecutively with primary antibodies against Keratin 14 (BAbCo), secondary antibody biotin-conjugated goat anti-rabbit IgG, and HRP-streptavidin complex, then visualized by diaminobenzidine. Afterwards, sections were counterstained with hematoxylin (18, 19).
PCR analysis of tumor and normal cells isolated by laser capture micro-dissection
Paraformaldehyde-fixed, paraffin-embedded tumors normal skin tissues were sectioned and stained with H&E. To determine the status of Mrad9 in presumably Mrad9 nondeleted (Tar/Tar) or deleted (−/−) tumors, micro-dissection of tumor and adjacent normal cells from thin sections on slides was performed using a Leica laser micro-dissection system (Leica As LMD). Dissected cells were digested overnight with proteinase K. DNA isolation and PCR were carried out as described above.
Preparation and in vitro culture of keratinocytes
Full-thickness skin removed from newborn mice was treated with 0.25% trypsin overnight at 4°C. The epidermis was peeled off from the dermis and dispersed by stirring into single cells that were then suspended in Keratinocyte-SFM medium with supplements (Invitrogen). Cells were first incubated in dishes coated with collagen type I at 34°C in 5% CO2 for 12 hours to allow cells to attach to the bottom. Afterwards, unattached cells were removed by washing with PBS. Attached cells were further cultured in fresh medium, which was replaced every two days.
Proliferation assay
Keratinocytes were isolated as described and seeded into 12-well plates (3.5×105 cells/well) containing Keratinocyte-SFM medium with supplements. Cells were counted every 2 days. The effect of antioxidant treatment was assessed by culturing Keratinocytes in medium containing 1µM EGCG added initially on the second day of culturing, and included in the fresh medium used during routine maintenance of the cells.
Cell cycle analyses
The profile of cells in different phases of the cell cycle was determined using previously established methods (21). For a simple analysis of cell cycle distribution, 1×107 keratinocytes were plated per 10 cm dish. After incubation for 4 days, cells were processed and stained with propidium iodide (PI), then analyzed by a FACSCalibur (Becton Dickinson). To assess DNA synthesis, 10 µM BrdU was added to medium and cells were pulse-labeled for 40 min. Cells were then processed and probed with FITC-conjugated anti-BrdU antibody (Becton Dickinson), and stained with PI. Flow cytometric analyses were performed on a FACSCalibur.
Apoptosis assays
Keratinocytes, were cultured for 5 days and trypsinized for 10 min using 0.1% trypsin at 37°C (Cat: T3924, Sigma), washed twice with cold PBS, then resuspended in 1× binding buffer (10 mM HEPES, pH 7.4; 140 mM NaCl; 2.5 mM CaCl2) at a concentration of 1×106 cells/ml. Then cells were stained with Annexin V-FITC (Jingmei Biotech, Beijing, China) and PI for 15 min at room temperature, before flow cytometric analysis.
Neutral comet assay
Keratinocytes were first cultured in standard medium for 2 days, then incubated in medium containing 0, 0.01, 0.025, 0.05, or 0.1µg/ml DMBA, respectively, for 24 hr before analysis. The comet assay was carried out according to the manufacturer’s instruction (Trevigen). Briefly, cells at a concentration of 1×105 /ml were mixed gently with pre-melted low temperature melting agarose at a volume ratio of 1 to 10 (v/v) and spread on glass slides. The slides were then submerged in pre-cooled neutral lysis buffer at 4°C for 30 min. After rinsing, the slides were equilibrated in TBE solution, electrophoresed at 1.0 V/cm for 20 min, and then stained with PI. Fluorescence images for at least 50 nuclei were captured using a Nikon microscope and analyzed by CASP-1.2.2 software (University of Wroclaw, Poland) for tail moment (i.e., the geometric mean of fluorescence on the tail from the nucleus).
Statistical analysis
All statistical analyses were performed using statistical software package SAS Version 9.1.3 (22). The Chi-square test was used to compare genotype ratio for newborn pups derived from matings between Mrad9+/− mice. The Kaplan-Meier PL method (23) was used for comparison of the relative risks of tumor development induced by DMBA among the mice with the three Mrad9 genotypes. We designed the tumor development experiment to meet a set of conditions so the Log Rank Test in the Kaplan-Meier PL method could be employed, and the number of animals used was reduced but results with statistical significance still could be achieved (22; refer to Results). The Student's t test was performed to determine statistical significance of the differences for the comet assay. In all the above analyses, p < 0.05 was considered statistically significant.
Results
Targeted deletion of Mrad9 in mouse keratinocytes
Mrad9−/− mice die during embryonic development, but relative to wild-type controls, Mrad9+/− animals have no overt defects in gross morphology or health up to 1.5 years of age (15). In this report, we describe the development of viable mice with a specific deletion of Mrad9 in keratinocytes. These animals were made by crossing mice containing loxP targeted to Mrad9 (15) with mice in which Cre transcription is under the control of the K5 promoter, specifically activated in keratinocytes starting on day 14.5 dpc (16). After repeated backcrosses, homologous Cre genotypes were obtained in mice with three different Mrad9 genotypes (Fig.1 A and B). Cre homozygous status of the mice was determined by crossing them with 2 mice bearing no Cre, which produced at least 15 Cre–positive pups and no Cre–negative pups (data not shown). Mating between Cre+/+ Mrad9Tar/+ mice yielded an offspring composition of Mrad9+/+ (147): Mrad9+/− (245): Mrad9−/− (141), statistically equal to 1:2:1 (Chi-square Test: P=0.845). Compared to animals with keratinocytes genetically Mrad9+/+ or Mrad9+/−, no obvious defects were observed in Mrad9−/− mice examined up to 1.5 years of age (data not shown).
Deletion of Mrad9 occurred exclusively in tissues (skin, forestomach, eye and esophagus) that contain keratinocytes (Fig 1B). Notably, PCR using DNA templates from Cre+/+ Mrad9Tar/Tar mouse skin or isolated skin keratinocytes yielded both Mrad9 deleted as well as targeted signature bands (Fig 1B and C), indicating that Mrad9 deletion in skin keratinocytes was not complete, or there was a significant contamination of other types of cells. In order to exclude the possibility that the targeted signature bands mainly derived from contaminating non-keratinocytes, the percentage of cells containing keratin 5 was assessed by flow cytometry. The isolated Cre+/+ Mrad9Tar/Tar skin keratinocytes stained with anti-keratin 5 antibody were 96% keratin 5-positive (data not shown). We also re-performed the PCR using the T1/T2 primer pair and DNA templates from keratinocytes with 5 cycles less of reaction. For these conditions, the targeted signature bands were reduced to 1/9 and 1/18 in Cre+/+ Mrad9Tar/+ and Cre+/+ Mrad9Tar/Tar keratinocytes, respectively; the wild type signature band was not detected in the same PCR reaction (data not shown). However, the targeted signature band in Cre+/+ Mrad9Tar/Tar cells was still 77% of that detected in Cre+/+ Mrad9Tar/+ cells. Taking all these data together, we conclude that a significant portion of skin keratinocytes did not have Mrad9 deleted.
We also monitored Mrad9 mRNA and protein levels in pup skins (Fig 1D). RT-PCR analysis indicated that the Mrad9/GADPH mRNA ratios were 1, 0.62 and 0.38 in Cre+/+ Mrad9+/+, Cre+/+ Mrad9Tar/+ and Cre+/+ Mrad9Tar/Tar skins, respectively. Western blotting illustrated that the Mrad9/beta-actin protein level ratios were 1, 0.69 and 0.51 in wild type, heterozygous and homozygous cells, respectively. Thus the Cre-mediated Mrad9 deletion significantly lowered Mrad9 expression in keratinocytes, and probably completely removed Mrad9 protein from a major portion of the Cre+/+ Mrad9Tar/Tar keratinocyte population.
For descriptive purposes, Mrad9+/+, Mrad9+/− and Mrad9−/− will be used to denote Cre+/+ Mrad9+/+, Cre+/+ Mrad9Tar/+ and Cre+/+ Mrad9Tar/Tar, respectively. However, as indicated by the results above, not all keratinocytes within the Cre+/+ Mrad9Tar/+ and Cre+/+ Mrad9Tar/Tar cell populations were deleted for Mrad9.
Susceptibility of Mrad9−/− mice to skin tumorigenesis
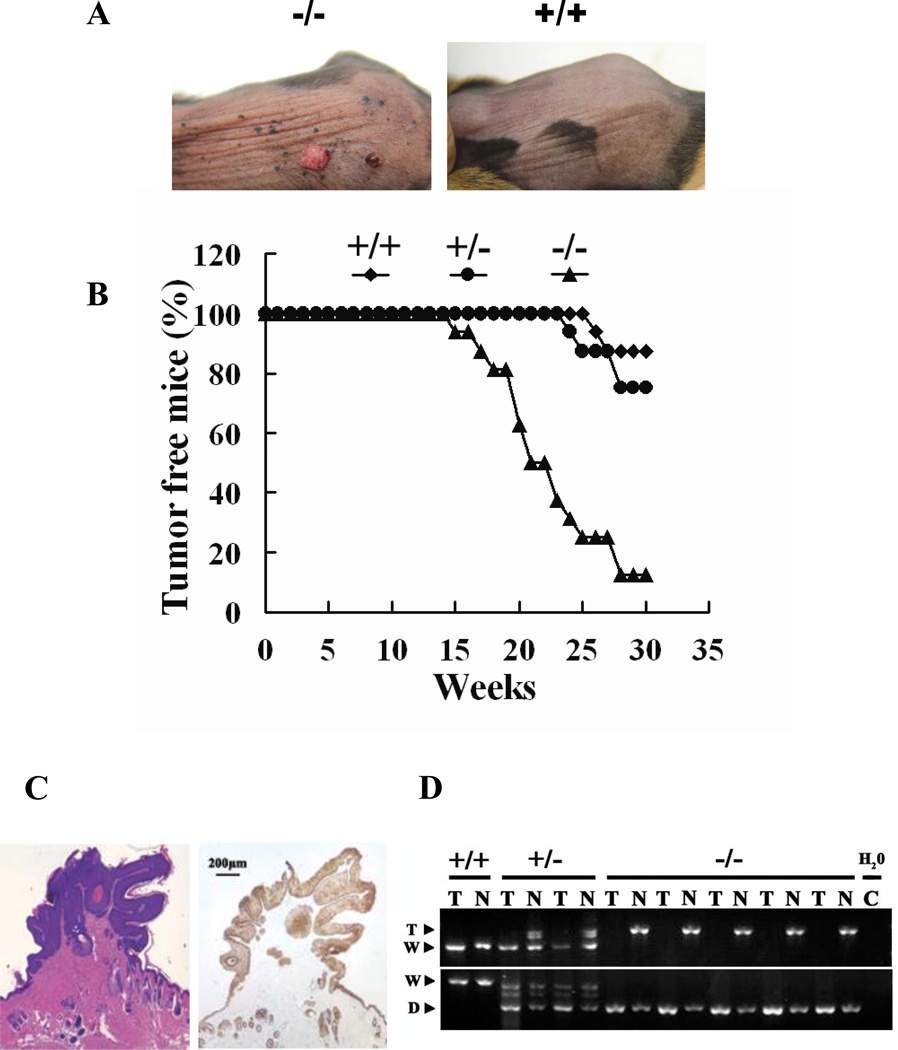
To determine if Mrad9 is important for tumorigenesis, the skin of mice with different status of Mrad9 was smeared with DMBA (7,12-dimethylbenzanthracene). A total of 48 mice were divided into 3 groups, each with the Mrad9+/+, Mrad9+/− or Mrad9−/− genotype, respectively. To increase the efficiency of the animal experiment statistically (24), we made a strict arrangement that the 3 groups of mice were from 16 litters, each litter consisting of 3 mice with 3 different Mrad9 genotypes respectively and identical sex, either female (10 liters) or male (6 liters). Under this setting, Log-Rank Test in the Kaplan-Meier PL method can be used for the statistical analysis on the significance of differences of tumor development among the three groups of animals (23, 24). DMBA is a chemical carcinogen that can induce tumors efficiently (25, 26). Two µg DMBA in 0.1 ml acetone was painted onto shaved mouse skin twice a week for 25 weeks. Skin tumors began to appear on Mrad9−/− mouse skin after DMBA treatment for 15 weeks whereas tumor onset occurred 25 and 27 weeks after the start of DMBA treatment in Mrad9+/− and Mrad9+/+ mice, respectively (Fig 2A and B). Staining skin specimens with H&E (hematoxylin and eosin) or anti-keratin 14 showed that the tumors were derived from keratinocytes and possessed characteristics of papillomas (Fig 2C) (27). Kaplan-Meier PL method (23) was used for comparison of the relative hazards of tumor development induced by DMBA among the three genotypes of mice. The rate of tumor development in Mrad9−/− mice was significantly higher than in Mrad9+/− and Mrad9+/+ animals (p=0.0004 and p=0.0003, respectively, Log-Rank Test). Although Mrad9+/− mice tended to be more prone to induced tumorigenicity than Mrad9+/+ mice, the difference is not statistically significant (p=0.4088, Log-Rank Test). Therefore, lower levels of Mrad9 caused by haploinsufficiency did not cause significantly increased cancer development, relative to wild type controls.
Figure 2. Skin tumor induction by DMBA.
Forty eight mice were divided into three groups, each with the Mrad9+/+ Mrad9+/− or Mrad9−/− genotype, respectively. The mice were subjected to DMBA treatment and tumor development was examined. (A) Papillomas induced by DMBA treatment in Mrad9−/− mouse skin (left), and treated Mrad9+/+ mouse control skin (right). (B) Tumor-free mice bearing one of the three genotypes. Application of 2 µg DMBA/0.1 ml acetone onto skin started at ages 7–8 weeks, and was given twice a week. Symbols: diamond, Mrad9+/+; circle, Mrad9+/−; triangle, Mrad9−/−. (C) H&E (hematoxylin and eosin, left) staining illustrates a papilloma, with connective tissues extending into the tumor. The tissue is also stained for Keratin 14 (right), thus indicating that it is derived from keratinocytes. (D) Tumor cells and adjacent normal tissue cells were isolated and genotyped by PCR to reveal Mrad9 statuses in the cells. +/+, +/− and −/− represent Mrad9+/+, Mrad9+/− and Mrad9−/− genotypes, respectively.
Since the Cre-mediated Mrad9 deletion was not complete, to confirm the importance of Mrad9 in tumor inhibition, tumor and nearby normal cells were isolated using laser capture micro-dissection and their genomic DNA was subjected to PCR. All five examined tumors from Mrad9−/− mice were Mrad9−/− while the normal cells on the sides of the tumors still contained undeleted but targeted Mard9 (Figure 2D) as expected, indicating that Mrad9 indeed plays a significant role in preventing tumor development.
Under this experimental setting, the number of induced tumors for all three Mrad9 genotypes was 1 or 2, and differences between mice with different Mrad9 genotypes were not observed.
Mrad9 deletion alters the cell cycle profile of keratinocytes and increases DNA damage
Since Rad9 plays important roles in DNA repair and cell cycle checkpoint controls, two processes important for maintaining genomic integrity and preventing carcinogenesis, we investigated these processes in keratinocytes bearing an Mrad9 deletion. Skin keratinocytes were isolated from one-day old pups. The cells were grown in defined Keratinocyte-SFM medium (Life Technologies, Inc.). The number of cells with each of the three genotypes dropped to less than 30% of the seeded (3.5×105/well) level after the first two days of incubation. Subsequently, the total number of wild type cells increased between day 2 and 6, then cell proliferation slowed down (Fig 3A; data not shown). In contrast, the total number of Mrad9−/− cells continued to decrease (Fig 3A; data not shown). The Mrad9−/− cells did not stop decreasing in number and did not begin to grow after extended culturing (data not shown). There were two types of Mrad9 heterozygous pups when mouse tail DNA was used for genotyping one day after birth, one with a low frequency of deletion of the Mrad9-targeted allele and the other with a high level of deletion (PCR data not shown). However, when genotyped again in 20-day and two-month old mice, there was only the latter type of deletion (data not shown). Furthermore, the cells isolated from all new born Mrad9+/− pups were found to have the latter deletion type after being cultured 3 days and then genotyped. The reason for having the subtle differences among new-born Mrad9+/− pups was not understood, but for the succinctness of experiments, the differences were not distinguished for in the rest of the study.
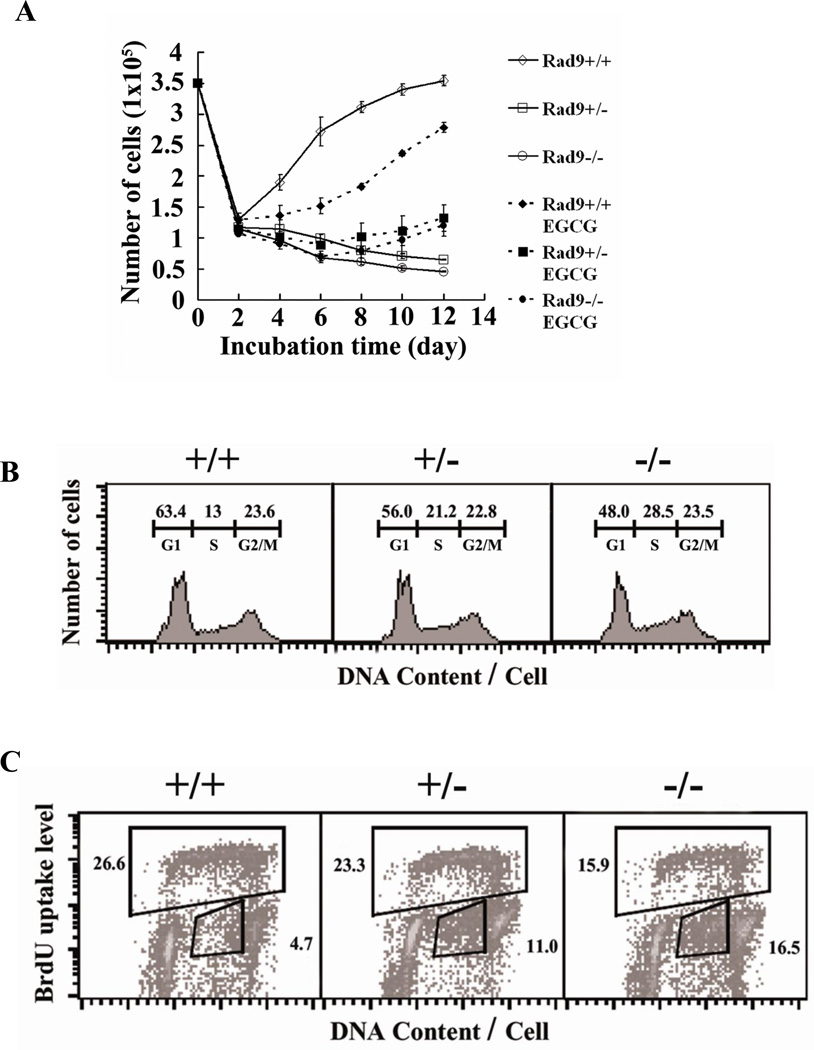
Figure 3. Proliferation and cell cycle distribution of wild type and Mrad9-deficient keratinocytes in culture.
(A) Proliferation of skin keratinocytes varying in Mrad9 status. Symbols for cultures without EGCG: open diamond, Mrad9+/+; open square, Mrad9+/−; open circle, Mrad9−/−. Deletion of either one or two Mrad9 loci retarded proliferation. Symbols for cultures with EGCG: solid diamond, Mrad9+/+; solid square, Mrad9+/−; solid circle, Mrad9−/−. The antioxidant EGCG was added to media. Retardation of Mrad9–deleted keratinocyte growth was alleviated by EGCG. (B) Flow cytometric analyses of cell cycle distributions of PI-stained keratinocytes with different Mrad9 genotypes. Deletion of either one or two Mrad9 loci led to reduced numbers of cells in G1 and an increased number in S phase. The numbers above each phase indicate the percentage of cells in that phase among the whole cell population. (C) Flow cytometric analyses of keratinocytes stained with both PI and BrdU. Mrad9 status is indicated on the top. Fewer Mrad9-deficient cells were BrdU-positive, and many of them were BrdU-negative but contained a late S phase amount of DNA. The number in the upper box is the percentage of BrdU-positive cells, and the number in the lower right box is the percentage of BrdU-negative cells with late S phase DNA content.
We tested whether reduction in number of Mrad9-deleted keratinocytes was caused by DNA lesions induced by reactive oxygen species (ROS), since it is known that Rad9 plays an important role in DNA damage repair. Conventionally, cells are cultured at 20% O2. Incubation at this O2 concentration loads cells with a higher O2 tension (thus higher ROS level) than within animals (28–30). We used the antioxidant EGCG to scavenge oxidative free radicals to assess the effect of the high oxygen tension present during cell culture, relative to in vivo in the animal, on cell proliferation. The addition of 1 µM of the antioxidant EGCG to the medium changed the direction of the cell growth curves of Mrad9−/− and Mrad9+/− keratinocytes upward from day 6 (Fig 3B). Therefore, high O2 tension was a major cause for the cell number reduction observed. Growth of the wild type cells also slowed down in the EGCG-containing medium, an established inhibitory effect of the drug on cell proliferation (31, 32).
Flow cytometric analyses of PI (propidium iodide)-stained keratinocytes indicated that more Mrad9−/− and Mrad9+/− cells, compared to the wild-type controls, accumulated in late S phase (Fig 3C). A measurement of BrdU uptake by replicative S phase cells in combination with DNA content via PI staining in individual cells can reveal more information on cell cycle distribution. Therefore, we investigated cell cycle profiles in more detail by pulse-labeling with BrdU and staining cells after 4 days incubation. A major population of Mrad9−/− and Mrad9+/− cells with DNA content in the range primarily of late S phase was not labeled by BrdU, and there were fewer BrdU-positive Mrad9−/− and Mrad9+/− cells than wild type cells in S phase (Fig 3D). The BrdU-negative cells with late S phase DNA content means that the cells were arrested in late S phase. Based on the above data, we conclude that the arrest of cells in late S phase is an important cause for the cell number reduction of Mrad9−/− and Mrad9+/− keratinocytes during in vitro incubation.
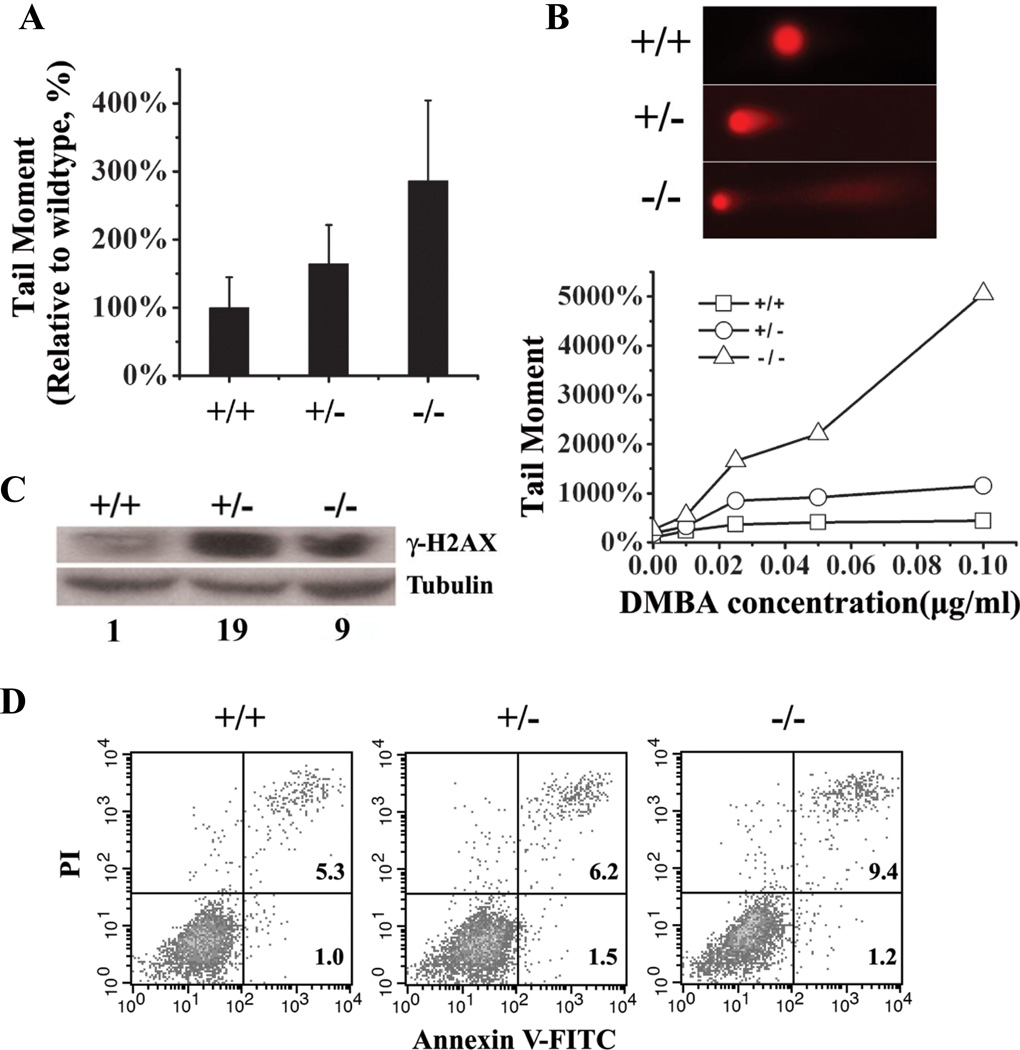
We used the neutral comet-assay to detect double-strand breaks (DSBs) in DNA. There were significantly more DSBs in incubated Mrad9+/− cells than in wild type cells, and more DSBs in Mrad9−/− cells than in Mrad9+/− cells (Fig 4A). Therefore, Mrad9 is critical for maintaining genomic integrity, and there is a dosage dependent effect. H2AX phosphorylation level is another established indicator of DNA double strand breaks. Consistent with the comet assay results, H2AX phosphorylation levels from lysates of Mrad9−/− cells as well as Mrad9+/− cells were much higher than in wild type cell lysates (Fig 4C). Interestingly, H2AX phosphorylation level from Mrad9−/− cells (arbitrary number 9) was lower than that from Mrad9+/− cells (arbitrary number 19). H2AX phosphorylation occurs predominantly in S phase and G2/M phase cells in vitro culturing (33). The lower ratio of normal S phase cells is likely a cause for the relatively lower H2AX phosphorylation level compared to that of Mrad9+/− cells (Fig 3C). In spite of possible influence by the changed cell cycle distribution, the overall H2AX phosphorylation level Mrad9−/− cells was still higher than that in wild type cells (arbitrary number 1).
Figure 4. Spontaneous and DMBA-induced DNA double strand breaks and apoptosis.
(A) Evaluation of spontaneous DNA double strand breaks in keratinocytes by the neutral comet assay. Cells were incubated for three days before collection and analysis. Both Mrad9+/− and Mrad9−/− cells contained significantly more double strand breaks (p = 1.4×10−6 and p = 3.0×10−17, respectively) as compared to the wild type control. (B) To evaluate DMBA-induced DNA double strand breaks in keratinocytes, cells were first incubated for 48 hours, then different concentrations of DMBA were added to the cells, which were subsequently cultured for an additional 24 hours. DMBA (0.1 µg/ml) induced apoptosis in Mrad9−/− cells as indicated by the prolonged tail, but not in the cells with the two other genotypes, shown on the top of the panel. (C) Western blotting analysis of γ-H2AX levels without DMBA treatment. Lysates were prepared from cells incubated for three days after initial isolation. The relative quantitative comparison of γ-H2AX levels of the three cell types is illustrated at the bottom of the panel (1:19:9). (D) Flow cytometric analysis of keratinocytes to assess spontaneous apoptosis using Annexin V labeling. Cells were incubated for 4 days before harvesting for apoptotic analysis. +/+, +/− and −/− represent Mrad9+/+, Mrad9+/− and Mrad9−/− genotypes, respectively.
As we showed above, tumors only appeared after DMBA treatment. Therefore, we asked if DMBA treatment causes more DSBs in Mrad9 deficient cells than in wild type cells. As predicted, incubation in medium containing 0.02 to 0.05µg/ml DMBA induced significantly more DSBs in Mrad9 deficient cells than wild type cells (Fig 4B). Further increase in the DMBA concentration to 0.1µg/ml DMBA greatly enhanced tail moment and coefficient variance of tail moment in the Mrad9−/− cells. The dramatic increases in both tail moment and its coefficient variance were because a subpopulation of Mrad9−/− cells had very long tails (Fig 4B and data not shown), a characteristic of apoptosis (34). No apoptosis was observed in Mrad9+/− and Mrad9+/+ cells treated with DMBA.
To confirm the effects of Mrad9 deletion on apoptosis, we stained the same cell populations for Annexin V-FITC and PI to provide supporting evidence. We found that the percentage of apoptotic cells among Mrad9+/− keratinocytes (7.74%) was higher than that of Mrad9+/+ cells (6.32%). The percentage of Mrad9−/− apoptotic cells (10.58%) was higher than that of Mrad9+/− cells (Fig 4D). The inhibitory effect of Rad9 on apoptosis was consistent with previously reported effects on murine embryonic stem cells (35) and chicken ET40 cells (36).
Effect of Mrad9 deletion on expression of other cell cycle checkpoint genes
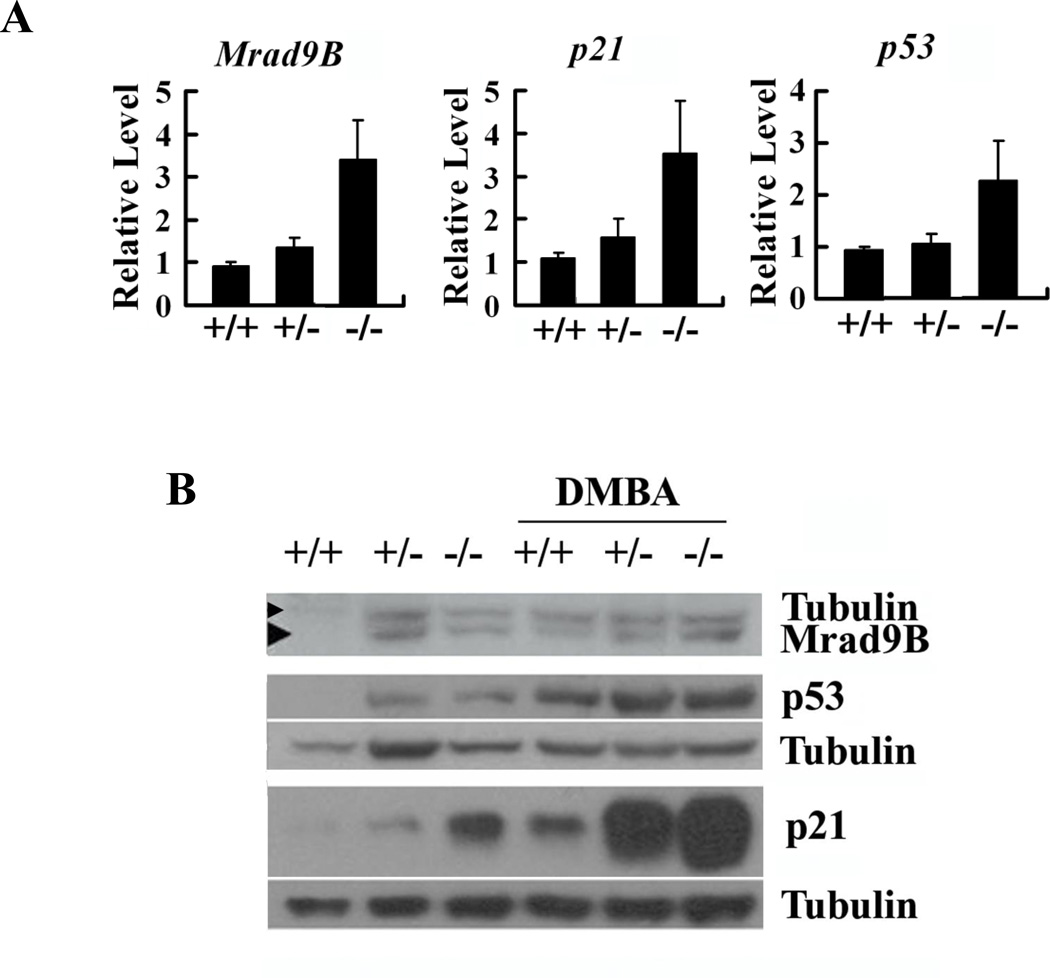
Expression of other cell cycle checkpoint genes, including p21, p53 and Rad9B, in Mrad9−/− and control keratinocytes was assessed by quantitative RT-PCR and western blotting to gain mechanistic insight into the function of Mrad9. Both heterozygous and homozygous deletion of Mrad9 induced expression of p21, p53 and Rad9B in skin keratinocytes (Fig 5A and 5B). Statistical analyses on the quantitative RT-PCR results indicate that the expressions of the three genes in Mrad9−/− cells are significantly higher than those in Mrad9+/− and Mrad9+/+ cells (Fig 5A). DMBA treatment (0.15µg/ml, 24 h) increased the levels of p21 and p53 proteins in cells with each of the three genotypes (Fig 5B). The same treatment also enhanced Mrad9B protein level in wild type cells, but not obviously in Mrad9−/− and Mrad9+/− cells. These results suggest that the genomic DNA damage stress response system is increased in cells with a deletion of Mrad9.
Figure 5. Expression of Mrad9B, p21 and p53 in keratinocytes differing in Mrad9 status.
(A) Real-time quantitative RT-PCR analysis of Mrad9B, p21 and p53 mRNA levels in one-day old mouse skin. Status of Mrad9 is indicated. Each relative level is the average ratio of the PCR results of an indicated gene relative to β-actin for three independent samples, and each PCR result is the mean of triplet PCR of the same sample. (B) Western blotting analysis of Mrad9B, p21 and p53 protein levels in keratinocytes incubated for three days after initial isolation. The first three lanes are the protein levels in cells without DMBA treatment, and the last three lanes are the protein levels in cells treated with 0.15 µg/ml DMBA for 24 hours. The data in the figure are three representative western blotting results, and each has an independent tubulin control. +/+, +/− and −/− represent Mrad9+/+, Mrad9+/− and Mrad9−/− genotypes, respectively.
Susceptibility of Mrad9−/− mice to the development of ageing skin
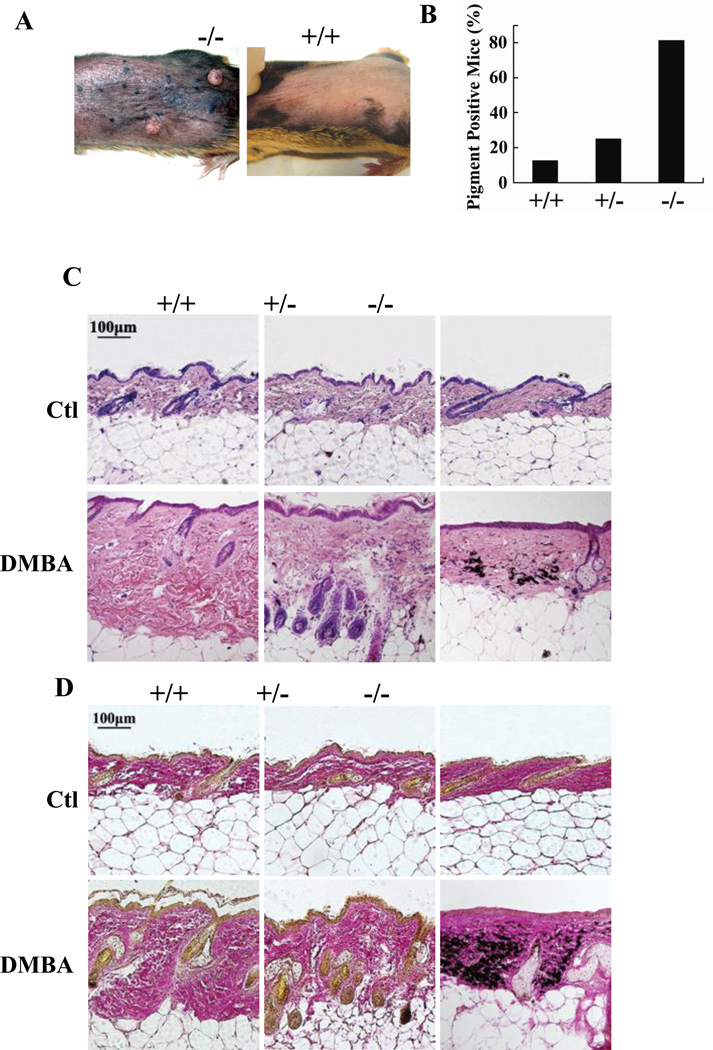
In the same set of mice used for tumor development experiments, DMBA treatment to skin readily induced skin pigment deposition in Mrad9−/− mice (Fig 5A). Within the DMBA treatment experimental period (30 weeks), skin pigment deposition occurred on 81.3% of Mrad9−/−, 25% of Mrad9+/− and 12.5% of Mrad9+/+ mice (Fig 5B). Pigment deposition occurred in 2 to 5 weeks after tumor formation in most mice and after the end of DMBA treatment in mice bearing any of the three genotypes (data not shown). H&E (hematoxylin and eosin) and VG (Van Gieson) staining on skin specimens demonstrated that pigment particles in the dermis were abnormally increased (Fig 5C and 5D); hair follicle number significantly decreased; and epidermis atrophied in Mrad9−/− mice. These phenotypes indicate that the skin of Mrad9−/− mice aged readily in response to DMBA treatment. However, the phenotypes did not appear without DMBA treatment (data not shown), suggesting that they are mediated by DNA damage.
Discussion
The Rad9 gene is evolutionarily conserved from yeast to human and critical for cell cycle checkpoint controls as well as DNA repair (6, 7). Its role as a tumor suppressor has not been reported thus far, although many DNA repair and cell cycle checkpoint genes are known to be required for preventing tumorigenesis. Here we report the establishment of a conditional Mrad9 knockout model using mouse keratinocytes to examine whether Mrad9 is inhibitory to tumor formation and the mechanisms involved (Fig 1). We demonstrated that Mrad9 is required for suppressing skin tumor formation induced by the DNA damaging agent DMBA (Fig 2). Furthermore, we show that Mrad9 also plays a key role in preventing skin ageing induced by DMBA treatment (Fig 6).
Figure 6. DMBA-induced skin ageing in wild type or Mrad9-deleted mice.
(A) Mrad9−/− (left) and Mrad9+/+ (right) mice shown were treated with DMBA for 25 weeks. (B) Skin pigment deposition in mice with different Mrad9 status treated with DMBA. (C) H&E (hematoxylin and eosin) staining of DMBA-treated mouse skins. DMBA caused a reduction of follicles, atrophied skin and abnormal pigment deposition on Mrad9−/− mouse skin while DMBA-treated skins of Mrad9+/+ and Mrad9+/− mice looked normal. (D) VG (Van Gieson) staining of DMBA treated skin. This treatment led to a thinner but dense dermis in Mrad9−/− but not in Mrad9+/+ or Mrad9+/− mouse skins. +/+, +/− and −/− represent Mrad9+/+, Mrad9+/− and Mrad9−/− genotypes, respectively.
Although DMBA treatment enhanced the incidence of papillomas in Mrad9 deleted skin keratinocytes statistically significantly more than in wild type keratinocytes, the Mrad9 deletion alone did not lead to an increase in spontaneous tumor formation. DNA repair, cell cycle checkpoint controls and apoptosis are three key functions in preventing the formation of primary tumors. There is an abundance of evidence indicating that Rad9 participates in multiple DNA repair pathways (8, 37–43) and cell cycle checkpoints (15, 44–46). Both anti-apoptotic and pro-apoptotic roles were reported using different human and animal cell lines (35, 47–49). The roles of Rad9 in DNA repair and apoptosis in keratinocytes have not been reported previously. We illustrated in this study that keratinocytes with Mrad9 deleted at either one or two loci had more DNA double strand breaks, and the increase of DNA lesions induced by DMBA was more significant in Mrad9 deleted cells than in wild type controls (Fig 4). Therefore, the function of Mrad9 in repairing DNA lesions is likely one key component of the ability of the protein to repress tumorigenesis. Mrad9 deletion led to higher apoptotic keratinocytes in culture either with DMBA treatment or not (Fig 4C), thus apoptosis is not a significant mechanism used by Mrad9 to serve as a gatekeeper to prevent tumor formation in skin keratinocytes.
Human Rad9 protein can bind the p21 promoter region and induce p21 transcription (50). Here we showed that Mrad9 deletion highly enhanced p21 expression (Fig 5A). However, Mrad9 deletion also induced p53 expression (Fig 5A), which could serve as the transactivator of p21 expression. Interestingly, Mrad9 deletion also enhanced Mrad9B (Mrad9 homologue) expression. Since Mrad9 deletion alone did not lead to tumor formation, it is possible that Mrad9B might have at least partially redundant functions related to Mrad9 activity. Treatment with DMBA increased Mrad9B protein level in wild type keratinocytes, but did not further enhance Mrad9B level in Mrad9−/− and Mrad9+/− keratinocytes. However, DMBA treatment increased p21 and p53 protein levels in keratinocytes with any of the three Mard9 genotypes (Fig 5B). The Mrad9B level seems to be limited to relatively low abundance compared with p21 and p53 levels, suggesting that the regulation of Mrad9B is different from that of p21 and p53.
DMBA treatment also caused mouse skin ageing in Mrad9 deleted animals more significantly than in wild type controls. Skin ageing was reflected as increased pigmentation, decreased hair follicles and atrophied epidermis. In cell culture, Mrad9-deleted keratinocytes have more double strand breaks and a net negative growth (Fig 4A). Detailed examination revealed that many Mrad9-deficient cells were arrested in late S phase and more tended to go into apoptosis (Fig 3D; Fig 4C). The Mrad9-deficient cells did not stop replicating, thus the arrest in late S phase and apoptosis are major contributing factors to the negative growth of Mrad9-deleted keratinocytes cultured in vitro. The negative growth was reversed by addition of the antioxidant EGCG to the medium (Fig 3B), suggesting that oxidative free radicals lead to the growth defect, probably due to an enhancement in the frequency of DNA lesions. Under in vivo conditions where oxygen tension is lower and keratinocytes are in a more compatible environment, it is conceivable that extra genomic stress such as DMBA treatment is needed to increase the number of DNA lesions and mediate the proliferation defect.
It is clear from our results that Rad9 effects cell cycle distribution in keratinocytes. How Mrad9 regulates cell cycle checkpoints in keratinocytes, and the relationship to the mechanism that arrests Mrad9-deleted keratinocytes in late S phase remain to be addressed. The mechanism underlying the latter novel phenotype, in particular, is worth further investigation.
In summary, we demonstrate that Rad9 is important for resisting the development of tumors in keratinocytes, and roles in several molecular mechanisms that stabilize the genome might be involved. This work underscores the need to understand the function of Rad9 and other cell cycle checkpoint or DNA repair proteins, especially in terms of their impact on tumorigenesis and potential for translational impact in the clinic.
ACKNOWLEDGMENTS
Grant support: National Natural Science Foundation of China 30470373 and 30530180 (HH), National Protein Project of Ministry of Science and Technology 2006CB910902 (HH), Knowledge Innovation Program of Chinese Academy of Sciences KSCX2-YW-R63 (HH); NIH grants GM079107 and CA130536 (HBL).
We thank Dr. Songmei Geng (Department of Dermatology, Second Hospital, Xi’An JiaoTong University, China)for helpful discussions and suggestions on histological analysis, Dr. Jianhua Liu (Department of Health Statistics, Chinese Centers for Disease Control and Prevention, China) for statistical analyses. We also thank Dr. Xiao Liang (State Key Laboratory of Molecular Oncology, Cancer Institute/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China) for technical assistance related to laser capture micro-dissection.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 4.Houtgraaf JH, Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med. 2006;7:165–172. doi: 10.1016/j.carrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nature reviews. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 6.Parrilla-Castellar ER, Arlander SJ, Karnitz L. Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair (Amst) 2004;3:1009–1014. doi: 10.1016/j.dnarep.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman HB. Rad9, an evolutionarily conserved gene with multiple functions for preserving genomic integrity. Journal of cellular biochemistry. 2006;97:690–697. doi: 10.1002/jcb.20759. [DOI] [PubMed] [Google Scholar]
- 8.Toueille M, El-Andaloussi N, Frouin I, et al. The human Rad9/Rad1/Hus1 damage sensor clamp interacts with DNA polymerase beta and increases its DNA substrate utilisation efficiency: implications for DNA repair. Nucleic Acids Res. 2004;32:3316–3324. doi: 10.1093/nar/gkh652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniwa Y, Yoshimura M, Bermudez VP, et al. Accumulation of hRad9 protein in the nuclei of nonsmall cell lung carcinoma cells. Cancer. 2005;103:126–132. doi: 10.1002/cncr.20740. [DOI] [PubMed] [Google Scholar]
- 10.Cheng CK, Chow LW, Loo WT, Chan TK, Chan V. The cell cycle checkpoint gene Rad9 is a novel oncogene activated by 11q13 amplification and DNA methylation in breast cancer. Cancer research. 2005;65:8646–8654. doi: 10.1158/0008-5472.CAN-04-4243. [DOI] [PubMed] [Google Scholar]
- 11.Maniwa Y, Yoshimura M, Bermudez VP, et al. His239Arg SNP of HRAD9 is associated with lung adenocarcinoma. Cancer. 2006;106:1117–1122. doi: 10.1002/cncr.21705. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Hsu CL, Ni J, et al. Human checkpoint protein hRad9 functions as a negative coregulator to repress androgen receptor transactivation in prostate cancer cells. Molecular and cellular biology. 2004;24:2202–2213. doi: 10.1128/MCB.24.5.2202-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu A, Zhang X, Lieberman HB. Rad9 has a functional role in human prostate carcinogenesis. Cancer Res. 2008;68:1267–1274. doi: 10.1158/0008-5472.CAN-07-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smilenov LB, Lieberman HB, Mitchell SA, Baker RA, Hopkins KM, Hall EJ. Combined haploinsufficiency for ATM and RAD9 as a factor in cell transformation, apoptosis, and DNA lesion repair dynamics. Cancer Res. 2005;65:933–938. [PubMed] [Google Scholar]
- 15.Hopkins KM, Auerbach W, Wang XY, et al. Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Molecular and cellular biology. 2004;24:7235–7248. doi: 10.1128/MCB.24.16.7235-7248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao CM, Yang X, Cheng X, Lu YX, Zhou J, Huang CF. Establishment of keratinocyte-specific Cre recombinase transgenic mice. Yi Chuan Xue Bao. 2003;30:407–413. [PubMed] [Google Scholar]
- 17.Hirao A, Cheung A, Duncan G, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Mao C, Teng Y, et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Itami S, Ohishi M, et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer research. 2003;63:674–681. [PubMed] [Google Scholar]
- 20.Culling CFA. Handbook of histopathological and histochemical techniques. 3rd ed. London: Butterworth; 1974. [Google Scholar]
- 21.Hang HYFM. Analysis of the mammalian cell cycle by flow cytometry. In: Lieberman HB, editor. Cell cycle checkpoint control protocols. Totowa (NJ): Humana Press; 2004. [Google Scholar]
- 22.Yasuhara S, Zhu Y, Matsui T, et al. Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J Histochem Cytochem. 2003;51:873–885. doi: 10.1177/002215540305100703. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. SAS/STAT®9.1 User's Guide. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 24.Lee ET, Wang JW. Statistical Methods for Survival Data Analysis. Third Edition. New York: John Wiley and Sons; 2003. [Google Scholar]
- 25.Michael FW, Festing PO, Das Rose Gaines, Borja Mario Cortina, Berdoy Manuel. Laboratory Animal Handbooks NO. 14. London: The Royal Society of Medicine Press Ltd; 2002. The Design of Animal Experiments. Reducing the use of animals in research through better experimental design. [Google Scholar]
- 26.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 27.Corominas M, Leon J, Kamino H, Cruz-Alvarez M, Novick SC, Pellicer A. Oncogene involvement in tumor regression: H-ras activation in the rabbit keratoacanthoma model. Oncogene. 1991;6:645–651. [PubMed] [Google Scholar]
- 28.Knutsen GL, Kovatch RM, Robinson M. Gross and microscopic lesions in the female SENCAR mouse skin and lung in tumor initiation and promotion studies. Environ Health Perspect. 1986;68:91–104. doi: 10.1289/ehp.866891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito H, Hammond AT, Moses RE. The effect of low oxygen tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp Cell Res. 1995;217:272–279. doi: 10.1006/excr.1995.1087. [DOI] [PubMed] [Google Scholar]
- 32.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Kurose A, Huang X, Traganos F, Dai W, Darzynkiewicz Z. Extent of constitutive histone H2AX phosphorylation on Ser-139 varies in cells with different TP53 status. Cell Prolif. 2006;39:313–323. doi: 10.1111/j.1365-2184.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu A, Zhou H, Leloup C, et al. Differential impact of mouse Rad9 deletion on ionizing radiation-induced bystander effects. Radiat Res. 2005;164:655–661. doi: 10.1667/rr3458.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 2007;21:1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnova E, Toueille M, Markkanen E, Hubscher U. The human checkpoint sensor and alternative DNA clamp Rad9-Rad1-Hus1 modulates the activity of DNA ligase I, a component of the long-patch base excision repair machinery. Biochem J. 2005;389:13–17. doi: 10.1042/BJ20050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandita RK, Sharma GG, Laszlo A, et al. Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol Cell Biol. 2006;26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt PD, Helt CE, Keng PC, Bambara RA. The Rad9 protein enhances survival and promotes DNA repair following exposure to ionizing radiation. Biochem Biophys Res Commun. 2006;347:232–237. doi: 10.1016/j.bbrc.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 40.Guan X, Bai H, Shi G, et al. The human checkpoint sensor Rad9-Rad1-Hus1 interacts with and stimulates NEIL1 glycosylase. Nucleic Acids Res. 2007;35:2463–2472. doi: 10.1093/nar/gkm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Lindsey-Boltz LA, Sancar A, Bambara RA. Mechanism of stimulation of human DNA ligase I by the Rad9-rad1-Hus1 checkpoint complex. The Journal of biological chemistry. 2006;281:20865–20872. doi: 10.1074/jbc.M602289200. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Brandt P, Rossi ML, et al. The human Rad9-Rad1-Hus1 checkpoint complex stimulates flap endonuclease 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16762–16767. doi: 10.1073/pnas.0407686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi G, Chang DY, Cheng CC, Guan X, Venclovas C, Lu AL. Physical and functional interactions between MutY glycosylase homologue (MYH) and checkpoint proteins Rad9-Rad1-Hus1. The Biochemical journal. 2006;400:53–62. doi: 10.1042/BJ20060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MJ, Lin YT, Lieberman HB, Chen G, Lee EY. ATM-dependent phosphorylation of human Rad9 is required for ionizing radiation-induced checkpoint activation. J Biol Chem. 2001;276:16580–16586. doi: 10.1074/jbc.M008871200. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M, Hirano A, Kumano T, et al. Critical role for chicken Rad17 and Rad9 in the cellular response to DNA damage and stalled DNA replication. Genes Cells. 2004;9:291–303. doi: 10.1111/j.1356-9597.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 46.Dang T, Bao S, Wang XF. Human Rad9 is required for the activation of S-phase checkpoint and the maintenance of chromosomal stability. Genes Cells. 2005;10:287–295. doi: 10.1111/j.1365-2443.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida K, Komatsu K, Wang HG, Kufe D. c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol Cell Biol. 2002;22:3292–3300. doi: 10.1128/MCB.22.10.3292-3300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida K, Wang HG, Miki Y, Kufe D. Protein kinase Cdelta is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. Embo J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu K, Miyashita T, Hang H, et al. Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nat Cell Biol. 2000;2:1–6. doi: 10.1038/71316. [DOI] [PubMed] [Google Scholar]
- 50.Yin Y, Zhu A, Jin YJ, et al. Human RAD9 checkpoint control/proapoptotic protein can activate transcription of p21. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8864–8869. doi: 10.1073/pnas.0403130101. [DOI] [PMC free article] [PubMed] [Google Scholar]