Abstract
Background
Alcohol affects many of the brain regions and neural processes that support learning and memory, and these effects are thought to underlie, at least in part, the development of addiction. Although much work has been done regarding the effects of alcohol intoxication on learning and memory, little is known about the effects of acute withdrawal from a single alcohol exposure.
Methods
We assess the effects of acute ethanol withdrawal (6 h post-injection with 4 g/kg ethanol) on two forms of fear conditioning (delay and trace fear conditioning) in C57BL/6J and DBA/2J mice. The influence of a number of experimental parameters (pre- and post-training withdrawal exposure; foreground/background processing; training strength; non-associative effects) is also investigated.
Results
Acute ethanol withdrawal during training had a bidirectional effect on fear conditioned responses, decreasing contextual responses and increasing cued responses. These effects were apparent for both trace and delay conditioning in DBA/2J mice and for trace conditioning in C57BL/6J mice; however, C57BL/6J mice were selectively resistant to the effects of acute withdrawal on delay cued responses.
Conclusions
Our results show that acute withdrawal from a single, initial ethanol exposure is sufficient to alter long-term learning in mice. In addition, the differences between the strains and conditioning paradigms used suggest that specific learning processes can be differentially affected by acute withdrawal in a manner that is distinct from the reported effects of both alcohol intoxication and withdrawal following chronic alcohol exposure. Thus, our results suggest a unique effect of acute alcohol withdrawal on learning and memory processes.
Keywords: Acute Ethanol Withdrawal, Fear Conditioning, Strain Comparison, Hippocampus
Introduction
Drugs of abuse, including alcohol, affect the brain regions and neural processes that support learning and memory (Hyman, 2005; Gould, 2010). By altering the activity of a number of ion channels and receptors, alcohol modulates synaptic function and plasticity (Zorumski et al., 2014). Additionally, the function of brain regions that play key roles in learning are significantly altered by ethanol exposure and withdrawal (White and Best, 2000; Chen et al., 2009; Holmes et al., 2012; DePoy et al., 2013). The effects of alcohol on long-term learning and memory are thought to underlie, at least in part, the development and maintenance of addiction. As a result, there is increased interest in how a first ethanol experience alters learning and how these changes contribute to alcohol addiction.
Investigations into how initial ethanol exposure affects learning have focused primarily on the role of intoxication. Ethanol intoxication disrupts learning in a number of tasks in both human and rodent models (Knowles and Duka, 2004; Ray et al., 2012; Sanday et al., 2013); however, little is known about the period of withdrawal following initial ethanol intoxication. Ethanol withdrawal can be observed following a single ethanol exposure (known as acute ethanol withdrawal; Buck et al., 1997), and is characterized by aversive physical and mental effects (Wiese et al., 2000; Karadayian et al., 2013). Although acute withdrawal has clear behavioral effects in rodent models, these changes are thought to be short-lived, lasting less than 24 h post-ethanol exposure in mice (Chen et al., 2011; Karadayian and Cutrera, 2013; Karadayian et al., 2013). It is unclear if a single round of acute withdrawal can alter long-term behavior and how those effects might contribute to addiction development.
Pavlovian fear conditioning, in which a neutral stimulus (conditioned stimulus; CS) is paired with an aversive stimulus (unconditioned stimulus; US) (Blanchard and Blanchard 1969; Fanselow, 1980), has been used to investigate the effects of alcohol on learning and memory (reviewed in Tipps et al., 2014a). Although much work has been done regarding the effects of alcohol intoxication on fear conditioning (e.g., Gould, 2003; Gulick and Gould, 2007; Lattal, 2007), the potential impact of acute withdrawal on this model is unclear.
Here, we examine the effects of acute ethanol withdrawal on fear conditioning in C57B6/J (B6) and DBA/2J (D2) mice. These strains differ in a variety of alcohol-related behaviors (Rhodes et al., 2007; Melon and Boehm, 2011), including the severity of acute withdrawal (Buck et al., 1997). These strains also differ in their performance on several learning tasks, including fear conditioning (Tipps et al., 2014b; Lattal and Maughan, 2012). The present studies use these strains to examine the potential effects of acute alcohol withdrawal in two fear conditioning procedures: delay fear conditioning and trace fear conditioning. Delay and trace conditioning are thought to rely on distinct neural pathways (Raybuck and Lattal, 2011), allowing us to assess effects across multiple learning processes. By applying an acute withdrawal resistant (B6) and sensitive (D2) strain, we can investigate both how acute withdrawal affects learning and how generalizable these effects are across different learning paradigms and genetic backgrounds.
Material and Methods
Animals
Male B6 and D2 mice (The Jackson Laboratory, Bar Harbor, ME) between 8 and 12 weeks of age were used. All mice were housed 4 per cage under standard housing conditions (12h:12h light:dark cycle) with access to food and water ad libitum. Mice were allowed to acclimate to the colony room for at least one week prior to any experimentation. Each experiment used a separate cohort of mice (n = 8-12 mice per group). All studies were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University and conducted in accordance with the guidelines provided by the National Institutes of Health (NIH Publications No. 8023).
Ethanol
Mice in the acute withdrawal groups were administered a single dose of 4 g/kg ethanol (i.p., 20% v/v ethanol solution in saline). The control groups received an equivalent volume of saline. In separate experiments, treatments were administered 6 h prior to training, 48 h prior to training, or 24 h after training.
Fear Conditioning Apparatus
Training and contextual testing were conducted in 14.5 cm circular Plexiglas chambers mounted on a rod floor. Each chamber was housed in an individual sound-attenuating box (Med-Associates, St. Albans, VT). An 85 dB white noise CS was administered through a sound generator (Coulbourn Instruments, Whitehall, PA), and a 0.35 mA footshock US was administered through the rod floor via a shock scrambler/generator (Coulbourn). The apparatus was cleaned with 1% acetic acid prior to conditioning and testing. The training and context testing sessions were controlled by an IBM-PC running Graphic State software (Coulbourn). Testing for cued fear conditioning was conducted in rectangular conditioning chambers with Plexiglas floors (Med-Associates). Each chamber was housed in an individual sound-attenuating box and located in a novel procedural room. The CS was generated with an ANL-926 programmable audio generator (Med-Associates) and administered through speakers mounted on the left wall of the chambers. The CS presentations were controlled by an IBM-PC running MED-PC IV (Med-Associates). The cue testing chambers were cleaned with 70% ethanol.
Fear Conditioning Procedure
Training
Each training session began with the activation of a house light. After 2 min, a 30-sec CS was activated. For delay training, the 30-sec CS co-terminated with a 2-sec footshock (US). Under the trace paradigm, the 30-sec CS was followed by a 30-sec trace interval and then the 2-sec US. All animals received two CS-US pairings separated by a 90-sec inter-trial interval in a 6.5-min session. For the context only condition, the CS was not presented, and for the No-US condition, the US was not presented, but the overall timing and session length were unchanged.
Testing
To evaluate contextual learning, mice were returned to the training chambers 24 h after training. The houselight was activated, and behavior was assessed for 12 min. To test cued learning, mice were placed in the novel cue testing apparatus 48 h after training. After a 3-min pre-CS interval, the animals were exposed to two 3-min CS presentations separated by a 3-min inter-CS interval and followed by a 3-min post-CS period, for a total test time of 15 min.
Scoring
The context and CS tests were handscored by an experimenter who was blind to the treatment condition of each subject. Animals were scored at 10-sec intervals for freezing, which was defined as the absence of all movement except respiration (Blanchard and Blanchard, 1969). The data are presented as the percentage of observations for each behavior (six observations per minute). For contextual learning we report the average total freezing for the full 12-min context test. Data for cued learning includes the average freezing during the non-CS periods (pre-CS, inter-CS, and post-CS) and during the two CS presentations.
Footshock Threshold
Animals were tested for footshock responses in the cue testing chambers with the Plexiglass floors removed. Each animal was placed into the chamber and allowed to acclimate for 2 min. Increasing intensities of 2-sec footshocks were administered with 30-sec intervals between each shock. The shock intensity began at 0.05 mA and was increased by 0.05 mA for each subsequent shock. The session ended when the mice responded to three shocks in a row, and the shock level at which the initial response was detected was recorded as the threshold for each animal. Responses included both locomotor changes (running or jumping) and vocalization.
Data Analysis
The data were analyzed using Excel (Microsoft Software) and Systat 13 (Cranes Software International). All graphs were generated using Prism (GraphPad Software Inc.). The data are reported as the mean ± standard error of the mean (SEM). All comparisons were made using univariate ANOVAs and Student's t-test to compare groups. A p-value less than 0.05 was considered significant.
Results
Effects of Acute Withdrawal on Delay and Trace Fear Conditioning
Six hours prior to training, each animal received a dose of either 4 g/kg ethanol or an equivalent volume of saline and was returned to their homecage. At this time point (6 h post-administration), D2 mice exhibit robust acute ethanol withdrawal as assessed by handling-induced convulsions (HIC), a common measure of withdrawal severity (Kozell et al., 2008). Although B6 mice show more modest acute withdrawal for this measure (Metten and Crabbe, 1994), making the timecourse for this strain less clear (Kozell et al., 2008), withdrawal is evident at this time point in B6 and D2 mice with extended ethanol exposure (Crabbe et al., 1998). For the experiments presented here, mice were trained during the acute withdrawal period (6 h post-administration), but the context and CS testing occurred post-withdrawal.
In the saline-treated groups, we observed significant differences in fear conditioning between the strains. For both delay and trace conditioning, B6 mice had greater responses to the context [delay (F1,14 = 25.5, p<0.001); trace (F1,14 = 19.9, p=0.001)] and the CS [delay (F1,14 = 5.3, p=0.038); trace (F1,14 = 5.6, p=0.033)] than D2 mice. These strain differences were expected and are consistent with previously reported fear conditioning differences in B6 and D2 mice (Tipps et al., 2014b). Due to the strain differences in our control groups, we analyzed the effects of acute withdrawal within each strain separately.
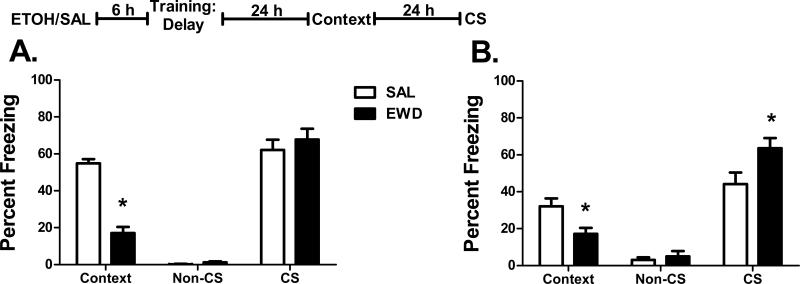
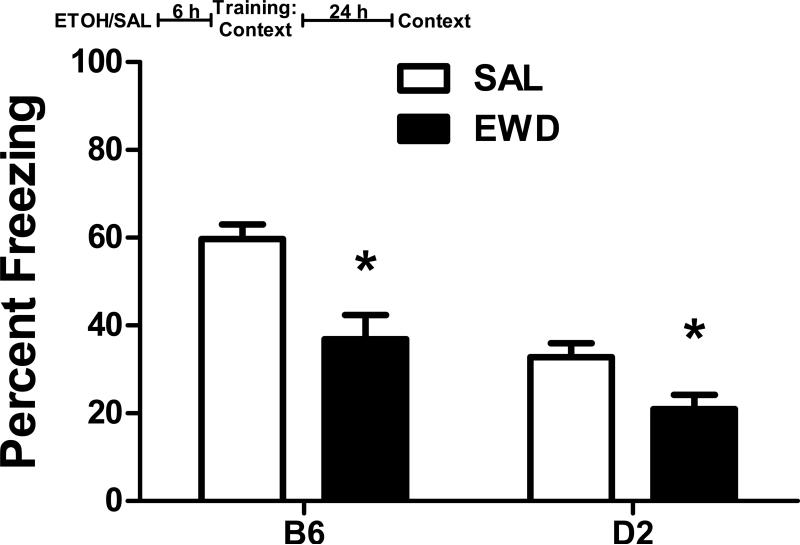
In delay fear conditioned mice, we found a significant effect of treatment on freezing to the context in both B6 (F1,18 = 81.3, p<0.001) and D2 mice (F1,18 = 7.9, p=0.012). Acute withdrawal during training significantly reduced contextual freezing in both strains (Figure 1 A and B). There was no effect of treatment on non-CS freezing during the CS test; however, there was a significant effect on CS freezing in D2 (F1,18 = 6.1, p=0.024), but not B6 mice (p=0.49). Interestingly, while context freezing was significantly reduced by acute withdrawal, the CS responses in the D2 mice were significantly enhanced in the withdrawal group compared to saline-treated mice.
Figure 1. Delay Fear Conditioning.
Percent freezing (mean ± SEM) during the context test and the non-CS and CS periods of the CS test for (A) B6 and (B) D2 mice trained with delay fear conditioning 6 h after the administration of ethanol (EWD) or saline (SAL). Insert: The experimental timeline for drug administration (ETOH – ethanol; SAL – saline) and fear conditioning. n=12 for EWD groups; n=8 for SAL groups; *p<0.05 vs. SAL.
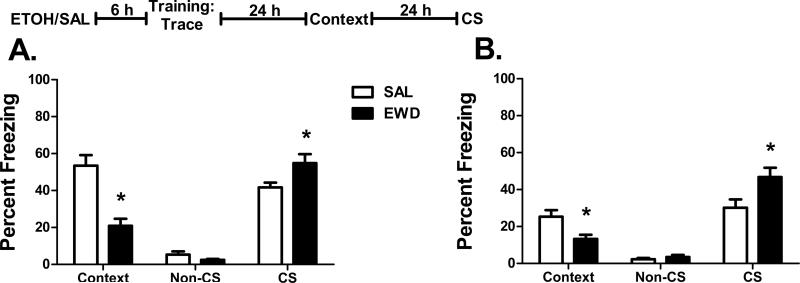
Similar to the results for delay conditioning, there was a significant effect of treatment on freezing to the context in trace fear conditioned mice. Again, both B6 (F1,18 = 27.6, p<0.001) and D2 mice (F1,18 = 10.3, p=0.005) in the acute withdrawal group showed a significant reduction in context freezing (Figure 2 A and B). There was also a significant effect of treatment on freezing to the CS for both B6 (F1,18 = 4.8, p=0.042) and D2 mice (F1,18 = 5.7, p=0.028) during the CS test. In contrast to our delay effects, both strains showed a significant increase in freezing to the CS following training under acute withdrawal in the trace paradigm.
Figure 2. Trace Fear Conditioning.
Percent freezing (mean ± SEM) during the context test and the non-CS and CS periods of the CS test for (A) B6 and (B) D2 mice trained with trace fear conditioning 6 h after the administration of ethanol (EWD) or saline (SAL). Insert: The experimental timeline for drug administration (ETOH – ethanol; SAL – saline) and fear conditioning. n=12 for EWD groups; n=8 for SAL groups;*p<0.05 vs. SAL.
Pre-training and Post-training Withdrawal Exposure
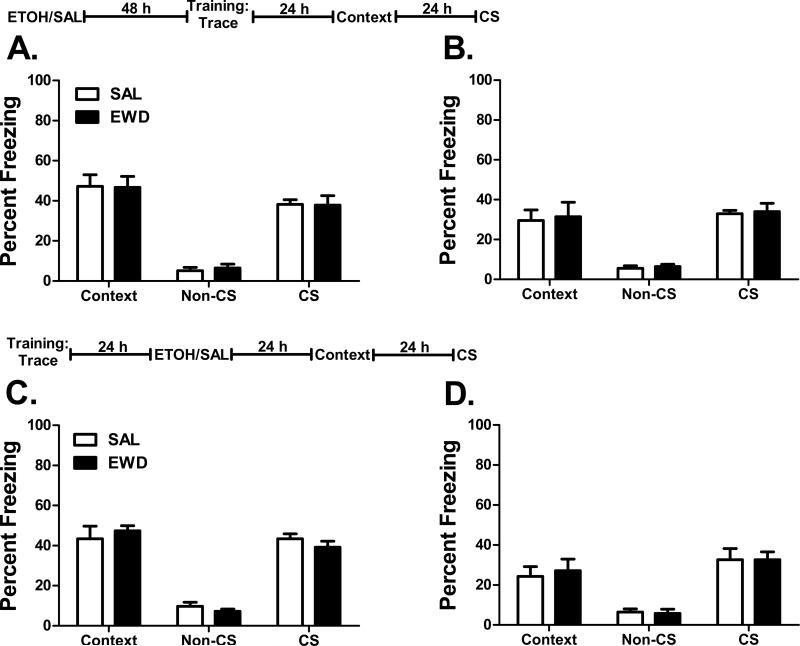
Our results suggest that acute ethanol withdrawal during training alters long-term learning in both B6 and D2 mice. However, it is also possible that exposure to a high dose of ethanol is sufficient to alter subsequent learning, regardless of the training/withdrawal timing. Alternatively, acute withdrawal could have long-term effects on recall or performance in this task rather than on learning itself. To address these possibilities, we dissociated the withdrawal period from the training period in two groups of trace fear conditioned mice. The first group received ethanol or saline 48 h prior to training to test the possibility that exposure to a high dose of ethanol results in long-term deficits in learning, even when training takes place after the withdrawal period. The second group received ethanol or saline 24 h after training and was tested 24 h later to assess any effects of ethanol withdrawal on recall and performance that are independent of withdrawal exposure during training.
Ethanol exposure and withdrawal prior to training had no effect on freezing behavior during either the context or CS test in either strain (Figure 3A and B) (p>0.8 for all measures). Similarly, ethanol administration 24 h after training did not alter freezing responses during either the context or CS test (Figure 3C and D) (p>0.25 for all measures). These data suggest that prior exposure to 4 g/kg ethanol is not sufficient to alter fear conditioning, and that acute alcohol withdrawal does not affect the recall or performance of previously learned conditioned fear behaviors.
Figure 3. Pre- and post-training acute withdrawal exposure.
Percent freezing (mean ± SEM) during the context test and the non-CS and CS periods of the CS test following acute withdrawal 48 h prior to training (A-B) or 24 h after training (C-D). Experimental timelines are presented above each data set (ETOH – ethanol; SAL – saline). (A) B6 and (B) D2 mice trained with trace fear conditioning 48 h after ethanol (EWD) or saline (SAL) administration. (C) B6 and (D) D2 mice trained with trace fear conditioning and then administered either ethanol or saline 24 h later. n=8 for all groups.
Weak Delay Training in B6 Mice
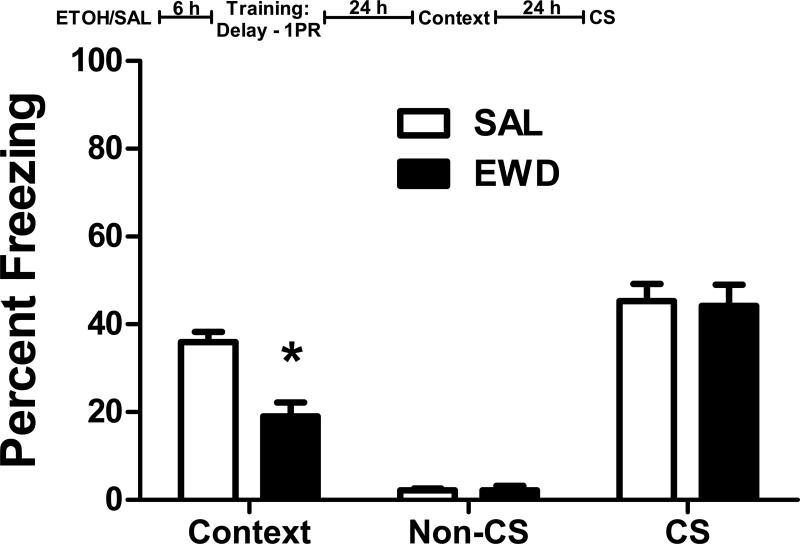
Although the trace fear conditioned B6 mice show effects of acute withdrawal on both contextual and cued learning (Figure 2), only the context responses were altered in delay-trained B6 mice (Figure 1). One potential explanation for this difference is that B6 mice are already showing maximal freezing to the CS in the delay condition. To test this, we trained a group of B6 mice with delay fear conditioning using only a single CS-US pairing. This weak training approach reduces overall freezing levels in B6 mice and has been used to demonstrate CS response enhancement by intoxication with low doses of ethanol that were not apparent under a stronger conditioning paradigm (Gulick and Gould, 2007).
After delay conditioning with a single CS-US pairing, there was still a significant effect of acute withdrawal on context freezing (F1,18 = 20.5, p<0.001; Figure 4), with the withdrawal group showing less freezing to the context than the saline group. However, there was no effect of treatment on non-CS or CS freezing during the CS test (p>0.85 for all measures). This experiment replicates the deficit in context freezing observed with our two pairing delay training (Figure 1) and suggests that the lack of a withdrawal effect on CS responses in delay-trained B6 mice is not due to a ceiling effect on their freezing level.
Figure 4. Weak Delay Fear Conditioning.
Percent freezing (mean ± SEM) during the context test and the non-CS and CS periods of the CS test in B6 mice trained with only a single CS-US delay pairing 6 h after the administration of ethanol (EWD) or saline (SAL). Insert: The experimental timeline for drug administration (ETOH – ethanol; SAL – saline) and fear conditioning (1PR – single CS-US pairing). n=8 for all groups; *p<0.05 vs. SAL.
Foreground Context Training
Our data also show bidirectional effects on contextual and cued learning. Although alcohol intoxication at some doses can differentially affect contextual and cued learning, this is typically due to a lack of effect in one measure, rather than contrasting effects in both (Gould and Lommock, 2003; Gulick and Gould, 2007). One potential explanation for the diverging context and cue effects observed here is a foreground/background effect on contextual processing. When a CS is paired with a shock, it becomes the primary predictor of the shock, while the context becomes a secondary (background) cue (Gulick and Gould, 2007), and these processes could be differentially affected by acute withdrawal. To investigate this, we trained mice in the absence of a CS, making the context the primary predictor of the shock.
Similar to the delay and trace paradigms, we found a significant effect of treatment on contextual freezing, with acute withdrawal decreasing context freezing in both B6 (F1,18=11.5, p=0.003) and D2 mice (F1,18=6.8, p=0.018) (Figure 5). The effect of acute alcohol withdrawal on contextual learning is unaffected by whether the context is processed as the primary predictor of the US or as a background cue, suggesting that the bidirectional effects of withdrawal on context and cue learning are not due to the difference in their ability to predict the US.
Figure 5. Foreground Context Training.
Percent freezing (mean ± SEM) during the context test in B6 and D2 mice trained in the absence of a CS 6 h after the administration of ethanol (EWD) or saline (SAL). Insert: The experimental timeline for drug administration (ETOH – ethanol; SAL – saline) and fear conditioning. n=8 for all groups; *p<0.05 vs. SAL.
No-US Exposure
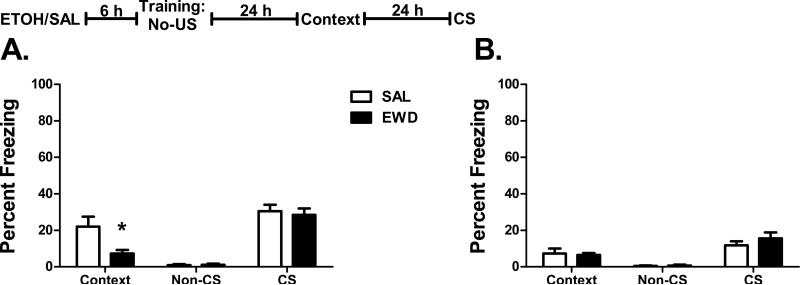
A second possible explanation for the bidirectional effects on context and cue responding is that acute withdrawal itself may serve as a US, which could make the CS more aversive, resulting in an increased response specifically to the CS, rather than enhancing the association between the CS and the footshock. To investigate this possibility, we exposed mice to the white noise CS in the absence of a footshock.
Consistent with previous reports (Tipps et al., 2014b), saline-treated B6 mice demonstrated a freezing response to the context even when trained in the absence of a US (Figure 6A). These non-associative B6 contextual responses were significantly decreased by acute withdrawal (F1,14 = 7.5, p=0.016) (Figure 6A). D2 mice showed no change in context freezing (Figure 6B); however, given the low freezing in the saline group, this is unsurprising. In the CS test, both B6 and D2 mice showed moderate levels of freezing behavior to the CS in the absence of the US. Neither strain showed any effect of acute withdrawal on these CS responses (B6 p=0.66; D2 p=0.31), suggesting that the CS enhancement observed in the trace- and delay-trained mice is not simply due to an enhancement of CS responding alone.
Figure 6. No-US Training.
Percent freezing (mean ± SEM) during the context test and the non-CS and CS periods of the CS test for (A) B6 and (B) D2 mice trained in the absence of a US 6 h after the administration of ethanol (EWD) or saline (SAL). Insert: The experimental timeline for drug administration (ETOH – ethanol; SAL – saline) and fear conditioning. n=8 for all groups; *p<0.05 vs. SAL.
Footshock Threshold
Finally, we assessed whether acute withdrawal alters responses to the US. Alcohol withdrawal has been shown to alter pain responses (Gatch, 2009, Karadayian et al., 2013), suggesting that a potential change in footshock sensitivity could account for some of the behavioral differences observed. There were no differences between the strains in the saline control mice in shock response threshold (B6 0.125 ± 0.012 mA; D2 0.141±0.009; p=0.25). When comparing the saline and ethanol-treated groups, we found a significant effect of strain (F1,20 = 40.9, p<0.001), treatment (F1,20 = 72.72, p<0.001), and a strain x treatment interaction (F1,20 = 4.544, p=0.045). For both strains, acute withdrawal significantly increased the level of footshock at which motor responses were observed (B6 0.15 ± 0.001, p=0.049; D2 0.183 ± 0.011 mA, p=0.011). This could suggest that acute withdrawal reduces sensitivity to the US; however, acute withdrawal has also been shown to decrease locomotion (Karadayian and Cutrera, 2013), suggesting that movement-based measures may be inaccurate. Typically, these strains respond to increasing footshocks with locomotor changes (startle, running, jumping) followed by vocalization at higher levels (Liu et al., 2003; Matthews et al., 2008), and the threshold is defined as the first observable locomotor response; however, when the threshold data were re-analyzed to assess the point at which any response (locomotor or vocalization) was observed, the treatment differences in both strains were non-significant (p>0.07 for all measures), suggesting that the footshock threshold is not altered by acute withdrawal, although the pattern of responding is.
Conclusions
To the best of our knowledge, these data are the first to demonstrate that acute alcohol withdrawal following a single injection of ethanol is sufficient to alter performance on a fear conditioning task. In addition to our primary finding, two aspects of the data are of particular interest. First, we observe a bidirectional effect of acute withdrawal in which context learning is impaired while CS learning is enhanced, suggesting that acute ethanol withdrawal does not generally impair all learning. Second, while some of the findings were consistent across the B6 and D2 strains, B6 mice were selectively resistant to the effects of acute withdrawal on CS responding in delay, but not trace, fear conditioning. Together with studies assessing other measures of acute withdrawal (Buck et al., 1997; Kozell et al., 2008), our results support the conclusion that D2 mice may be generally sensitive to acute ethanol withdrawal, while B6 mice are sensitive to some, but resistant to other, acute withdrawal effects. These findings raise the possibility that individual learning processes are differentially affected by acute ethanol withdrawal in a strain dependent manner and point to specific brain regions that may be contributing to these differences.
Hippocampal Processes are Sensitive to Disruption by Acute Ethanol Withdrawal
The present studies suggest that processes involving the hippocampus may be more sensitive to acute withdrawal than processes that do not. Across different conditioning procedures and strains, the impairment of contextual learning was robust and consistent. This is particularly interesting given the documented strain differences in both acute withdrawal severity (Buck et al., 1997) and contextual learning (Andre et al., 2012; Tipps et al., 2014b), and suggests that acute withdrawal robustly impairs the processes underlying contextual learning, regardless of differences in other measures of withdrawal severity or the baseline levels of contextual learning.
Contextual processing is thought to be primarily driven by the hippocampus (Lovett-Barron et al., 2014), which is severely disrupted by ethanol exposure (White and Best, 2000; Weitemier and Ryabinin, 2003). A number of cellular processes in this region, including neuronal proliferation (Nixon and Crews, 2002) and the formation of long-term potentiation (Matthews and Silvers, 2004), are inhibited by ethanol, and these changes in hippocampal function are associated with impaired memory (Matthews et al., 1999; Givens et al., 2000; Alijan-pour et al., 2012). The impairment in contextual learning observed here points to the possibility that acute withdrawal also impairs hippocampal function.
The strain differences observed in CS responding also suggest differential withdrawal sensitivity in hippocampal and non-hippocampal tasks. While D2 mice showed a consistent withdrawal-induced increase in CS responses, this effect was only apparent in B6 mice following trace fear conditioning. The absence of a CS response increase in B6 mice was likely not due to a ceiling effect given that no enhancement was observed following training with either a single conditioning trial or under non-associative conditions. Interestingly, repeated intermittent ethanol exposure in rats also selectively impairs trace CS responses (Yttri et al., 2004), and trace CS responses are more sensitive to impairment by acute intoxication than delay CS responses (Weitemer and Ryabinin, 2003), illustrating that ethanol can differentially affect these two learning types.
Delay and trace fear conditioning rely on distinct neural substrates for CS processing (reviewed in Raybuck and Lattal, 2014). Trace fear conditioning requires the hippocampus and prefrontal areas to maintain the trace memory necessary to form the CS-US association (Raybuck and Lattal, 2011; Gilmartin et al., 2013), whereas delay fear conditioning relies primarily on the amygdala for these associations (Raybuck and Lattal, 2011). It is possible that acute withdrawal impairs hippocampal processing to a greater extent than non-hippocampal processing. As a result, trace fear conditioning and contextual learning would be more sensitive to acute ethanol withdrawal than delay CS learning. This could also explain why the withdrawal resistant B6 mice show selective withdrawal effects for processes involving the hippocampus (i.e., context learning, trace CS learning) but show no effect on hippocampus-independent behaviors (delay CS). However, it should be noted that the bidirectionality of the effects observed here argue against a simple hippocampal impairment hypothesis, which would predict that both contextual learning and trace fear conditioning should be impaired by acute withdrawal. Rather, our data suggest that the specific pathways underlying contextual and cued fear conditioning are differentially affected by acute withdrawal and that the involvement of the hippocampus alters the sensitivity of these processes, but does not dictate the direction of the effects.
Acute Withdrawal Effects are Distinct from Those Reported for Intoxication or Chronic Withdrawal
The bidirectional effects observed in our study represent an interesting and unique modulation of these learning processes by acute withdrawal. Acute intoxication studies have shown that contextual and cued memory can be differentially affected by alcohol (Gould, 2003); however, for studies in which context and cue responses differ, there was an absence of effect for one measure, rather than the bidirectional effects on both observed in our study. Other intoxication studies have found that high doses of ethanol can alter both contextual and cued learning (Gulick and Gould, 2007), but, unlike the present data, the observed changes were in the same direction. Thus, although our data are in agreement with previous work suggesting that context and cue processes can be differentially affected by ethanol, the acute withdrawal effect observed here seems to be distinct from the effects of acute intoxication.
Our results also differ from those reported in studies examining the effects of withdrawal following chronic ethanol. At short withdrawal durations (3 days after ethanol cessation), an increase in contextual fear conditioning is observed (Bertotto et al., 2006; 2010; 2011), while a study using a longer withdrawal period (2 weeks after ethanol cessation) reported no effect on contextual learning (Borlikova et al., 2006). For studies in which chronic withdrawal effects on contextual fear conditioning are observed, the results are opposite those reported here for acute withdrawal. Similarly, studies examining the effects of withdrawal from chronic ethanol exposure on CS learning in a response inhibition task have reported that the ability to learn the CS association is impaired in withdrawn rats (Stephens et al., 2001; Ripley et al., 2003), whereas our acute data show an enhancement in CS learning. It should be noted that the majority of reports on chronic alcohol withdrawal use rats rather than mice, and CS learning is typically assessed in a different fear conditioning paradigm than the one used in the current study. To fully address the relationship between acute and chronic withdrawal on learning processes, an assessment of the effects of withdrawal from chronic ethanol exposure in mice using delay and trace fear conditioning is needed; however, based on the currently available literature, the effects of acute withdrawal on fear conditioning appear to differ from those of withdrawal following chronic ethanol exposure.
Future Directions
Our data highlight a number of interesting questions for future work to investigate. We focused on a single time point within the acute withdrawal period (6 h post-administration), but the various withdrawal symptoms can differ in their timecourses (Metten and Crabbe, 1994; Karadayian et al., 2013; Karadayian and Cutrera, 2013). While we found that ethanol exposure 48 h prior to training has no effect on subsequent fear conditioning, this leaves a large window in which to explore the timing component of withdrawal effects on learning and compare the relationship of learning effects with the various withdrawal symptoms observed. Our work was also used a single dose of ethanol. Typically, high doses of ethanol (3.5 – 4 g/kg) are used to see robust acute withdrawal effects; however, the learning changes observed here may be more sensitive than other acute withdrawal measures. The dose dependence of our observed effects would also be an interesting avenue for future work. Finally, the duration of our withdrawal effects were tested 24 and 48 h after training, but it is possible that these effects could last much longer. The effects of intoxication have been shown to last at least 1 week following training (Gulick and Gould, 2007), suggesting that the effects of acute withdrawal could be likewise long-lasting.
Conclusions
In conclusion, our data show that acute ethanol withdrawal impairs contextual learning while increasing CS learning and that a subset of these effects are sensitive to individual differences in acute withdrawal sensitivity. This work illustrates the ability of acute withdrawal to differentially alter individual learning processes and highlights specific brain regions and pathways that could potentially contribute to withdrawal in B6 and D2 mice. Finally, our data suggest that the acute withdrawal period may contribute to the effects of ethanol on learning and memory in a way that is distinct from both acute intoxication and withdrawal from chronic ethanol exposure.
Acknowledgements
This work was supported by NIH 1F32 AA022011 and T32 AA07468 (MET); NIH F32DA031537 and T32 DA07262 (JDR); NIH R01 AA011114, P60 AA10760, R24 AA020245, R01 DA005228, and the VA (KJB); NIH DA02592 and US Department of the Army/DODTATRC:W81XWH-12-2-0048 (KML).
References
- Alijan-pour J, Abrari K, Bluki TL, Ghorbanian MT, Goudarzi I, Salmani ME, Mirshekar M. Acute ethanol administration affects memory reactivation: a look at the neuronal density and apoptosis in the rat hippocampus. Pharmacol Biochem Behav. 2012;102:321–328. doi: 10.1016/j.pbb.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Andre JM, Cordero KA, Gould TJ. Comparison of the performance of DBA/2 and C57BL/6 mice in transitive inference and foreground and background contextual fear conditioning. Behav Neurosci. 2012;126:249–257. doi: 10.1037/a0027048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotto ME, Bustos SG, Molina VA, Martijena ID. Influence of ethanol withdrawal on fear memory: Effect of D-cycloserine. Neuroscience. 2006;142:979–990. doi: 10.1016/j.neuroscience.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Bussolino DF, Molina VA, Martijena ID. Increased voluntary ethanol consumption and c-Fos expression in selected brain areas induced by fear memory retrieval in ethanol withdrawn rats. Eur Neuropsychopharmacol. 2010;20:568–581. doi: 10.1016/j.euroneuro.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Maldonado NM, Bignante EA, Gorosito SV, Cambiasso MJ, Molina VA, Martijena ID. ERK activation in the amygdala and hippocampus induced by fear conditioning in ethanol withdrawn rats: modulation by MK-801. Eur Neuropsychopharmacol. 2011;21:892–904. doi: 10.1016/j.euroneuro.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Elbers NA, Stephens DN. Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: evidence for effect on frontal cortical but not hippocampal function? Eur J Neurosci. 2006;24:205–216. doi: 10.1111/j.1460-9568.2006.04901.x. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43:411–420. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kozell LB, Buck KJ. Substantia nigra pars reticulata is crucially involved in barbiturate and ethanol withdrawal in mice. Behav Brain Res. 2011;218:152–157. doi: 10.1016/j.bbr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of a quantitiative loci for chronic withdrawal severity in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1998;286:263–271. [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Gatch MB. Ethanol withdrawal and hyperalgesia. Curr Drug Abuse Rev. 2009;2:41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B, Williams JM, Gill TM. Septohippocampal pathway as a site for the memory-impairing effects of ethanol. Hippocampus. 2000;10:111–121. doi: 10.1002/(SICI)1098-1063(2000)10:1<111::AID-HIPO12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethnaol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Karadayian AG, Busso MJ, Feleder C, Cutrera RA. Alterations in affective behavior during the time course of alcohol hangover. Behav Brain Res. 2013;253:128–138. doi: 10.1016/j.bbr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Karadayian AG, Cutrera RA. Alcohol hangover: type and time-extension of motor function impairments. Behav Brain Res. 2013;247:165–173. doi: 10.1016/j.bbr.2013.03.037. [DOI] [PubMed] [Google Scholar]
- Knowles SK, Duka T. Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol. 2004;15:111–121. doi: 10.1097/00008877-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Kozell LB, Belknap JK, Hofstetter JR, Mayeda A, Buck KJ. Mapping a locus for alcohol physical dependence and associated withdrawal to a 1.1 Mb interval of mouse chromasome 1 syntenic with human chromasome 1q23.2-23.3. Genes Brain Behav. 2008;7:560–567. doi: 10.1111/j.1601-183X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- Lattal KM. Effects of ethanol on the encoding, consolidation, and expression of extinction following contextual fear conditioning. Behav Neurosci. 2007;121:1280–1292. doi: 10.1037/0735-7044.121.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Maughan DK. A parametric analysis of factors affecting acquisition and extinction of contextual fear in C57BL/6 and DBA/2 mice. Behav Processes. 2012;90:49–57. doi: 10.1016/j.beproc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Singh RP, Khan AH, Bhavsar K, Lusis AJ, Davis RC, Smith DJ. Identifying loci for behavioral traits using genome-tagged mice. J Neurosci Res. 2003;74:562–569. doi: 10.1002/jnr.10765. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Ilgen M, White AM, Best PJ. Acute ethanol administration impairs spatial performance while facilitatitng nonspatial performance in rats. Neurobiol Learn Mem. 1999;72:169–179. doi: 10.1006/nlme.1998.3900. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem. 2004;82:299–308. doi: 10.1016/j.nlm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Chesler EJ, Cook MN, Cockroft J, Philip VM, Goldowitz D. Genetic mapping of vocalization to a series of increasing acute footshocks using B6.A consomic and B6.D2 congenic mouse strains. Behav Genet. 2008;38:417–723. doi: 10.1007/s10519-008-9210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav Pharmacol. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Mun EY, Buckman JF, Udo T, Bates ME. Memory for emotional picture cues during acute alcohol intoxication. J Stud Alcohol Drugs. 2012;73:718–725. doi: 10.15288/jsad.2012.73.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Bridging the interval: Theory and neurobiology of trace conditioning. Behav Processes. 2014;101C:103–111. doi: 10.1016/j.beproc.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr., Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ripley TL, O'Shea M, Stephens DN. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur J Neurosci. 2003;18:441–448. doi: 10.1046/j.1460-9568.2003.02759.x. [DOI] [PubMed] [Google Scholar]
- Sanday L, Patti CL, Zanin KA, Fernandes-Santos L, Oliveira LC, Kameda SR, Tufik S, Frussa-Filho R. Ethanol-induced memory impairment in a discriminative avoidance task is state-dependent. Alcohol Clin Exp Res 37 Suppl. 2013;1:E30–39. doi: 10.1111/j.1530-0277.2012.01905.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci. 2001;14:2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Lattal KM. Substance abuse, memory, and post-traumatic stress disorder. Neurobiol Learn Mem. 2014a;112:87–100. doi: 10.1016/j.nlm.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, Lattal KM. Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learn Mem. 2014b;21:380–393. doi: 10.1101/lm.035261.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- White AM, Best PJ. Effects of ethanol on hippocampal place-cell and interneuron activity. Brain Res. 2000;876:154–165. doi: 10.1016/s0006-8993(00)02629-9. [DOI] [PubMed] [Google Scholar]
- Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med. 2000;132:897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: dose-dependent impairments in trace conditioning. Alcohol Clin Exp Res. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]