Abstract
A model with which to elucidate the mechanism of Ca2+ release from, and Ca2+ loading in the sarcoplasmic reticulum (SR) by Ca2+ current (ICa) in cardiac cells is proposed. The SR is assumed to be comprised of three functional subcompartments: (1) the main calcium store (MCS), which contains most of the calcium (both free and bound); (2) the releasable terminal (RT), which contains the calcium readily available for release; and (3) the longitudinal network of the SR (LSR), which sequesters and the transfers the sarcoplasmic calcium to the RT. A rapid increase of the Ca2+ concentration at the outer surface of the SR (Cae) due to the fast component of ICa activates and inactivates this surface, inducing the release of Ca2+ from the RT to the sarcoplasmic space. The RT in turn is further activated and inactivated by a increase in the concentration of sarcoplasmic Ca2+. The Ca2+ in the sarcoplasmic space is then sequestered by the LSR, leading to the reactivation of the RT. Further increase of Cae due to the slow component of ICa enhances the entry of Ca2+ into the MCS to be bound by the binding substance. The free Ca2+ released from the Ca–binding substance complex is transferred to the RT for subsequent release. The activation, inactivation and reactivation are Ca2+-mediated and time-dependent. The proposed model yields simulation of the many events qualitatively similar to those observed experimentally in skinned cardiac cells.
Introduction
A transient increase of intracellular calcium is an important step coupling membrane excitation to muscular contraction. In mammalian cardiac muscle, calcium entry through the sarcolemma during the plateau of the action potential have been considered necessary for contraction. More than a decade ago, Bassingthwaighte and Reuter (1972) postulated that the trans-sarcolemmal Ca2+ influx was insufficient for activating the myofilaments and that additional Ca2+ released from the internal stores was required.
Experiments on skinned cells (Fabiato and Fabiato, 1978; Fabiato, 1982) demonstrated that no stimulated trans-sarcolemmal influx of Ca2+ of any magnitude could activate the myofilaments directly. In isolated rat ventricular cells, the computed [total Ca2+] was 7 µM and 10 µM during the initial 10 and 20 ms respectively of the fast Ca2+ current (Fabiato, 1983). This Ca2+ influx and the resting [Ca2+] (7 µM) resulted in pCa values of 6.8 and 6.71 respectively. This meager rise in [free Ca2+] is insufficient to induce any direct activation of the myofilaments.
The role of the trans-sarcolemmal Ca2+ seems to be to induce the release of Ca2+ from the intracellular Ca store—the sarcoplasmic reticulum (SR). The Ca2+-induced release of Ca2+ had been demonstrated in skinned cardiac cells (Fabiato and Fabiato, 1975, 1977, 1978). Recent advances in single-channel conduction and gating experiments on canine (Rousseau et al., 1986) and human cardiac SR (Holmberg and Williams, 1989) strongly support the concept of Ca2+-induced Ca2+ release. These single-channel experiments did not elucidate the release mechanism or processes. However, Fabiato’s experiements have suggested that the release mechanism is not an all-or-none process but is determined by three factors:
the level of preload of the SR with Ca2+;
the rate of change of trigger [free Ca2+];
the level of [free Ca2+] used as trigger.
Fabiato (1985b) demonstrated that in skinned Purkinje cells, the mechanism of Ca2+-induced release of Ca2+ from the SR appeared to be via a channel with time- and Ca2+-dependent activation and inactivation. Based on this concept, a preliminary model (Wong et al., 1987) for Ca2+-dependent Ca2+ release from the SR was proposed. Although the model fits well all experimental data tested, it does not include the mechanism involved in Ca2+ release from the SR by the fast component and Ca2+ loading in SR by the slow component of the trans-sarcolemmal Ca2+ influx (Fabiato, 1985c). Thus, the present model is an improved version in that it not only produces results quantitatively similar to the experimental observations, but also includes some processes not considered in the previous model.
This model formulation contains some features which have not been demonstrated experimentally, but which appear to be needed to provide behavior similar to the observed behavior of cardiac single cells and fiber bundle preparations. Thus, the model should be regarded as descriptive overall, and explicative in only certain well-defined features. The main innovative feature is the desription of Ca2+-dependent Ca2+ release, which is expressed in a fashion analogous to Hodgkin–Huxley kinetics, but the channel “conductance” is time- and [Ca2+]-dependent rather than time- and voltage-dependent.
Overall Description of the Proposed Model
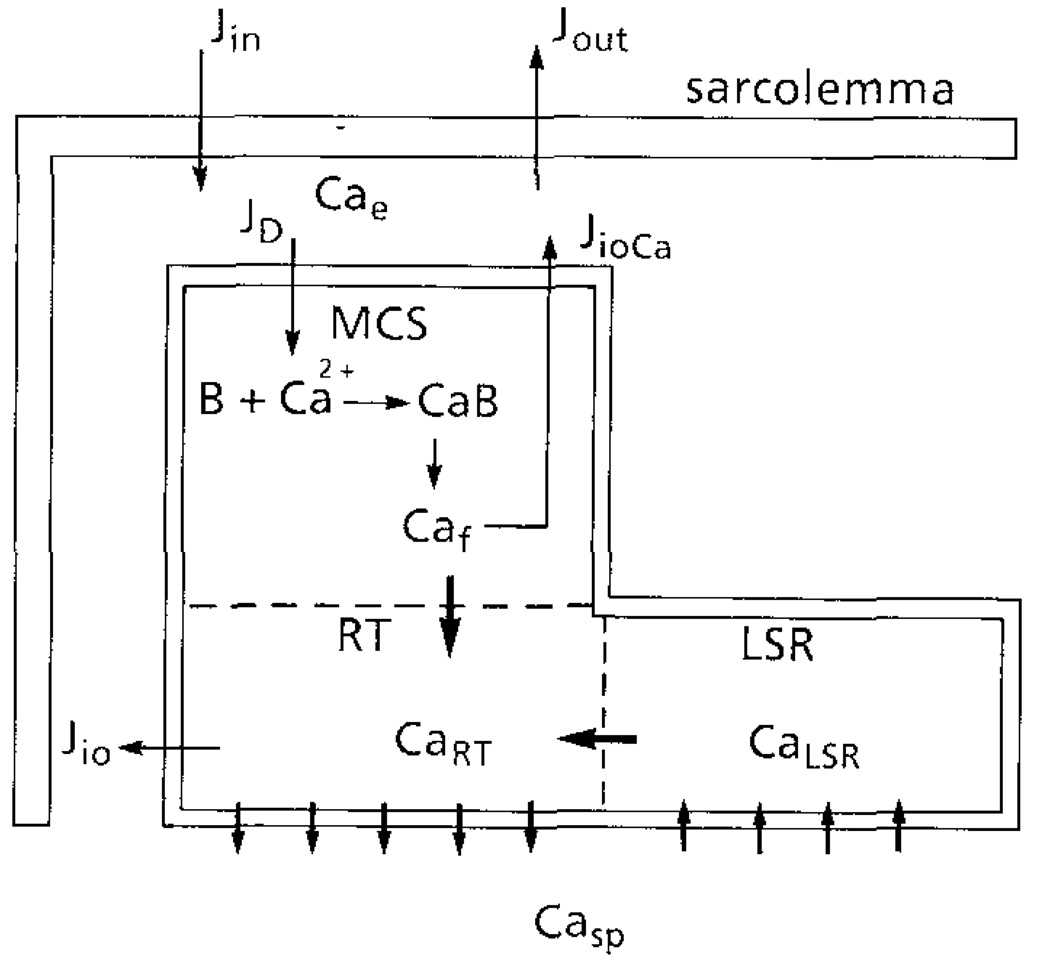
The schematic diagram shown in Fig. 1 represents a cardiac cell with intact sarcolemma. The SR comprises three subcompartments: (1) the main calcium store (MCS), which contains most of the calcium store in the SR and in which the calcium is predominantly bound; (2) the “releasable” terminal (RT), in which the calcium is mostly free and which releases a fraction of its calcium content to the myofibrils to activate the contractile machinery; and (3) the longitudinal network (LSR), which sequesters the calcium from the sarcoplasmic space and transfers it to the RT.
Figure 1.
Schematic diagram of the model. The sarcoplasmic reticulum (SR) comprises of: (1) Main Calcium Store (MCS) into which Ca2+ entering by diffusion is bound by the binding substance B; (2) a Releasable Terminal (RT) which releases Ca2+ to the sarcoplasmic space; (3) the longitudinal SR which sequesters Ca2+ from the sarcoplasmic space. Free Ca from MCS (Caf) and LSR (CaLSR) are transferred to RT with time constants τfr and τLSRJin and Jout are the trans-sarcolemmal influx and efflux respectively. JioCa and Jio are Ca–Ca exchange between Cae and CaRT. Cae is the [Ca2+] between sarcolemma and SR; Casp is the sarcoplasmic Ca2+.
To simulate experiments on skinned cardiac cells, the membrane in Fig. 1 is removed. At resting state, the calcium concentration at the outer surface of the SR (Cae) is low and this surface is completely inactivated. No calcium is released from the RT. When Cae increases rapidly, the outer surface of the SR is activated and inactivation of this surface is also initiated at a slower rate. Both the activation and inactivation are calcium (Cae)- and time-dependent, as described by Hodgkin–Huxley kinetics.
The activation of the SR outer surface triggers the release of calcium from the RT, resulting in an increase of calcium concentration in the sarcoplasmic space (Casp). As the activation and inactivation of the RT is also Casp- and time-dependent, more calcium release can be induced until inactivation becomes dominant. While Ca2+ is being released from the RT, [free Ca2+] (Caf) in the MCS is increased due to the diffusion of Ca2+ from outside the SR and the dissociation of the Ca–binding substance complex. The free Ca2+ from the MCS and the LSR is transferred to the RT for subsequent release. The Ca2+ in the sarcoplasmic space is sequestered by an active process (Jpump). The decrease of Casp results in the activation and reactivation of the RT.
Assumption and Formulation
(1) Calcium concentration at the outer surface of the SR (Cae). The calcium concentration at the outer surface of the SR is determined by:
the rate and the amount of increase or decrease of Ca2+ from the external source (Jin);
the diffusion (JD) of Ca2+ across the outer SR membrane into the MCS; and
the calcium efflux (JioCa) from the MCS.
The rate of change of Cae is
In experiments on skinned cells, the calcium source and sink can be considered very large. Therefore:
| (1) |
where Jin represents the rate of injection or aspiration of trigger Ca2+.
(2) Free calcium concentration in the MCS (Caf). Calcium-binding substance exists in the SR (Solaro and Briggs, 1974). It is assumed that the free Ca2+ that enters the MCS binds to the free binding substance B (calsequestrin) by a simple first-order absorption, one Ca2+ molecule per site. The free calcium concentration in the MCS can be described in relation to the concentration of free binding substance B and bound Ca2+ (CaB) within the MCS:
| (2) |
where JD = D(α) (Cae – Caf), D(α) is the diffusion coefficient, τfr is the time constant associated with the transfer of Caf to the RT, the Kfr is a constant of proportionality.
Binding is assumed at all times to be essentially at an equilibrium (Bassingthwaighte and Reuter, 1972), i.e.:
| (3) |
where Ka is a dissociated constant. Taking the derivative of equation (3) and substituting in equation (2) gives:
| (4) |
(3) Calcium concentration in the releasable terminal (CaRT). The RT is assumed to differ only functionally from the MCS. The calcium content in the RT is free and is replenished by the free calcium transferred from the MCS and the LSR. The rate of release is determined by:
the calcium gradient between the RT and the sarcoplasmic space;
the degree of activation and inactivation of the outer SR membrane, described by the kinetic parameters p(t) and q(t); and
the degree of activation and inactivation of the RT, which is described by the parameter r(t), itself a function of Casp.
The rate of change of CaRT is given by:
| (5) |
where Kfr and KLSR are constants, τfr and τLSR are time constants associated with the process of calcium transfer, gCa is the maximal rate of release, and Jio is the calcium efflux from the RT to the outside of the cell.
(4) Calcium concentration in the sarcoplasmic space (Casp). The calcium concentration in the sarcoplasm is determined by the amount of calcium released from the SR, the uptake of calcium and the passive diffusion of calcium between the sarcoplasm and the outside of the cell. The rate of change of Casp is:
| (6) |
where Jpump is the uptake rate of the LSR and De is the diffusion coefficient.
(5) Calcium concentration in the longitudinal sarcoplasmic reticulum (CaLSR). The calcium content in the LSR depends on the Ca2+ uptake rate and the transfer of its content to the RT. This model assumes transmembrane transport to be the uptake mechanism. Kitazawa (1976) demonstrated that the Ca2+ uptake of fragmented chicken cardiac SR increased sigmoidally with free Ca2+ concentrations. At a certain free Ca2+ concentration, the Ca uptake increased exponentially with time and the initial uptake rate varies with the free Ca2+ concentration. The Ca uptake data suggests a process empirically described as:
and
| (7) |
where Vmax, V′max are maximum pump rates and Km, K′max are the concentrations at which the half-maximal pump rate occurs; KLSR is a constant; τLSR, a time constant, is related to the apparent diffusion coefficient.
Both x(t) and f(t) are kinetic parameters describing the “conductance” of the channels of the RT surface. As x(t) is slow-varying and its value is significant only at relatively high Casp, Ca2+ uptake by the LSR starts near the end of Ca2+ release. The second term in Jpump accounts for the removal of Ca2+ during the relaxation phase.
(6) Activation, inactivation and reactivation. The activation and inactivation of the outer SR surface are characterized by two parameters—p and q. These parameters are governed by the first-order kinetic equation:
The rate constants αy, βy are functions of Cae. Thus, the steady-state parameters p∞, q∞ and the time constants τp, τq are functions of Cae. To explain the experimental observations that the release and accumulation of Ca2+ are influenced by the rate (α) of change of Cae, it is necessary to assume that τp is inversely proportional to, and τq proportional to, the rate of change of Cae. Thus:
| (8) |
| (9) |
where Cao is the maximal calcium concentration injected or aspired and α0 is a certain rate constant chosen as standard. Equation (8) indicates that τp, τq are linearly and inversely proportional to the rate of change of Cae respectively. When dCae/dt is high, the outer SR surface is rapidly activated, but slowly inactivated. At low rate, the opposite occurs.
The activation, inactivation and reactivation of the RT is described by the parameter r(t). However, r(t) is comprised of four parameters: d, f, X̅, and x, such that:
The steady-state parameters d∞, f∞, x∞ and the time constants τd, τf and τx are functions of Casp; X̅ and τX̅ are functions of the trigger calcium concentration (Cae). The parameter r(t) should be considered as a variable that varies with Casp and Cae, rather than as a Hodgkin–Huxley-type kinetic parameter. The formulations of the kinetic equations for X̅ and x are similar to those used by Standen and Stanfield (1982).
(7) Calcium efflux. Calcium efflux is assumed to take place at two locations within the SR: the MCS and the RT. At the MCS, free Ca2+ is extruded across the SR membrane by a Ca2+–Ca2+ exchange process (Wong and Bassingthwaighte, 1981):
where ϕ(Nao) is a function of [Na+]o in the oppositional space or outside the SR, and K1, K2 are constants.
In the present model, the RT is preplenished by free Ca2+ from the LSR and the MCS. When high [Ca2+] outside the cell is maintained, there will be a continuous production of free Ca2+ in the MCS and a continuous transfer of Ca2+ to the RT. If there were no Ca2+ extrusion from the RT to the outside of the cell, the RT would be loaded infinitely with Ca2+—an impossible situation.
To prevent an unreasonable overloading of RT, a Ca2+ extrusion mechanism is assumed. This mechanism is described as:
The function F(CaRT) should be such that Jio is insignificant when CaRT is equal or below its resting value (14 µM) but increases otherwise. An exponential function of the form:
seems to give the most satisfactory results.
(8) Time constant associated with the calcium transfer. The two time constants τfr and τLSR associated with the transfer of Caf and CaLSR to the RT are assumed to be inversely proportional to the apparent diffusion coefficient for Ca. Based on the study of Safford and Bassingthwaighte (1977), the apparent diffusion coefficient for Caf is related to the degree of binding of Ca2+ by the binding substance. Thus:
The rate of transfer of Ca2+ from LSR to RT is again assumed to be related to [Ca2+] in the LSR. This is based on the assumption that some degree of binding occurs in the LSR and that the apparent diffusion coefficient for CaLSR varies nonlinearly with CaLSR. Therefore:
Numerical Parameters
All model responses in this study were simulated with the same set of kinetic parameters, time constants and the following numerical constants:
The above numerical values were chosen to produce an optimal performance of the model. The only variable in all simulations was the trigger [Ca2+] at the outer surface of the SR, described by equation (9).
Kinetic Parameters and Time Constants
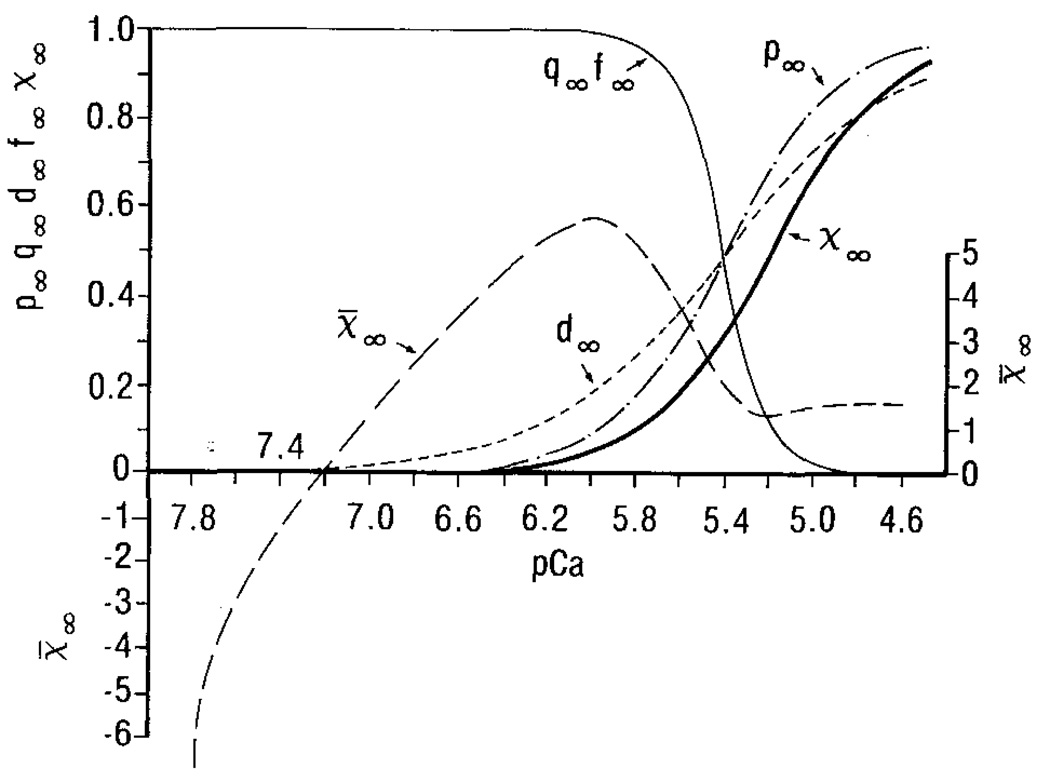
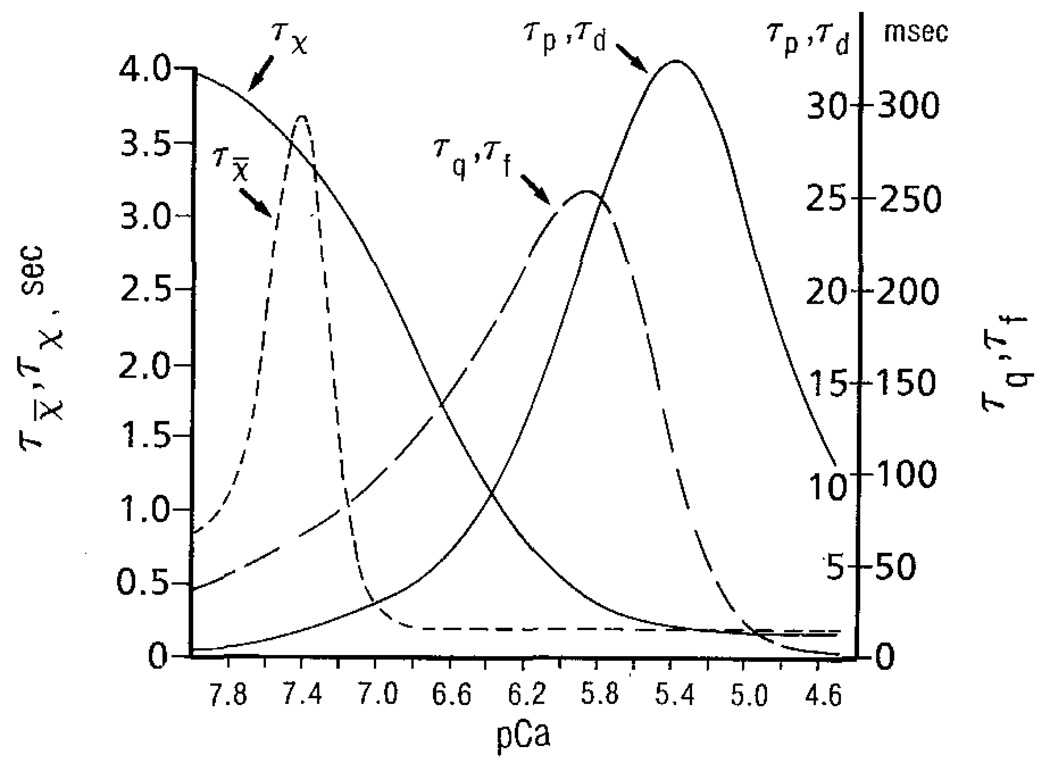
Figure 2 depicts the steady-state parameters as functions of trigger pCa or sarcoplasmic pCa. The time constants, as functions of pCa, are shown in Fig. 3. These functions are chosen after trial and error so that the model produces results qualitatively similar to those observed experimentally.
Figure 2.
The steady-state values for the activation (q∞) and inactivation (p∞, λx̅) parameters as functions of pCa at the outer SR surface. The activation (d∞) and inactivation (f∞x∞) parameters for the RT are functions of the pCa of the sarcoplasmic space.
Figure 3.
Time constants τp, τq, and τX̅ are functions of the pCa of the outer SR surface; τf, τd, and τx are functions of the pCa of the sarcoplasmic space.
Cyclic Release
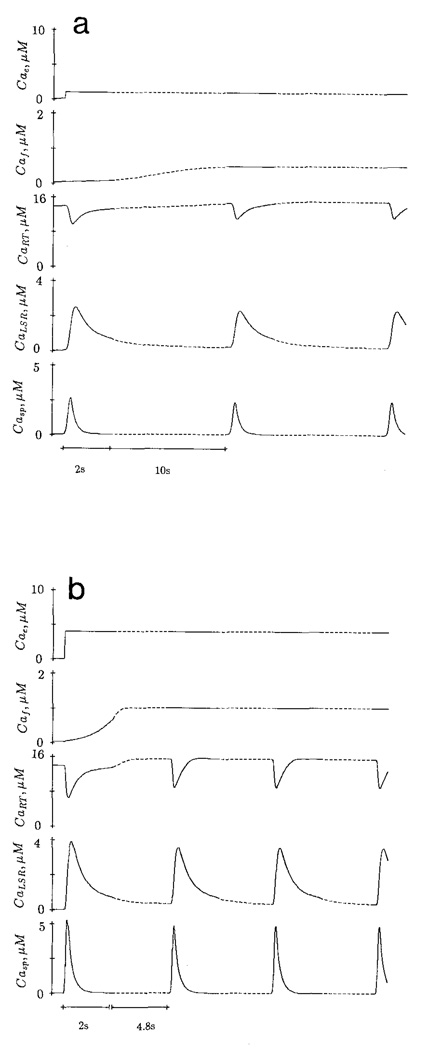
Cyclic release of Ca from the SR of skinned cardiac cells of rat (Fabiato and Fabiato, 1975) and Purkinje cells (Fabiato, 1985b) occurs when the trigger Ca2+ above a critical level is maintained outside the cell. The amplitude and frequency of the cyclic release depends on the trigger Ca concentration. The cycle release of Ca was simulated with the present model. With a trigger [Ca] of 1 µM (pCa = 6.0), the peak sarcoplasmic Ca (Casp) is 2.62 µM with a cycle period of 11.6 s (Fig. 4a). At trigger pCa = 5.4, the peak Casp is 5.27 µM and the cycle period is 6.8 s (Fig. 4b). At supraoptimal trigger Ca (pCa = 5.2), both peak Casp (4.38 µM) and cycle period (5.25 s) are reduced (Fig. 4c). The simulation is in agreement with the experimental observations on skinned ventricular cell of rat (Fig. 8 of Fabiato and Fabiato, 1975).
Figure 4.
Simulated cyclic release of Ca at constant trigger Ca2+ (Cae). The cycle period is 11.6 s at pCae 6.0 (a), 6.8 s at pCae 5.4 (b) and 5.25 s at pCae 5.2 (c). The change of time scale (– – – –) should be noted.
Effect of Concentration and the Rate of Increase of Trigger Ca (Cae) on Ca Release
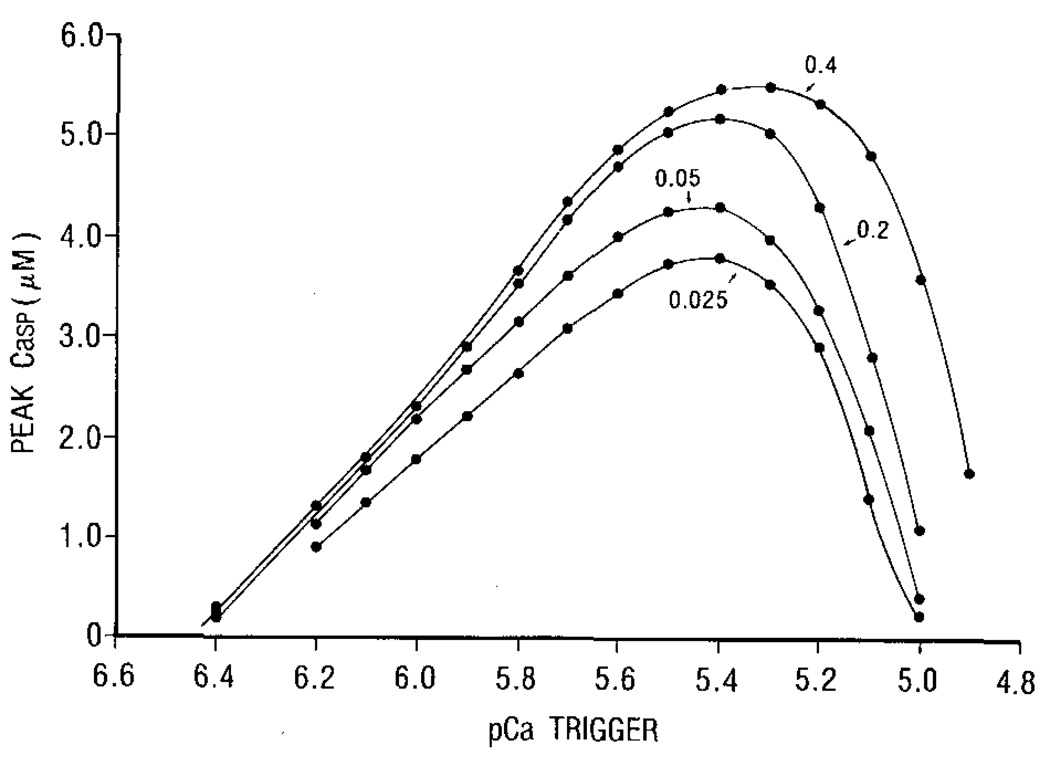
The bell-shaped relationship between the trigger pCa and peak sarcoplasmic Ca transient (Fig. 5) is mainly due to the activation p and the inactivation q of the outer SR surface. This is equivalent to the relation of peak Ca current and voltage. The effect of the rate of increasing trigger Ca on Ca release is indicated by the value of α [see equation (9)]. A higher value of α means a faster rate of increase.
Figure 5.
The relation between the trigger pCa, the rate of rise of trigger PCa and the peak Casp in the sarcoplasmic space. The numbers indicate the value of rate constant (α) in Cae = Cao[1 – exp(–αt)]. The larger the number, the faster the increase of Cae.
The higher the rate of increase of trigger Ca, the greater the peak sarcoplasmic Ca, similar to the peak tension and trigger pCa relation shown by Fabiato (1985b). These are the consequences of the assumption that the time constants of activation (τp) and inactivation (τq) are functions of α (equation (8)]. When trigger Ca is increased slowly (α is small), the outer SR surface is slowly activated, but rapidly inactivated, resulting in a reduction of the value of p · q.
Rate of Inactivation and of Removal of Inactivation
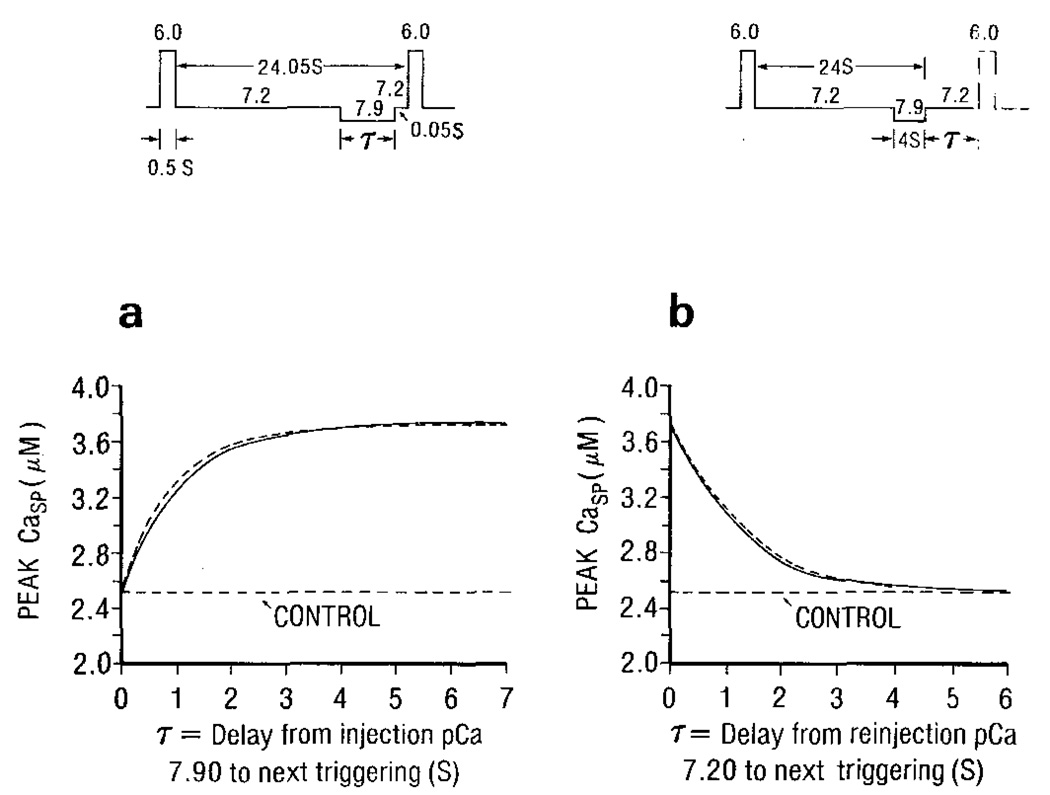
Fabiato’s experiments (1985b, Fig. 18) to demonstrate the rate of inactivation and of removal of inactivation were mimicked. The model response, shown in Fig. 6, is qualitatively similar to the experimental observations. The curve in Fig. 6a can be expressed as:
| (10) |
where Cac is the control peak Casp; Cam is the maximal peak Casp; τ is the delay from injection pCa 7.9 to the next triggering; T is the time constant of the exponential curve.
Figure 6.
The simulated protocols (upper panels) and the model’s results (lower panel) demonstrate the rate of removal of inactivation (a) and the rate of inactivation (b). The duration of trigger Ca at pCa 6.0 is 0.5 s. The dotted curve in (a) is described by equation (10) with T = τX̅ (7.9) = 0.903 s, and in (b) by equation (11) with T = τX̅ (7.9) = 1.43 s.
According to the present model scheme, it can be speculated that Cam reflects the magnitude of X̅∞ and T, the time constant, τX̅ at pCae = 7.9. The speculation is based on the release expression: p · q · r (CaRT – Casp), r = d · f – X̅ · x. At pCae = 7.2, p · q ≈ 0, d · f ≈ 0, X̅ · x ≠ 0 but slowly decreases toward zero in 24 s or longer. Therefore, r is negative and the releasable terminal is inactivated. When pCae of 7.9 is introduced at a delay (τ) from the next trigger, X̅(τ) at the end of τ is:
where X̅0(7.2) is the value of X̅ just before pCae = 7.9 is introduced.
In the present model, X̅0(7.2)≈0, and x, being very slow-varying, does not vary much within τ. Thus:
As X̅∞ is negative, r has a positive value. The degree of activation of the releasable terminal increases exponentially with a time constant = τX̅(7.9). To show that the time constant (T) in Fig. 6a is equal to τX̅(7.9), Ca in equation (10) was computed (dotted curve) with Cac = 2.51 µM, (Cam – Cac) = 1.20 µM and T = τX̅(7.9) = 0.903 s, a value used in the present model.
The curve in Fig. 6b can be described as:
| (11) |
where τ is the delay from reinjection of pCa 7.2 to the next triggering, and T is the time constant; Cam is the peak Casp at τ = 0.
When pCa 7.2 is reinjected after τ seconds, the value of X̅ at the end of τ is:
where X̅0(4, 7.9) is the value of X̅ at the end of 4 s at pCa = 7.9.
As X̅(7.2) = 0, X̅(τ) = X̅0(4, 7.9) exp[−τ/τX̅(7.2)] indicates that before the next triggering, the degree of activation of the releasable terminal decays exponentially with a time constant of τX̅(7.2) The dotted curve in Fig. 6b is described by equation (11) with Cac = 2.51 µM, (Cam – Cac) = 1.20 µM and T = τX̅(7.2) = 1.43 s, which is the value chosen in this model.
Simulation of Ca2+ Current
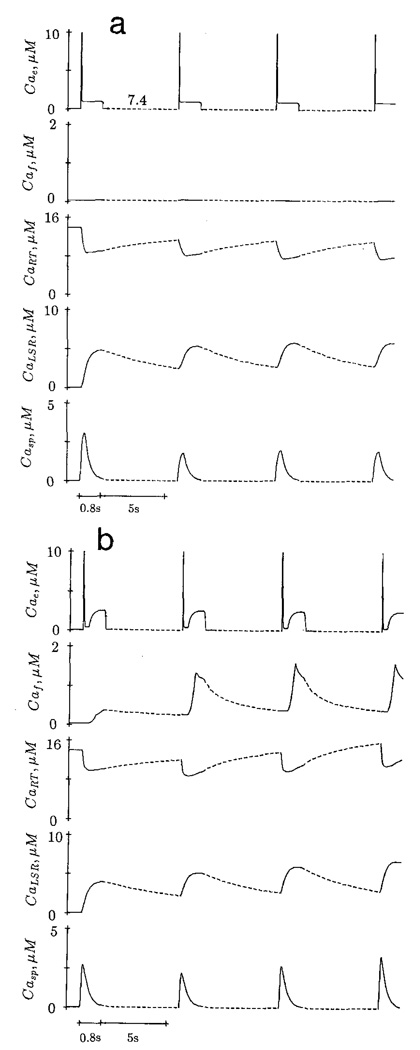
To simulate the fast component of Ca2+ current, Fabiato (1985c) injected trigger Ca2+ at pCa 5.0 in 10 ms and mixed with a volume of solution at pCa 7.0 injected at a slow rate. The slow component was made by mixing pCa 5.0 and pCa 7.0 solution at a slower rate. In the present study, the fast component was simulated by increasing the [free Ca2+] from pCa 7.4 to pCa 5.0 in 10 ms (i.e. α = 0.6 in equation (9)], which decayed to either pCa 6.4 or pCa 6.0 in 40 ms (α = 2.0), and lasted for 200 ms. The [free Ca2+] was slowly increased to a final pCa of 5.6 at two rates: α = 0.01 for 300 ms and α = 0.025 for the next 300 ms. This [free Ca2+] was rapidly reduced to resting pCa level (e.g. 7.4). The total duration of fast and slow components was 810 ms.
Potentiation of Subsequent Ca2+ Transient by the Slow Component
Figure 7 shows the effect of adding a slow component to the trigger Ca2+ on the subsequent sarcoplasmic Ca2+ transient (Casp). The first trigger Ca2+ (i.e. third pulse), comprising of fast and slow components, does not potentiate the sarcoplasmic Ca2+, but increases the [free Ca2+] sequestered by LSR are then transferred to the releasable terminal and become available for subsequent release.
Figure 7.
Demonstration of the potentiation of subsequent Ca transient by the slow component of trigger Ca. The first two trigger Ca pulses (uppermost panel) consist of fast component only. The third pulse, comprising of fast and slow component, does not modify the corresponding sarcoplasmic Ca transient immediately. The fourth and fifth trigger Ca pulses potentiate the subsequent transient. Note the change of time scale (– – –).
Positive and Negative Staircase
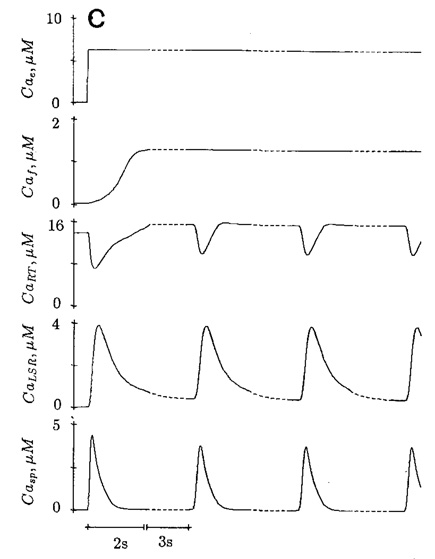
An increase of frequency of stimulation by trigger Ca2+ with fast component only results in a negative staircase. In Fig. 8a, the first sarcoplasmic Ca2+ (Casp) transient is triggered by a fast component after a long rest period. Subsequent stimulation at one per 6 s produces decreasing Ca2+ transient. In the absence of slow component, the [free Ca2+] production in MCS (Caf) is minimal. The higher frequency of stimulation reduces the time for the releasable terminal to be replenished by the [Ca2+] from MCS and LSR, resulting in a progressive reduction of calcium content in this compartment. In contrast, trigger Ca2+ with fast and slow components elicits a positive staircase (Fig. 8b) at the same frequency of stimulation (one per 6 s). The calcium content of the releasable terminal progressively increases due to the increase of [Ca2+] in the MCS.
Figure 8.
Negative staircase (a) is elicited by trigger Ca2+ with fast component only, and stimulated at a frequency of one per 6 s. Positive staircase (b) is caused by trigger Ca2+ with fast and slow components, stimulated at the same frequency. Change of time scale is indicated by the dotted line.
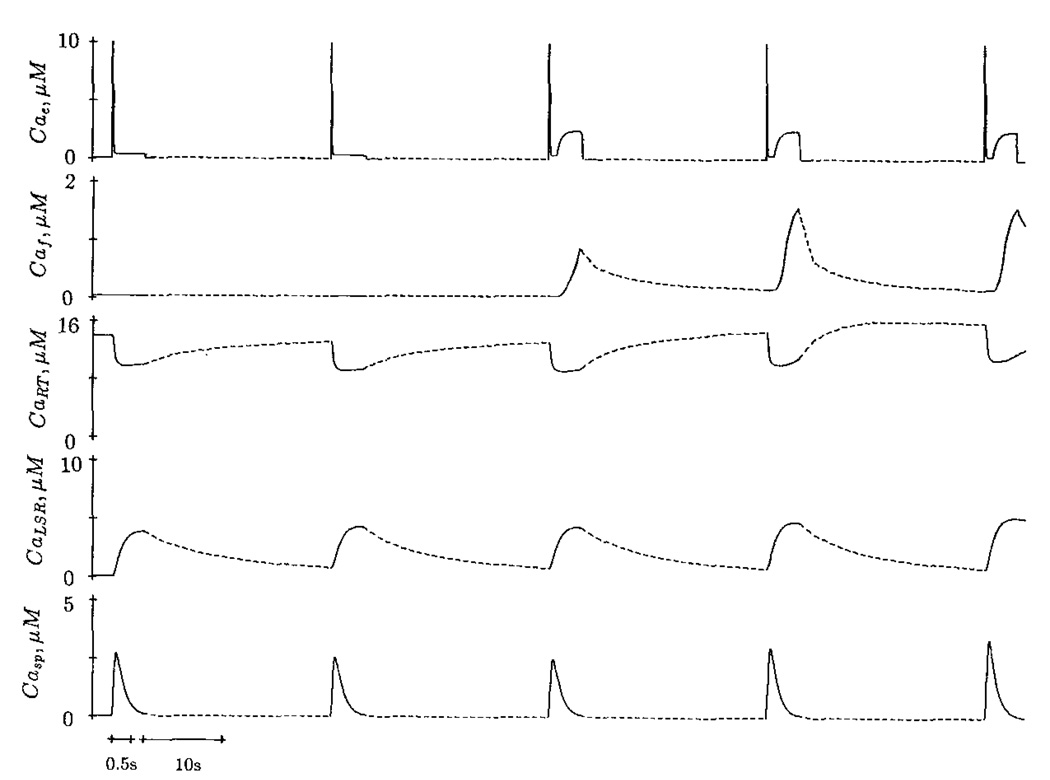
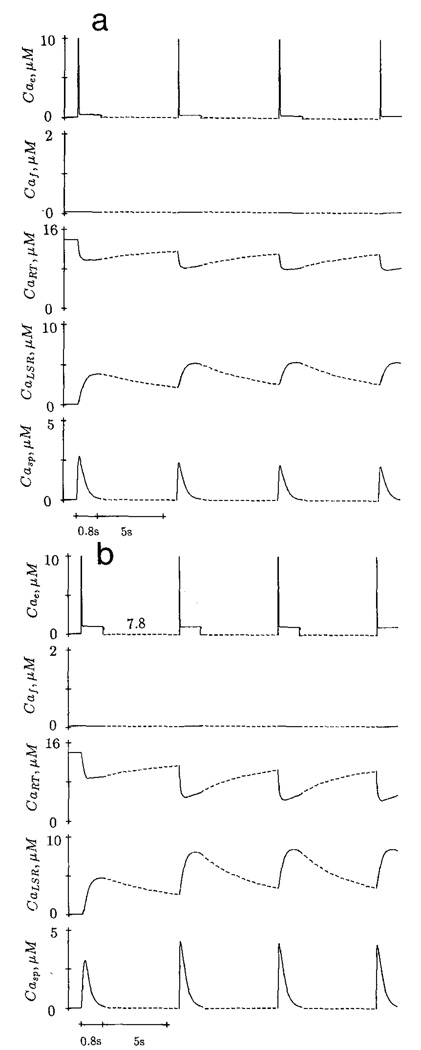
Effect of Resting [free Ca2+] on the Subsequent Calcium Release
The initial conditions in Fig. 9a,b are identical. The trigger Ca2+ consists of a fast component only; a 10 ms pCa 5.0 decays rapidly to pCa 6.0 in 40 ms and lasts for 800 ms. The first sarcoplasmic Ca2+ transient is elicited by the trigger Ca2+ after a long rest period; it has a peak value of 3.1 µM. When the resting [Ca2+] during the interval between stimuli is lowered to pCae = 7.8 and stimulation frequency is increased (one per 6 s), the subsequent sarcoplasmic Ca2+ transient is potentiated (Fig. 9a). However, at a resting [free Ca2+] of pCae = 7.4, higher stimulation frequency produces a reduced Ca2+ transient in the next beat.
Figure 9.
Effect of resting pCae during the interval between two stimuli on subsequent Ca2+ transient. The initial resting pCae is 7.4. After the first Ca2+ transient, the resting pCae is changed to 7.8. Dotted lines indicate smaller time scale.
Fabiato (1985c) reasoned that when the resting [free Ca2+] was higher, the negative staircase elicited was caused by a progressive reduction in the degree of refilling of the SR during the interval between tension transients resulting from the increase of frequency. According to the model response, the potentiated Ca2+ transient at higher resting [free Ca2+] is not due to the loading of the releasable terminal. In fact, the calcium content in this compartment is reduced. The potentiation is caused by the elevated degree of reactivation of the releasable terminal (r = d · f – λX̅ · x).
At resting pCae = 7.8, X̅∞ is more negative, resulting in a larger positive value of r during the interval between stimuli. At pCae = 7.4, X̅∞ is less negative. The releasable terminal at the end of the of the resting period (6 s) has a smaller value of r and thus less Ca2+ is released in the subsequent beat.
Discussion
The present model has demonstrated that trans-sarcolemmal Ca2+ influx, such as Ca2+ current, can trigger Ca2+ release from and load the sarcoplasmic reticulum in cardiac cells. The demonstration is made possible by assuming that the activation, inactivation and the reactivation of the SR membrane is Ca2+-mediated and time-dependent.
In many aspects, the proposed model is phenomenological as some of the assumptions do not seem to have a molecular basis. For example, it is difficult to explain how the rate of change of trigger Ca2+ affects τp, τq, the diffusion of Ca2+ into the MCS, or how trigger [Ca2+] can influence τx, which is primarily a function of sarcoplasmic [Ca2+]. Another objectionable feature is Jio, the calcium efflux from the RT to the outside of the cell. Jio can be eliminated by (1) making the free Ca2+ transferred from LSR to MCS instead of to RT. By modifying the numerical values of parameters of constants in Fig. 2, the free Ca2+ in RT can be limited to about 18 µM (Adler et al., 1985). (2) Considering the MCS as the compartment which can store, release and sequester Ca2+, then LSR is not necessary.
In the above two cases, it is necessary to modify or adjust the parameters and constants so that the amount of free Ca2+ produced in MCS is equal to the amount of Ca2+ sequestered, otherwise the free [Ca2+] in RT cannot be maintained. In other words, it is necessary to assume a high free Ca2 in the MCS during resting state, as in the other E–C coupling models (Cannell and Allen, 1984; Adler et al., 1985). If the free Ca2+ in the MCS were high, calcium would be extruded continuously across the SR membrane to the outside diffusion. High resting free Ca2+ within the MCS could be maintained only if the SR membrane was nondiffusible at the resting condition, or some “calcium pump” similar to the Na–K pump was was assumed. However, these assumptions do not seem reasonable.
The releasable terminal, being part of the SR, does contain relatively high free Ca2+; this calcium is not extruded by diffusion. The separation of the SR into a “main calcium store” and the Releasable Terminal is for the convenience of mathematical formulation. One should perceive the MCS and RT together as one single compartment (i.e. SR), in which the resting free Ca2+ is high and calcium is extruded by a Ca–Ca exchange mechanism.
Cannell et al. (1987) reported the effect of depolarization duration on the time course of calcium transient (indicated by the fluorescent Ca2+ indicator fura-2) in single rat cardiac cells. They suggested that Ca release might be mediated by Calcium-Induced Calcium Release (CICR) and modulated directly by changes in voltage. An alternative scheme of Ca2+ release from the SR based on CICR and a voltage-dependent mechanism (Hilgemann and Noble, 1987) should be considered. However, in a review article, Fabiato (1985d) cited studies which demonstrated no change of SR potential during Ca2+ release induced by Ca2+ or caffeine, or no Ca2+ release induced by change of potential in single skinned cardiac cells. The effect of depolarization duration on calcium transient is equivalent to the effect of decreasing trigger [Ca2+] at different phases of Ca2+ transient (Fabiato, 1985b) and can be explained by the present model. The release parameter r(t) = (f · d – λX̅ · x) is dependent on the sarcoplasmic calcium (Casp) or intracellular Ca2+ and trigger Ca2+ (Cae) concentration. In a cardiac cell with sarcolemma, repolarization at different phases of the Ca2+ transient alters Ca2+ influx into the appositional space, changing Cae and X̅(t). Subsequently, r(t) is altered, affecting the amount of Ca2+ released and intracellular Ca2+ concentration.
Studies by Winegrad (1979, 1982) showed no evidence for different sites for Ca2+ release and accumulation in cardiac muscle. Experiments on skinned cardiac cells (Fabiato, 1981, 1983) suggest that the longitudinal SR is capable of both accumulating and releasing Ca2+. A recent study by Fabiato (1985c) suggests a mechanism through which Ca2+ release and accumulation could take place with only a functional compartment caused by the time and Ca2+ dependence of activation and inactivation of Ca2+ release from the SR and of the Ca2+ accumulation into the SR.
The present model appears to be in conflict with the above concept as the release and accumulation of Ca2+ occurs at different sites or compartments (RT and LSR). However, the kinetic parameters f(t) and x(t) are involved in Ca2+ release and uptake. Furthermore, the LSR transfers free Ca2+ directly to RT for subsequent release. Arbitrarily, both RT and LSR could be lumped together as one functional unit capable of releasing and accumulating Ca2+.
The model is consistent with the concept that the trans-sarcolemmal Ca2+ influx loads the SR with Ca2+ when it is slow and occurs during the refractory period of the process of Ca2+-induced release of Ca2+ (Fabiato, 1985c). The refractory period in the model is determined by the release expression (d · f – λX̅ · x). After the fast component of the Ca2+ influx triggers the release of Ca2+, x(t) increases and f(t) decreases, making (d · f – λX̅ · x) negative and model refractory. Within the refractory period, the slow component of the influx increases the [Ca2+] outside the SR. Subsequently, [Ca2+] enters the MCS, increasing its free Ca2+ content.
In many aspects, the kinetics of the proposed Ca release mechanism are similar to those of cardiac action potential (Beeler and Reuter, 1977). The parameters p, q, d and f can be considered equivalent to the activation and inactivation of inward currents, and X · x to the outward current. The rate of Ca released from releasable terminal, given as:
differs little conceptually from the kinetics of ionic current, I = g(E – Er), in which g is the conductance and (E – Rr) the driving force. The first term of the Ca release equation is equivalent to conductance, the second term to the driving force.
The expression for Jpump, despite its empirical nature, has its physiological function. As X(t) is slow-varying and its value is small when Ca2+ is being released, Ca uptake is not significant, thus allowing the increase of sarcoplasmic Ca2+ at a faster rate to activate the myofilaments. Near the end of release, when x(t) has become relatively large and the sarcoplasmic Ca2+ is high, the Ca uptake is accelerated. This is in agreement with the experimental observation (Fabiato, 1985b) that Ca uptake starts at the end of the release.
The delay of Ca uptake is physiologically significant. If release and uptake occurred simultaneously, more Ca2+ had to be released than necessary in order to be sufficient to activate the myofilaments; energy expenditure would be greater to keep the uptake process operating all the time.
An alternative and realistic scheme for Jpump is:
In this equation, ATP promotes Ca uptake while ADP and Pi inhibits it. If ATP is depleted early there is an accumulation of Pi and ADP, for example during moderate or severe hypoxia (Nayler et al., 1979), and the Ca accumulation by the LSR and the transport of Ca to the releasable terminal will be depressed. However, the variation of local concentrations of ATP, Pi and ADP with time on a beat-to-beat basis is not clear.
Despite the fact that the “steady state” kinetic parameters and the time constants are “guessed” or speculated, the model is able to simulate many events qualitatively similar to those observed experimentally (Fabiato, 1985c). Perhaps the model can be refined if the kinetic parameters are determined more realistically. The amplitudes and rate of rise of aequorin light data vs trigger pCa of the Fabiato’s experiments (1985a,b) on skinned cells can provide clues for estimating p, q, d, f and their associated time constants. X̅, x and τx can be estimated from the periodicity and amplitude of the cyclic release.
The present model can be applied directly to cardiac cells with intact sarcolemma by replacing the space at the outer surface of the SR with the oppositional space (APS). Thus equation (1) becomes:
Jin is the transmembrane calcium influx during a depolarization such as the calcium current. Jout is the transmembrane calcium efflux from APS to the extracellular space. The efflux is probably due to Ca–Na exchange and/or calcium pump. Both JD and JioCa have the same meaning as defined in equation (1).
A small number of experiment observations on skinned cells cannot be simulated by the present model. This is because many mechanistic and molecular details of Ca2+ release and accumulation are still lacking in this study. Nevertheless, the model appears to confirm the hypothesis that the activation, inactivation and reactivation of Ca2+ release and accumulation processes are calcium-mediated and time-dependent, analogous in principle to Hodgkin–Huxley channel regulation by voltage.
Conclusion
A model has been proposed to describe the mechanism of calcium-induced calcium release from cardiac sarcoplasmic reticulum (SR). Based on the assumption that the activation, inactivation and reactivation of the SR calcium channels are Ca2+-mediated and time-dependent, the model satisfactorily explains (a) how an increase of [free Ca2+] outside the SR membrane induces a release of Ca2+ from the lumen of the SR, (b) why this phenomenon is graded rather than all-or-none, and (c) why trans-sarcolemmal Ca2+ influx such as Ca2+ current can trigger Ca2+ release from and also load the SR.
Acknowledgments
The authors thank Mr P. King, Editorial Service, Dalhousie University for editing this manuscript.
This work was supported by a program grant from MRC (PG 30) and a grant from the Nova Scotia Heart Foundation.
Contributor Information
Alan Y. K. Wong, Department of Physiology and Biophysics, Dalhousie University Halifax, Nova Scotia, Canada B3H 4H7
Alexandre Fabiato, Department of Physiology, Medical College of Virginia, Richmond, VA 23298, U.S.A..
James B. Bassingthwaigthe, Center for Bioengineering, University of Washington, Seattle, Washington, U.S.A.
LITERATURE
- Adler D, Wong AYK, Mahler Y, Klassen GA. Model of calcium movements in the mammalian myocardium: interval-strength relationship. J. theor. Biol. 1985;113:379–394. doi: 10.1016/s0022-5193(85)80233-2. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte JB, Reuter H. Calcium movements and excitation–contraction coupling in cardiac cell. In: DeMello WC, editor. Electric Phenomena in the Heart. New York: Academic Press; 1972. pp. 353–395. [Google Scholar]
- Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibers. J. Physiol. 1977;268:117–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Allen DG. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys. J. 1984;45:913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Berlin JR, Lederer WJ. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987;238:1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. gen. Physiol. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium release in skinned cardiac cells: variation with species, tissues and development. Fed. Proc. 1982;41:2238–2244. [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J. gen. Physiol. 1985a;85:189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the d-sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. gen. Physiol. 1985b;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. gen. Physiol. 1985c;85:391–420. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Appraisal of the hypothesis of the “depolarization-induced” release of calcium from the sarcoplasmic reticulum in skinned cardiac cells from the rat or pigeon ventricle. In: Fleischer S, Tonomura Y, editors. Structure and Function of Sarcoplasmic Reticulum. New York: Academic Press; 1985d. [Google Scholar]
- Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cell. J. Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ. Res. 1977;40:119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cell from adult, human, cat, rabbit, rat and frog hearts and from fetal and new-born rat ventricles. Ann. N.Y. Acad. Sci. 1978;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Noble D. Excitation–contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc. R. Soc. Lond. 1987;B230:163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- Holmberg SRM, Williams AJ. Single channel recordings from human cardiac sarcoplasmic reticulum. Circ. Res. 1989;65:1445–1449. doi: 10.1161/01.res.65.5.1445. [DOI] [PubMed] [Google Scholar]
- Kitazawa T. Physiological significance of Ca uptake by mitochondria in the heart in comparison with that by cardiac sarcoplasmic reticulum. J. Biochem. 1976;80:1129–1147. doi: 10.1093/oxfordjournals.jbchem.a131369. [DOI] [PubMed] [Google Scholar]
- Nayler WG, Poole-Wilson PA, Williams A. Hypoxia and calcium. J. molec. Cell. Cardiol. 1979;11:683–706. doi: 10.1016/0022-2828(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Henderson JS, Meissner G. Single channel and 45Ca flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys. J. 1986;50:1009–1014. doi: 10.1016/S0006-3495(86)83543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford RE, Bassingthwaighte JB. Calcium diffusion in transient and steady states in muscle. Biophys. J. 1977;20:113–135. doi: 10.1016/S0006-3495(77)85539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A binding site model for calcium channel inactivation that depends on calcium entry. Proc. R. Soc. Lond. 1982;B217:101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Briggs FN. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle: calcium binding. Circ. Res. 1974;34:531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Electromechanical coupling in heart muscle. In: Berne RM, Sperelakis N, Geiger SR, editors. Handbook of Physiology. Section 2: The Cardiovascular System: Vol. 1: The Heart. Bethesda, MD: American Physiological Society; 1979. pp. 393–428. [Google Scholar]
- Winegrad S. Calcium release from cardiac sarcoplasmic reticulum. Ann. Rev. Physiol. 1982;44:451–462. doi: 10.1146/annurev.ph.44.030182.002315. [DOI] [PubMed] [Google Scholar]
- Wong AYK, Bassingthwaighte JB. The kinetics of Ca-Na exchange in excitable tissue. Math. Biosci. 1981;53:275–310. doi: 10.1016/0025-5564(81)90022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AYK, Fabiato A, Bassingthwaighte JB. Model of Ca release mechanism from the sarcoplasmic reticulum: Ca-mediated activation, inactivation and reactivation. In: Sideman S, Beyar R, editors. Activation, Metabolism and Perfusion of the Heart. The Hague: Martinus Nijhoff; 1987. pp. 281–295. [Google Scholar]