Abstract
Chick muscle cultures infected with wild-type Rous sarcoma virus form myotubes, but these myotubes vacuolate and by day 6 most have degenerated, leaving only large numbers of transformed mononucleated, replicating cells. Muscle cultures infected with a temperature-sensitive mutant (TS) at permissive temperatures behave as cells infected with wild-type Rous sarcoma virus. TS-infected cells reared for 8 days at nonpermissive temperature form contracting myotubes, plus large numbers of fibroblastic cells. If these cultures are lowered to permissive temperature, within 72 hr the myotubes vacuolate and degenerate, whereas the mononucleated cells transform. If replicating TS-transformed cells after 8 days at permissive temperature are shifted to nonpermissive temperature, within 72 hr many cells fuse and form contracting, post-mitotic myotubes. Creatine kinase (ATP:creatine N-phosphotransferase, EC 2.7.3.2) levels parallel the formation and degeneration of myotubes during these temperature shifts. If the viral transforming gene is expressed in the post-mitotic myotubes it is lethal, whereas it is not lethal if expressed in replicating percursor myogenic cells. The viral gene expression at permissive temperature blocks further myogenesis depending on the position of the cells in the myogenic program. The virus does not cancel the replicating, transformed myogenic cells' commitment to, or position in, the myogenic lineage. When the transforming action of the virus is suppressed, the normal myogenic program resumes.
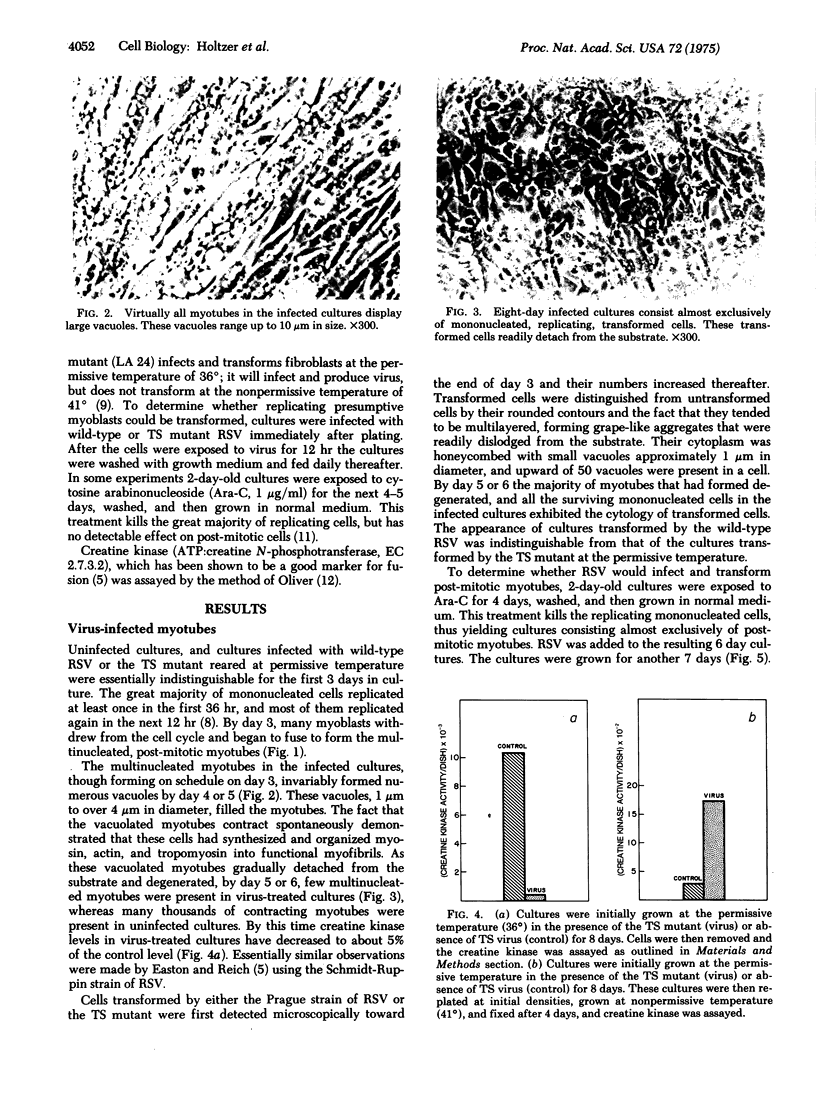
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Schiltz J., Dienstman S., Holtzer H. The phenotypic complexity of myogenic clones. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1506–1510. doi: 10.1073/pnas.71.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Mitosis and the processes of differentiation of myogenic cells in vitro. J Cell Biol. 1969 Apr;41(1):188–200. doi: 10.1083/jcb.41.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton T. G., Reich E. Muscle differentiation in cell culture. Effects of nucleoside inhibitors and Rous sarcoma virus. J Biol Chem. 1972 Oct 25;247(20):6420–6431. [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Fogel M., Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma). Proc Natl Acad Sci U S A. 1967 Sep;58(3):967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Fogel M. The relationship of polyoma virus-induced tumor (T) antigen to activation of DNA synthesis in rat myotubes. Dev Biol. 1973 Nov;35(1):180–186. doi: 10.1016/0012-1606(73)90016-x. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Groudine M., Holtzer H., Scherrer K., Therwath A. Lineage-dependent transcription of globin genes. Cell. 1974 Nov;3(3):243–247. doi: 10.1016/0092-8674(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., King D., Yang S. J. Quantitative changes in unscheduled DNA synthesis in rat muscle cells after differentiation. Nat New Biol. 1971 Apr 21;230(16):242–244. doi: 10.1038/newbio230242a0. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Croop J., Dienstman S., Ishikawa H., Somlyo A. P. Effects of cytochaslasin B and colcemide on myogenic cultures. Proc Natl Acad Sci U S A. 1975 Feb;72(2):513–517. doi: 10.1073/pnas.72.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Dienstman S., Chi J., Biehl J., Somlye A. Perspectives in myogenesis. Biochimie. 1974;56(11-12):1575–1580. doi: 10.1016/s0300-9084(75)80282-3. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Strahs K., Biehl J., Somlyo A. P., Ishikawa H. Thick and thin filaments in postmitotic, mononucleated myoblasts. Science. 1975 May 30;188(4191):943–945. doi: 10.1126/science.1138363. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Kaighn M. E., Ebert J. D. Induction of thymidine-3H incorporation in multinucleated myotubes by Rous sarcoma virus. Int J Cancer. 1968 Jan 15;3(1):126–136. doi: 10.1002/ijc.2910030115. [DOI] [PubMed] [Google Scholar]
- Nameroff M. A., Reznik M., Anderson P., Hansen J. L. Differentiation and control of mitosis in a skeletal muscle tumor. Cancer Res. 1970 Mar;30(3):596–600. [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessac B., Calothy G. Transformation of chick embryo neuroretinal cells by Rous sarcoma virus in vitro: induction of cell proliferation. Science. 1974 Aug;185(4152):709–710. doi: 10.1126/science.185.4152.709. [DOI] [PubMed] [Google Scholar]
- Simons P. J., Tuffery A. A., McCully D. J., Aw E. J. Cellular interactions in vitro with cells derived from tumors induced by murine sarcoma virus (Harvey). J Natl Cancer Inst. 1971 Jun;46(6):1229–1242. [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]
- Weintraub H., Campbell G. L., Holtzer H. Differentiation in the presence of bromodeoxyuridine id "all-or-none". Nat New Biol. 1973 Aug 1;244(135):140–142. doi: 10.1038/newbio244140a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Campbell G. L., Holtzer H. Identification of a developmental program using bromodeoxyuridine. J Mol Biol. 1972 Sep 28;70(2):337–350. doi: 10.1016/0022-2836(72)90543-8. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967 Jul 22;215(5099):421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]








