Abstract
Differentiation induction is currently considered as an alternative strategy for treating chronic myelogenous leukemia (CML). Our previous work has demonstrated that Sprouty-related EVH1 domainprotein2 (Spred2) was involved in imatinib mediated cytotoxicity in CML cells. However, its roles in growth and lineage differentiation of CML cells remain unknown. In this study, we found that CML CD34+ cells expressed lower level of Spred2 compared with normal hematopoietic progenitor cells, and adenovirus mediated restoration of Spred2 promoted the erythroid differentiation of CML cells. Imatinib could induce Spred2 expression and enhance erythroid differentiation in K562 cells. However, the imatinib induced erythroid differentiation could be blocked by Spred2 silence using lentiviral vector PLKO.1-shSpred2. Spred2 interference activated phosphorylated-ERK (p-ERK) and inhibited erythroid differentiation, while ERK inhibitor, PD98059, could restore the erythroid differentiation, suggesting Spred2 regulated the erythroid differentiation partly through ERK signaling. Furthermore, Spred2 interference partly restored p-ERK level leading to inhibition of erythroid differentiation in imatinib treated K562 cells. In conclusion, Spred2 was involved in erythroid differentiation of CML cells and participated in imatinib induced erythroid differentiation partly through ERK signaling.
Introduction
Chronic myelogenous leukemia (CML) arises mostly from a pluripotent hematopoietic stem cell that contains thereciprocal t(9;22)(q34;q11) chromosomal translocation coding BCR/ABL fusion oncoprotein. BCR/ABL kinase activates a variety of downstream survival pathways and inhibits cell differentiation [1,2]. The CML is currently successfully treated with BCR-ABL inhibitors, such as imatinib and dasatinib [3–5]. However, clinical resistance to these drugs has also been widely reported in CML patients [6–9].
CML is a clonal hematopoietic stem cell disorder that the malignant clone progressively loses the capacity for terminal differentiation. Thus, differentiation induction has been considered as an alternative approach for CML therapy. Some valuable progress has been achieved in biological or chemical agents that could induce terminal differentiation [10–13]. It has been reported that low concentration of imatinib induces proliferation arrest and erythroid differentiation of CML cells [14,15]. The RAS-ERK pathway is known to contribute to myeloid differentiation of CML cells [16]. Notably, CML treatment lead to terminal differentiation of leukemia cell lines or primary cells, as well as proliferation arrest and cell apoptosis, by regulating RAS-ERK cascade [17–20].
Sprouty-related EVH1 domainprotein 2 (Spred2) proteins are identified as a family of membrane-associated negative regulators of growth factor-induced RAS-ERK activation [21]. Our previous studies demonstrated that Spred2, a subset of Spreds family, was involved in imatinib-induced cytotoxicity in CML cells. Imatinib treatment upregulates Spred2 expression, leading to apoptosis and growth arrest in CML cells [17]. However, whether Spred2 is implicated in CML cell differentiation remains unclear. In this study, we clarified the expression and potential roles of Spred2 protein in erythroid differentiation of CML cells and its mechanisms.
Methods
Cell lines and primary cells
The human myelogenous leukaemia cell line K562 were obtained from America Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 (Sigma, St. Louis, MO, USA) medium containing 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT, USA), 100 unit/ml penicillin and 100 μg/ml streptomycin in a humidified 5% CO2 atmosphere at 37°C.
The bone marrow (BM) samples were obtained from healthy donor or CML patients undergoing diagnostic procedures at Peking university first hospital. Written informed consent was obtained from each healthy donor and CML patient. All the procedures were approved by the Ethics Committee of Beijing Institute of Radiation Medicine. Mononuclear cells were isolated from heparinized samples by centrifugation through a Ficoll-Hypaque density gradient (Amersham Biosciences, Piscataway, NJ, USA). Then, CD34+ cells were isolated by using human CD34 positive selection kit (Stem Cell Technology, Vancouver BC, Canada).
Lentiviral vector production
Lentiviral shRNA vector targeting Spred2 (PLKO.1-shSpred2) was constructed according to the protocol of PLKO.1-puro vector (Addgene, Cambridge, MA). Briefly, the forward oligo, 5’ccggtggtattggaatgctatgtaactcgagttacatagcattccaataccatttttg 3’ and reverse oligo, 5’aattcaaaaatggtattggaatgctatgtaactcgagttacatagcattccaatacca3’ were annealed and inserted into the PLKO.1-puro vector, which was digested by AgeI and EcoRI. And, control vector PLKO.1-shScramble was also purchased from addgene. Then, the 1406 bp fragment between XbaI and BamHI was obtained from plasmid pHIV7-SF-RFP, and cloned into the corresponding sites (SpeI and BamHI) of PLKO.1-shSpred2 or PLKO.1-shScramble, respectively.
293T cells (ATCC) were cultured in RPMI 1640 (Sigma) medium supplement with 10% FCS (Hyclone) and plated at 6×106 cells per 10-cm plate 1 day before transfection. Transfer vector PLKO.1-shSpred2 or PLKO.1-shScramble, packing plasmid psPAX2 and envelope plasmid pMD2.G were co-transfected by using the phosphate coprecipitation kit (Promega, Madison, WI, USA) according to manufacturer’s protocol and culture medium was replaced by fresh growth medium 6h after transfection. The virus containing media were collected at 36h and 48h after transfection. Viruses were purified and concentrated by PEG, followed by determination of viral titers on HT1080 cells.
Virus transduction
Before transduction, CD34+ cells were cultured in SFEM medium (Stem Cell Technologies. Inc., Vancouver, Canada) supplement with 50ng/ml stem cell factor (SCF), 100ng/ml thrombopoietin (TPO), 100ng/ml FMA-like tyrosine kinase 3 ligand (Flt-3L), 100 ng/ml interleukin (IL) -6, and 50ng/ml IL-3 (Peprotech, Rocky Hill, NJ) for 48 hours. CD34+ cells and K562 cells were plated in 24-well plate at a density of 2×105 per well, and then were infected by lentiviral vectors at multiplicity of infection (MOI) of 10 or by adenoviral vector at MOI of 150. The gene transduction efficiency of lentiviral vectors, indicated by RFP expression, was detected by flow cytometry (Becton Dickinson, Mountain View, CA).
Differentiation assay
For differentiation assay, CD34+ cells infected by viruses were cultured in Iscove’s Modified Dulbecco Medium (IMDM) supplement with 30% FCS, 50 ng/ml SCF, 50 ng/ml IL-3, 200 ng/ml granulocyte colony-stimulating factor (G-CSF), 200 ng/ml granulocyte-macrophage colony stimulating factor (GM-CSF) (Peprotech), 63 μM β-mercaptoethanol and 3 unit/ml erythropoietin. At day 0, 3 and 7 post-infection, cells were collected and labeled with allophycocyanin (APC)-conjugated anti-human CD34 antibody, fluorescein isothiocyanate (FITC)-conjugated anti-human CD235a antibody and Phycoerythrin (PE) Cy7 conjugated anti-human CD11b antibody (BD Biosciences, San Jose, CA), and then detected by flow cytometer.
The differentiation of K562 cells transduced by lentivrial vectors or adenoviral vectors was induced by 10ng/ml Phorbol-12-myristate-13-acetate (PMA, Sigma Chemical Co., St. Louis, MO) or 1μM imatinib (sigma), respectively. At indicated time points, cells were collected and labelled with PE/FITC-conjugated CD235a antibody, and then detected by flow cytometer.
And, the mRNA expression of Spred2, CD235a and differentiation related transcription factors GATA1 were also detected in CD34+ cells and K562 cells by using real-time reverse transcription polymerase chain reaction (RT-PCR).
Colony-Forming Cell (CFC) assay
Two days after transduced by lentiviral vectors, CD34+ cells were plated in 24-well plate at a density of 500 per well, and cultured in 1% methylcellulose medium supplemented with 30% FCS, 50ng/ml SCF, 50ng/ml IL-3, 200ng/ml G-CSF, 200ng/ml GM-CSF, 63μM β-mercaptoethanol and 3 unit/ml erythropoietin, which is formulated to support optimal growth of erythroid progenitors (CFU-E and BFU-E), granulocyte-macrophage progenitors (CFU-GM, CFU-G, and CFU-M) and multi-potential granulocyte, erythroid macrophage and megakaryocyte progenitor (CFU-GEMM). Fourteen days later, the presence of colonies (>40 cells) was counted and scored. The colonies formation scoring and erythroid colonies scoring were calculated from the numbers of colonies/total number of cells seeded.
Real-time RT-PCR
Total RNA was isolated from CD34+ cells or K562 cells by using TRIzol reagent (Invitrogen, Carlsbad, CA), and the cDNA was synthesized using a First Strand cDNA Synthesis Kit (Thermo Scientific, Wilmington, DE) according to the manufacturer’s instructions. Then, the mRNA expression was quantified by using SYBR Green Real-Time kit (Takara Bio Inc., Otsu, Shiga, Japan) on 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The primers for homo sapiens CD235a, spred1, spred2, gata binding protein 1 (globin transcription factor 1) (GATA1) and beta-actin (β-actin) were shown in Table 1. And the expression levels were normalized by β-actin or the target gene expression at day 0 after cultured in differentiation medium. The results were showed as the mean± s.d. of triplicates and were representative of three independent experiments.
Table 1. Primers for real-time PCR detection.
| genes | Sequence |
|---|---|
| CD235a | Sense: 5’-aagggtacaacttgcccatca-3’ |
| Antisense: 5’-ttcaacagaacttaaaggcacgtc-3’ | |
| Spred1 | Sense: 5’-ggaagcactagaaactggcattatt-3’ |
| Antisense: 5’-cacctggctgctaggcaaac-3’ | |
| Spred2 | Sense: 5’-ctcatccatggtgaacgacagaa-3’ |
| Antisense: 5’-tgtcaaaggctcgggcatc-3’ | |
| GATA1 | Sense: 5’-ctgcggcctctatcacaagatg-3’ |
| Antisense: 5’-actgagtacctgcccgtttactgac3’ | |
| PU.1 | Sense: 5’-tgaaggacagc atctggtg-3’ |
| Antisense: 5’-ccgtcttgccgtagttgc-3’ | |
| β-actin | Sense: 5’-gcgggaaatcgtgcgtgac-3’ |
| Antisense: 5’-ggaaggaaggctggaagag-3’ |
Western blotting
After indicated treatment, Spred2 over-expressed or slicenced K562 cells were collected and the protein was extracted. Then, the expression of Spred2 was detected by rabbit anti-human Spred2 antibody (Sigma). And, the activation of MAPK signalling pathway was detected by anti-phospho-ERK1/2 antibody and anti-ERK-1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 6h, 12h, 18h, 24h after treatment with 10mg/ml PMA or at 1h after treated by 0.1, 0.5 or 1.0μM imatinib.
Statistical analysis
All results are representative of at least three independent experiments. Values were presented as the mean ± SD. One-way analysis of variance was used to compare the means of two or more experimental groups, followed by the Dunnett post hoc test. The difference was considered to be statistically significant as p<0.05.
Results
Spred2 induced erythroid differentiation of NBM CD34+ cells
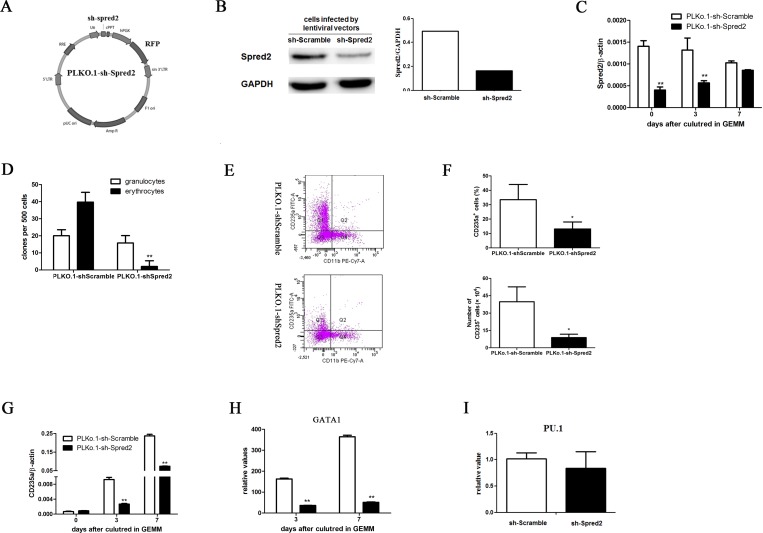
To clarify the roles of Spred2 in erythroid differentiation of normal hematopoietic stem/progenitor cells, the NBM CD34+ cells were transduced with PLKO.1-shSpred2, a lentivirus vector with shRNA specifically targeting Spred2 (Fig. 1A–1B), or PLKO.1-shScramble. Spred2 expression was downregulated during the differentiation in PLKO.1-shScramble transduced NBM CD34+ cells, while PLKO.1-shSpred2 stably silenced Spred2 expression until 7 days after cultured in GEMM medium (Fig. 1C). Our data showed that Spred2 interference inhibited the CFU-E (erythroid colony-forming units) obviously, but not CFU-G (granulocyte colony-forming units) (Fig. 1D). Moreover, CD235a expression could be induced by GEMM differentiation medium in NBM CD34+ cells, while Spred2 silence reduced the CD235a expression obviously (Fig. 1E–1G). Our results also showed that erythroid related transcription factor GATA1 was downregulated by Spred2 silence (Fig. 1H). However, Spred2 interference had little effect on the expression of transcription factor PU.1, which supports myeloid cell lineage differentiation, at day 3 post-infection (Fig. 1I).
Fig 1. Spred2 interference suppressed the eryhtroid differentiation of human normal bone marrow (NBM) CD34+ cells.
The scheme of lentivirus vector with shRNA specifically targeting Spred2, PLKO.1-shSpred2, was shown in A. 48h after transduced with 5 multiplicity of infection (MOI) PLKO.1-shSpred2 or PLKO.1-shScramble, the expression of Spred2 was detected to confirm the interference efficiency by Western-blotting (B) in human NBM CD34+ cells. Human NBM CD34+ cells were infected by PLKO.1-sh-Scramble or PLKO.1-sh-Spred2 at a MOI of 10 and cultured in GEMM medium. At day 0, 3 and 7 post-infection, the expression Spred2 was confirmed by real-time PCR (C), and the expression of CD34, CD235a and CD11b were analyzed by flow cytometer (E-F). Furthermore, the mRNA expression of CD235a (G), GATA1 (H) and PU.1 were detected by real-time RT-PCR. The expression of GATA1 expression was normalized by the expression level at day 0. And, the PU.1 expression at day 3 post-infection was normalized by the expression in PLKO.1-sh-Scramble group (I). PLKO.1-sh-Scramble or PLKO.1-sh-Spred2 transduced CD34+ cells were plated in 24-well plateand cultured in GEMM medium plus 1% methylcellulose for 14 days the presence of colonies (>40 cells) was counted and scored (D). Data are mean±s.d. of three independent experiments. *, p<0.05; **, p<0.01 vs the PLKO.1-sh-Scramble group at the same time point.
Spred2 restoration promoted erythroid differentiation of CML CD34+ cells
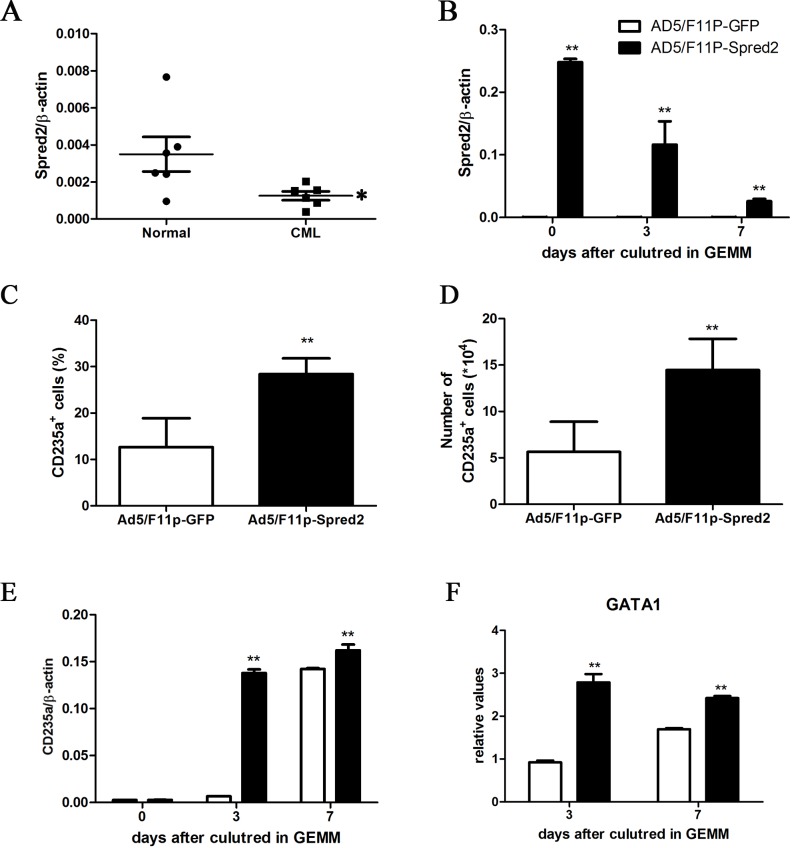
Compared to that in NBM CD34+ cells, the Spred2 expression was lower notably in CML CD34+ cells (Fig. 2A), which was consistent with the impaired differentiation ability of these cells. To clarify the effect of Spred2 restoration on erythroid differentiation of CML cells, the CML CD34+ cells were transduced with Ad5/F11p-Spred2, and assayed for erythorid differentiation ability. As shown in Fig 2B, Ad5/F11p-Spred2 transduction resulted in Spred2 restoration in CML CD34+ cells cultured in GEMM medium for 7 days. Spred2 restoration increased the generation of CD235a+ cells by CML CD34+ cells at 3 day after cultured in GEMM system (Fig. 2C–2D). The mRNA expression of CD235a (Fig. 2E) and GATA1 (Fig. 2F) were also upregulated obviously after Ad5/F11p-Spred2 transduction.
Fig 2. Spred2 induced erythroid differentiation of CML CD34+ cells.
The mRNA expression of Spred2 in CML patient derived CD34+ cells and NBM CD34+ cells was detected by using real-time RT-PCR (A). CML CD34+ cells were transduced with adenoviral vector Ad5/F11p-Spred2 or Ad5/F11p-GFP at a MOI of 150 and cultured in GEMM medium for 7 days, the expression of Spred2 (B), CD235a (E) and GATA1 (F) was detected by using real-time RT-PCR at day 0, 3 and 7. 2×105 transduced cells were cultured in GEMM medium for 3 days, the percentage of CD235a+ cells (C) was detected by flow cytometer and number of generated CD235a+ cells were calculated (D). Data are shown as mean±s.d. of three independent experiments. *, p<0.05, vs NBM CD34+ cells in A. *, p<0.05; **, p<0.01 vs the Ad5/F11p-GFP group at the same time point in B-F.
Spred2 was involved in imatinib induced erythroid differentiation of K562 cells
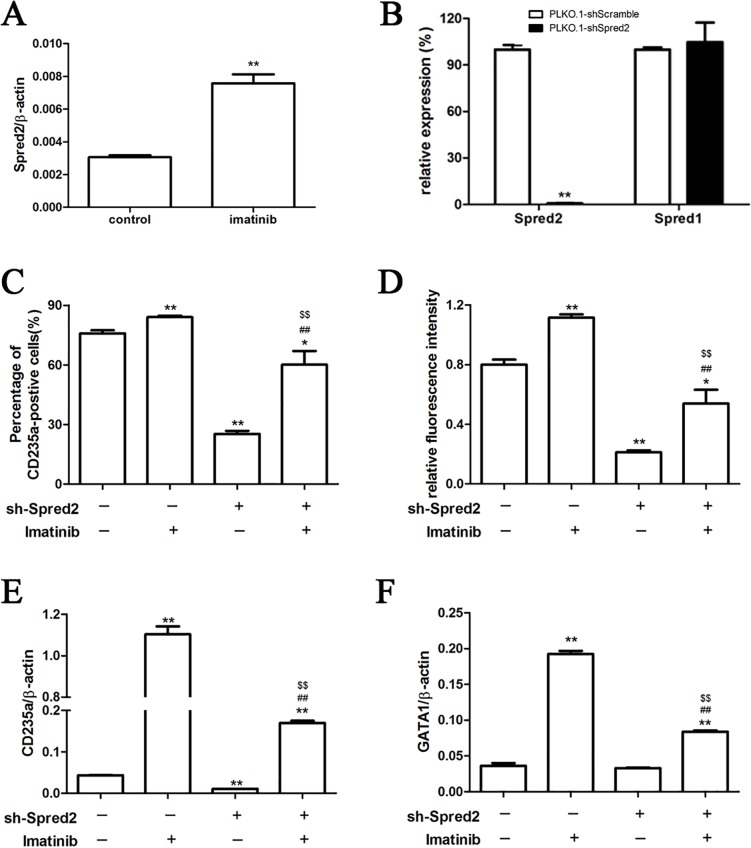
We investigated the influence of imatinib treatment on Spred2 expression of K562 cells using real-time RT-PCR, and the results showed that Spred2 could be induced by imatinib (Fig. 3A). PLKO.1-shSpred2 transduction inhibited Spred2 expression obviously in K562 cells, while had no effect on Spred1 (Fig. 3B). Interestingly, Spred2 interference could partially block imatinib-induced erythroid differentiation of K562 cells. As shown in Fig. 3C–3D, imatinib treatment increased CD235a expression in K562 cells, whereas Spred2 silence downregulated the expression of CD235a both in presence or absence of imatinib. Furthermore, mRNA expression of CD235a (Fig. 3E) and GATA1 (Fig. 3F) was also downregulated in PLKO.1-sh-Spred2 transduced cells. These results indicated that Spred2 was involved in imatinib induced erythroid differentiation of CML cells.
Fig 3. Spred2 was involved in imatinib induced erythroid differentiation.
K562 cells were induced by 1 μM imatinib for 48 hours, and the expression of Spred2 was detected by real-time reverse-transcription polymerase chain reaction (RT-PCR) (A). The K562 cells were transduced with PLKO.1-shSpred2 or PLKO.1-shScramble and cultured for 48 hours. The interference efficiency was confirmed by real-time RT-PCR (B). These transduced cells were cultured in the absence or presence of imatinib at a concentration of 1μM for 48 hours, the percentage of CD235a positive cells and relative fluorescence intensity were detected by flow cytometer (C-D), and the mRNA expression of CD235 (E) and GATA1 (F) were also detected by real-time RT-PCR. Data are shown as mean±s.d. of three independent experiments. *, p<0.05,**, p<0.01 vs the first column; #, p<0.05; ##, p<0.01 vs the second column; $, p<0.05,$$, p<0.01 vs the third column.
Spred2 over-expression enhanced erythroid differentiation induced by imatinib in K562 cells
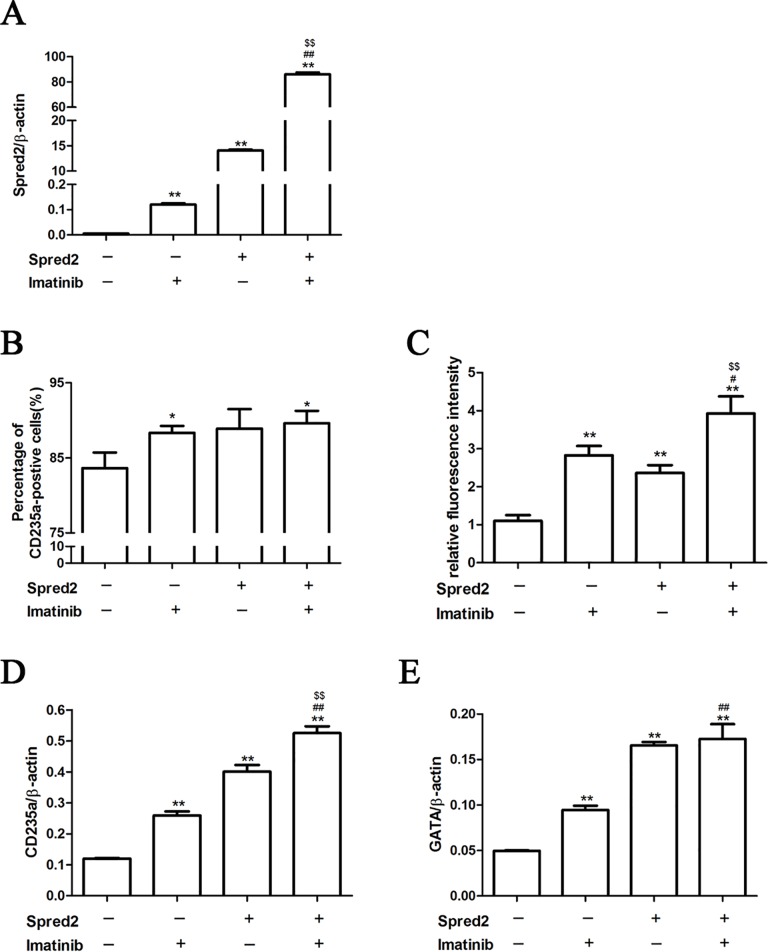
The effects of Spred2 over-expression on erythroid differentiation of K562 cells were also investigated in this study. Ad5/F11p-Spred2 transduction increased Spred2 expression of K562 cells in absence or presence of imatinib (Fig. 4A). Our results showed that imatinib or Spred2 over-expression could increase the CD235a and GATA1 expression, while the combination of imatinib and Spred2 over-expression was much more impressive (Fig. 4B–4E), suggesting the combination might be a potential strategy for CML therapy.
Fig 4. Spred2 over-expression enhanced imatinib-induced erythroid differentiation of K562 cells.
K562 cells were infected by adenoviral vector (Ad5/F11p-Spred2 or Ad5/F11p-GFP) at a MOI of 150 and cultured with or without 1μM imatinib for 48 hours. The mRNA expression of Spred2 (A), CD235a (D) and GATA1 (E) were detected by real-time RT-PCR. The percentage of CD235a positive cells (B) and relative fluorescence intensity (C) were detected by flow cytometer. Data are shown as mean±s.d. of three independent experiments. *, p<0.05, **, p<0.01 vs the first column; #, p<0.05; ##, p<0.01 vs the second column; $, p<0.05, $$, p<0.01 vs the third column.
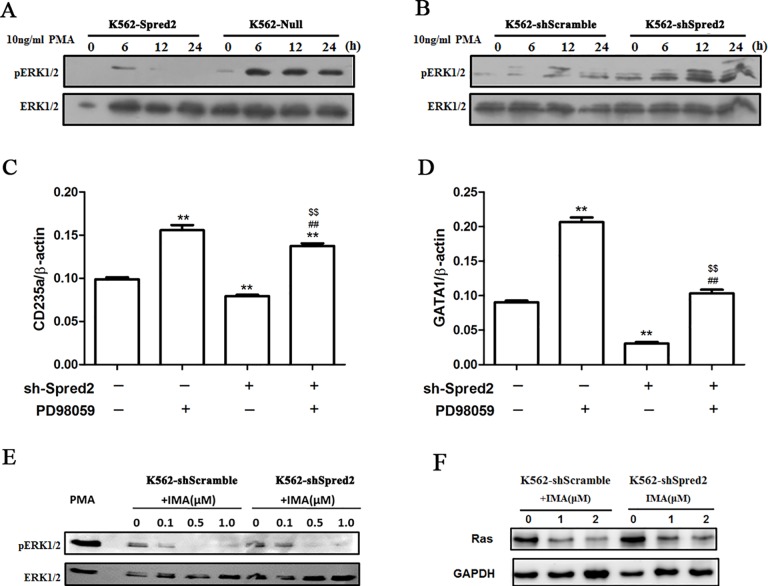
Spred2 regulated erythroid differentiation through targeting ERK signaling in K562 cells
Spred2 mediated inhibition of ERK signaling has been reported in K562 cells. In this study, we demonstrated that Spred2 could inhibit PMA induced ERK phosphorylation, while Spred2 interference enhanced PMA induced activation of ERK signaling (Fig. 5A-B). It has been demonstrated above that Spred2 silence could inhibit erythroid differentiation of K562 cells. However, ERK inhibitor, PD98059, partly restore the erythroid differentiation in PLKO.1-shSpred2 transduced K562 cells, suggesting PLKO.1-shSpred2 inhibit erythroid differentiation partly through ERK signaling (Fig. 5C–5D). Furthermore, we also found that imatinib treatment resulted in inactivation of ERK signaling, while Spred2 silence partly restored ERK phosphorylation in imatinib treated K562 cells (Fig. 5E), indicating that imatinib and Spred2 might synergistically inhibit the ERK signaling to regulate erythroid differentiation of K562 cells. Moreover, Spred-2 knockdown also increases Ras expression in K562 cells treated with imatinib, which is consistent to the changes of ERK signals (Fig. 5F).
Fig 5. Spred2 mediated erythroid differentiation partly through ERK signalling.
Spred2 over-expression cells (K562-Spred2) (A), Spred2 silencing K562 cells (K562-shSpred2) (B) and corresponding control cells (K562-Null and K562-shScramble) were starved deprived of serum overnight and stimulated with 10 ng/ml PMA for the indicated time points. Phosphorylated ERK1/2 and total ERK1/2 were detected by Western blotting. On the other hand, K562-shSpred2 and K562-shScramble cells were treated with imatinib at indicated concentrations for 1 hour, the phosphorylated ERK1/2 and total ERK1/2(E), and Ras (F) were detected. K562 cells were treated by 30μM ERK inhibitor PD98059 1h before lentiviral vector (PLKO.1-sh-Spred2 and PLKO.1-sh-Scramble) transduction. 24h after transduction, the culture medium was replaced by flesh complete culture medium containing 30μM PD98059. Then, the mRNA expression of CD235a and GATA1 were analysed by real-time RT-PCR at 48h post-infection (C and D). Data are shown as mean±s.d. of three independent experiments. *, p<0.05, **, p<0.01 vs the first column; #, p<0.05; ##, p<0.01 vs the second column; $, p<0.05, $$, p<0.01 vs the third column.
Discussion
Spred proteins, a class of selective inhibitors of the Ras-ERK cascade, inhibit cell motility, proliferation, tumor metastasis and Rho-mediated actin reorganization [22–24]. Spred2, a member of Spred proteins, is expressed in the aorta-gonad-mesonephros (AGM) region and functions as a negative regulator in AGM hematopoiesis [25]. In this study, we demonstrated that Spred2 was involved in erythroid differentiation of CML cells induced by imatinib.
Spred2 lies downstream of FoxO3a, which was involved in imatinib-induced cytotoxicity and erythroid differentiation [26–28]. Restored expression of Foxo3a and Spred1 was induced by tyrosine kinase inhibitors, such as imatinib and disatinib [29].Previous reports also showed that Spred2 down-regulation in hematopoietic stem cells of FoxO3a-deficient mice hyper-activated ERK and resulted in hyper-proliferation of neutrophils [30]. We also found the implication of Spred2 in imatinib-induced cell killing of CML cells [17]. However, the role of Spred2 in regulation of erythroid differentiation of CML cells and its mechanisms remain to be fully clarified.
CML is clinically characterized by three phases: an initial chronic phase displaying almost normal myeloid differentiation, followed by an accelerated phase and then the final blast crisis, in which myeloid and lymphoid blasts failed to differentiate and led to abnormal accumulation of immature leukemic blast cells in blood and bone marrow [31]. Our data showed that the expression of Spred2 was down-regulated significantly in CML CD34+ cells, and Spred2 over-expression could restore the ability of erythroid differentiation. These data indicated that Spred2 was involved in differentiation of CML cells and might be a candidate target for CML therapy.
Imatinib could induce both cytotoxicity and erythroid differentiation of CML cells [17,32,33]. We further investigated whether Spred2 was involved in imatinib induced erythroid differentiation in CML cells. K562 cell is a bipotent cell line established from a patient in a blast crisis of chronic myeloid leukemia, it possesses variable capacities of differentiation toward erythroid and megakaryocytic cell lineages. We assayed the effects of imatinib and Spred2 on differentiation potential of CML cells. Our results showed that Spred2 over-expression enhanced the erythroid differentiation induced by imatinib, whereras Spred2 silence partly blocked this process. We also demonstrated that imatinib induced Spred2 expression both in primary CML and K562 cells.
Several reports suggested that blockade of BCR-ABL and downstream Ras-ERK pathway by imatinib, geldanamycin, RNA interference of BCR-ABL, herbimycin A, U0126, butyrateand ara-C caused erythroid differentiation of K562 cells [8,34–38]. Others indicated that inhibition of signaling through ERK in K562 cells might be needed to enter the erythroid differentiation process, while the erythroid differentiation after initiation could be enhanced by both activation and inhibition of ERK signaling depending on inducing compound [36]. Based on the previous data that Spred2 inhibited phosphorylated-ERK (p-ERK) in K562 cells, we further demonstrated that Spred2 interference could partly reverse imatinib induced down-regulation of p-ERK level. Our data also showed that MEK-1 inhibitor, PD98059, not only enhanced the erythroid differentiation in K562 cells, but also reversed PLKO.1-sh-Spred2 induced inhibition of erythroid differentiation, indicating that Spred2 interference blocks erythroid differentiation partly through activation of ERK signaling.
Conclusion
We here demonstrated that Spred2 participated in erythroid differentiation of CML cells. Spred2 was involved in imatinib induced erythroid differentiation partly through inhibition of ERK signaling. These data might provide valuable insights into the mechanisms of differentiation of CML cells and present novel target for developing therapy strategies.
Acknowledgments
The work was supported by Chinese National Basic Research and Development (973 Program) (No.2012CB518205), the National Natural Science Foundation of China (No.81170460), and the National High Technology Research and Development Program of China (863 Program) (Nos.2012AA02A211 and SS2014AA020515).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Chinese National Basic Research and Development (973 Program) (No.2012CB518205), National Natural Science Foundation of China (No.81170460), and National High Technology Research and Development Program of China (863 Program) (Nos.2012AA02A211 and SS2014AA020515). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Marega M, Piazza RG, Pirola A, Redaelli S, Mogavero A, et al. (2010) BCR and BCR-ABL regulation during myeloid differentiation in healthy donors and in chronic phase/blast crisis CML patients. Leukemia 24: 1445–1449. 10.1038/leu.2010.101 [DOI] [PubMed] [Google Scholar]
- 2. Sell S (2005) Leukemia: stem cells, maturation arrest, and differentiation therapy. Stem Cell Rev 1: 197–205. [DOI] [PubMed] [Google Scholar]
- 3. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, et al. (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 4. Jain N, O'Brien S (2013) The frontline treatment of chronic myeloid leukemia in the chronic phase: current clinical decisions and future prospects for treatment. Expert Rev Hematol 6: 575–586. 10.1586/17474086.2013.835697 [DOI] [PubMed] [Google Scholar]
- 5. Hochhaus A, Kantarjian H (2013) The development of dasatinib as a treatment for chronic myeloid leukemia (CML): from initial studies to application in newly diagnosed patients. J Cancer Res Clin Oncol 139: 1971–1984. 10.1007/s00432-013-1488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. La Rosee P, Deininger MW (2010) Resistance to imatinib: mutations and beyond. Semin Hematol 47: 335–343. 10.1053/j.seminhematol.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Zhou M, Qiu HY, He GS, Xu Y, Cen JN, et al. (2013) [Multivariate analysis of imatinib resistance-related factors during the treatment of chronic myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi 34: 395–398. 10.3760/cma.j.issn.0253-2727.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 8. Milojkovic D, Apperley J (2009) Mechanisms of Resistance to Imatinib and Second-Generation Tyrosine Inhibitors in Chronic Myeloid Leukemia. Clin Cancer Res 15: 7519–7527. [DOI] [PubMed] [Google Scholar]
- 9. Jabbour EJ, Cortes JE, Kantarjian HM (2013) Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk 13: 515–529. 10.1016/j.clml.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Q, Harrison JS, Uskokovic M, Kutner A, Studzinski GP (2005) Translational study of vitamin D differentiation therapy of myeloid leukemia: effects of the combination with a p38 MAPK inhibitor and an antioxidant. Leukemia 19: 1812–1817. [DOI] [PubMed] [Google Scholar]
- 11. Meshkini A, Yazdanparast R (2007) Induction of megakaryocytic differentiation in chronic myelogenous leukemia cell K562 by 3-hydrogenkwadaphnin. J Biochem Mol Biol 40: 944–951. [DOI] [PubMed] [Google Scholar]
- 12. Yin L, Ahmad R, Kosugi M, Kawano T, Avigan D, et al. (2010) Terminal differentiation of chronic myelogenous leukemia cells is induced by targeting of the MUC1-C oncoprotein. Cancer Biol Ther 10: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnekenburger M, Grandjenette C, Ghelfi J, Karius T, Foliguet B, et al. (2011) Sustained exposure to the DNA demethylating agent, 2'-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem Pharmacol 81: 364–378. 10.1016/j.bcp.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 14. Gomez-Casares MT, Garcia-Alegria E, Lopez-Jorge CE, Ferrandiz N, Blanco R, et al. (2013) MYC antagonizes the differentiation induced by imatinib in chronic myeloid leukemia cells through downregulation of p27(KIP1.). Oncogene 32: 2239–2246. 10.1038/onc.2012.246 [DOI] [PubMed] [Google Scholar]
- 15. Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, et al. (2003) Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. FASEB J 17: 2160–2162. [DOI] [PubMed] [Google Scholar]
- 16. Miranda MB, Johnson DE (2007) Signal transduction pathways that contribute to myeloid differentiation. Leukemia 21: 1363–1377. [DOI] [PubMed] [Google Scholar]
- 17. Liu XY, Yang YF, Wu CT, Xiao FJ, Zhang QW, et al. (2010) Spred2 is involved in imatinib-induced cytotoxicity in chronic myeloid leukemia cells. Biochem Biophys Res Commun 393: 637–642. 10.1016/j.bbrc.2010.02.044 [DOI] [PubMed] [Google Scholar]
- 18. Yan J, Li QF, Wang LS, Wang H, Xiao FJ, et al. (2012) Methyl-beta-cyclodextrin induces programmed cell death in chronic myeloid leukemia cells and, combined with imatinib, produces a synergistic downregulation of ERK/SPK1 signaling. Anticancer Drugs 23: 22–31. 10.1097/CAD.0b013e32834a099c [DOI] [PubMed] [Google Scholar]
- 19. Zhu J, Li Z, Zhang G, Meng K, Kuang W, et al. (2011) Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3 /AKT signalings. PLoS One 6: e23720 10.1371/journal.pone.0023720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Packer LM, Rana S, Hayward R, O'Hare T, Eide CA, et al. (2011) Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell 20: 715–727. 10.1016/j.ccr.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, et al. (2001) Spred is a Sprouty-related suppressor of Ras signalling. Nature 412: 647–651. [DOI] [PubMed] [Google Scholar]
- 22. Miyoshi K, Wakioka T, Nishinakamura H, Kamio M, Yang L, et al. (2004) The Sprouty-related protein, Spred, inhibits cell motility, metastasis, and Rho-mediated actin reorganization. Oncogene 23: 5567–5576. [DOI] [PubMed] [Google Scholar]
- 23. Murphy T, Hori S, Sewell J, Gnanapragasam VJ (2010) Expression and functional role of negative signalling regulators in tumour development and progression. Int J Cancer 127: 2491–2499. 10.1002/ijc.25542 [DOI] [PubMed] [Google Scholar]
- 24. Yoshida T, Hisamoto T, Akiba J, Koga H, Nakamura K, et al. (2006) Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene 25: 6056–6066. [DOI] [PubMed] [Google Scholar]
- 25. Nobuhisa I, Kato R, Inoue H, Takizawa M, Okita K, et al. (2004) Spred-2 suppresses aorta-gonad-mesonephros hematopoiesis by inhibiting MAP kinase activation. J Exp Med 199: 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, et al. (2005) Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24: 2317–2329. [DOI] [PubMed] [Google Scholar]
- 27. Corrado P, Mancini M, Brusa G, Petta S, Martinelli G, et al. (2009) Acetylation of FOXO3a transcription factor in response to imatinib of chronic myeloid leukemia. Leukemia 23: 405–406. 10.1038/leu.2008.186 [DOI] [PubMed] [Google Scholar]
- 28. Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, et al. (2010) TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463: 676–680. 10.1038/nature08734 [DOI] [PubMed] [Google Scholar]
- 29. Birkenkamp KU, Essafi A, van der Vos KE, da Costa M, Hui RC, et al. (2007) FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem 282: 2211–2220. [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, et al. (2008) FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood 112: 4485–4493. 10.1182/blood-2008-05-159848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dorsey JF, Cunnick JM, Mane SM, Wu J (2002) Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood 99: 1388–1397. [DOI] [PubMed] [Google Scholar]
- 32. Kuzelova K, Grebenova D, Marinov I, Hrkal Z (2005) Fast apoptosis and erythroid differentiation induced by imatinib mesylate in JURL-MK1 cells. J Cell Biochem 95: 268–280. [DOI] [PubMed] [Google Scholar]
- 33. Lee YL, Chen CW, Liu FH, Huang YW, Huang HM (2013) Aclacinomycin A sensitizes K562 chronic myeloid leukemia cells to imatinib through p38MAPK-mediated erythroid differentiation. PLoS One 8: e61939 10.1371/journal.pone.0061939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawano T, Horiguchi-Yamada J, Iwase S, Furukawa Y, Kano Y, et al. (2004) Inactivation of ERK accelerates erythroid differentiation of K562 cells induced by herbimycin A and STI571 while activation of MEK1 interferes with it. Mol Cell Biochem 258: 25–33. [DOI] [PubMed] [Google Scholar]
- 35. Ida C, Ogata S, Okumura K, Taguchi H (2009) Induction of differentiation in k562 cell line by nicotinic acid-related compounds. Biosci Biotechnol Biochem 73: 79–84. [DOI] [PubMed] [Google Scholar]
- 36. Woessmann W, Zwanzger D, Borkhardt A (2004) ERK signaling pathway is differentially involved in erythroid differentiation of K562 cells depending on time and the inducing agent. Cell Biol Int 28: 403–410. [DOI] [PubMed] [Google Scholar]
- 37. Radujkovic A, Schad M, Topaly J, Veldwijk MR, Laufs S, et al. (2005) Synergistic activity of imatinib and 17-AAG in imatinib-resistant CML cells overexpressing BCR-ABL—Inhibition of P-glycoprotein function by 17-AAG. Leukemia 19: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 38. Schuster C, Forster K, Dierks H, Elsasser A, Behre G, et al. (2003) The effects of Bcr-Abl on C/EBP transcription-factor regulation and neutrophilic differentiation are reversed by the Abl kinase inhibitor imatinib mesylate. Blood 101: 655–663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.