Abstract
Background
Helping alcohol dependent individuals to cope with, or regulate, cue-induced craving using cognitive strategies is a therapeutic goal of cognitive behavioral therapy (CBT) for alcohol dependence. An assumption that underlies this approach is that alcohol dependence is associated with deficits in such cognitive regulation abilities. To date, however, the ability to utilize such strategies for regulation of craving has never been tested in a laboratory setting.
Methods
Here we compared 19 non-treatment-seeking, alcohol dependent drinkers (AD) to 21 social drinkers (SD), using a laboratory task that measured the ability to reduce cue-induced alcohol craving by thinking about long-term negative consequences of drinking, which is a specific cognitive regulation strategy that is taught in CBT. The task also assessed the ability to reduce food craving elicited by high-calorie food cues using a similar strategy.
Results
The reduction in craving when using this cognitive regulation strategy was approximately double in SD, compared to AD, for both alcohol and food cues. Furthermore, in SD but not AD, the ability to regulate cue-induced alcohol craving was correlated with the ability to regulate food craving. There were no significant correlations found between the ability to regulate cue-induced alcohol craving and a number of self-report measures related to severity of alcohol dependence, baseline craving, impulsivity and general self-regulation ability, for either AD or SD.
Conclusions
The results suggest that alcohol dependence is associated with deficits in cognitive regulation of cue-induced craving, and that these deficits are not specific to the regulation of alcohol craving, but generalize to the regulation of other appetitive states, such as food craving. Future studies may use similar procedures to address the neural and cognitive processes that underlie such regulation deficits, as well as the effects of treatments such as CBT on these processes.
Keywords: Craving, self-regulation, coping skills, cue-exposure, cognitive behavioral therapy
Introduction
A hallmark of alcohol dependence is craving, or the strong subjective desire to drink (A.P.A., 2013). Cognitive behavioral therapies (CBT) for alcohol dependence, such as Cognitive Behavioral Coping Skills Therapy and Relapse Prevention Therapy, teach alcohol dependent individuals to “cope” with craving when faced with temptations to drink by using a variety of cognitive strategies. Such strategies include “challenging and changing” thoughts about alcohol by focusing on long-term, negative consequences of drinking instead of short-term, pleasurable consequences (Kadden, 2007). This is part of a broader approach employed by CBT, aimed at teaching strategies for self-regulating affective states that promote relapse, such as craving and negative affect. According to the theoretical framework underlying CBT for addiction disorders (Larimer et al., 1999), alcohol dependent individuals possess deficits in their intrinsic coping ability, and use alcohol as a maladaptive coping strategy to compensate for these deficits. In keeping with this account, deficits in coping ability have been identified in alcohol dependent individuals using self-report scales (Berking et al., 2011). However, attempts to relate the mechanism of CBT to the remediation of self-reported coping skills deficits have been largely unsuccessful (Morgenstern and Longabaugh, 2000). This may be due to limitations inherent in clinical self-report scales, such as recall bias and poor reliability, as well as a lack of specificity vis-à-vis the psychological processes that underlie a complex construct such as coping ability (Morgenstern et al., 2013). Laboratory measures allow for a finer-grained, real-time parsing of the multiple cognitive, affective, motivational and, ultimately, neural processes that underlie coping, how these processes are disrupted by alcohol dependence, and their role in treatment (Ochsner, 2008, Ochsner et al., 2012).
Cue-induced craving is a specific form of craving triggered by stimuli that have been previously associated with alcohol (Niaura, 2000). Cue-induced craving occurs in “high-risk” situations where relapse tends to occur, such as social settings where alcohol is being consumed, and is thus a focus of coping skills training in CBT for addictions (Kadden, 2007). Although the specific role of cue-induced craving in compulsive drinking and relapse is a matter of debate (Tiffany and Conklin, 2000), cue-induced craving is a widely studied laboratory phenomenon, associated with a well-characterized set of subjective, behavioral, autonomic and neural responses (Carter and Tiffany, 1999, Schacht et al., 2013). While previous laboratory studies have addressed how cue-induced alcohol craving is modulated by various psychological factors, such as opportunities to drink (Wilson et al., 2004), expectancies of alcohol’s hedonic effects (Carter, 2006) and mood (Rubonis et al., 1994), no laboratory study yet has examined how cue-induced alcohol craving is affected by the utilization of cognitive coping strategies.
Coping with cue-induced alcohol craving using cognitive strategies, such as thinking about long-term, negative consequences of drinking instead of short-term, positive consequences, is a form of cognitive emotion regulation. Specifically, an emotionally charged stimulus (an alcohol cue) is thought about in a way that changes its semantic associations (pleasure, enjoyment, relaxation vs. illness, conflict, depression), altering its emotional impact (the incentive value alcohol), leading to a reduction in the level of felt emotion (subjective craving). A large number of functional neuroimaging studies have provided converging evidence that cognitive regulation of negative emotions, such as fear, sadness and disgust, increases activity in prefrontal cortical regions involved in various aspects of cognitive control, leading to a down-modulation of activity in subcortical regions involved in triggering affective and motivational states, which in turn leads to a reduction in emotional response (reviewed in Ochsner et al., 2012). This prefrontal circuitry has also been shown to play a role in the cognitive regulation of appetitive emotions, including cue-induced cigarette craving (Kober et al., 2010b), cue-induced cocaine craving (Volkow et al., 2010), and cue-induced food craving (Yokum and Stice, 2013). Neuropsychological and functional neuroimaging studies have shown that alcohol dependence is associated with abnormalities in this circuitry that are correlated with impairments in fundamental self-regulatory processes that may underlie cognitive emotion regulation in general and cognitive regulation of craving specifically. These include conflict monitoring (Field et al., 2007), response inhibition (Lawrence et al., 2009, Bjork et al., 2004), reward learning (Sjoerds et al., 2013), delayed discounting (Field et al., 2007) and reward-based decision-making (Xiao et al., 2013). Thus, if the ability to cognitively regulate cue-induced alcohol craving is dependent upon the integrity of this neural circuitry, then it is likely that alcohol dependence is associated with impairments in this ability.
In this study, we employed a laboratory task that indexed subjects’ ability to reduce cue-induced alcohol craving by thinking about negative consequences of drinking, which is a specific coping/cognitive regulation strategy that is taught in CBT for alcohol dependence. The task was adapted from previous studies in cigarette smokers examining their ability to cognitively regulate cue-induced cigarette craving (Kober et al., 2010a, Kober et al., 2010b). We compared performance on this task between a group of alcohol dependent drinkers (AD) and a group of social drinkers (SD) who were matched on a number of demographic variables. The task included high-calorie food cues in addition to alcohol cues, in order to control for general regulation processes that modulate all appetitive emotional states, not just those that regulate cue-induced alcohol craving. We addressed three hypotheses: (1) thinking about long-term, negative consequences of drinking will reduce cue-induced alcohol craving; (2) AD will be relatively impaired in this form of cognitive regulation of craving, compared to SD; and (3) this impairment will be specific to alcohol cues, i.e. it will not affect regulation of cue-induced food craving. In order to understand the sources of variability in regulation ability among AD, we also performed exploratory analyses examining the correlations between individual differences in the ability to cognitively regulate alcohol craving and self-report measures of heavy drinking, alcohol dependence severity, baseline alcohol craving, impulsivity, and self-regulation.
Materials and Methods
Participants
Nineteen individuals with alcohol dependence (AD) and 21 social drinkers (SD) were recruited as follows: Advertisements were posted in the local community inviting “individuals who drink alcohol” to undergo “tests of decision-making.” Participants underwent brief telephone screening, in order to ascertain basic demographics and the quantity of drinking. Treatment seekers were excluded. For recruitment purposes, participants were initially categorized as heavy drinkers (>14/24 drinks per week for women/men) or light drinkers (<15/25 drinks per week for women/men) and invited to participate based upon their age, sex and education level in order to facilitate matching between groups. After providing informed consent, participants underwent the Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM; Robins et al., 1988). Participants were then categorized as either AD or SD using the results of the CIDI-SAM. Specifically, AD were defined as participants meeting DSM-IV criteria for alcohol dependence during the last year, according to the CIDI-SAM, while SD were defined as participants not meeting DSM-IV criteria for either alcohol dependence or alcohol abuse, irrespective of the quantity of alcohol consumed. Participants with other DSM-IV substance use disorders, with the exception of nicotine dependence, were excluded using the CIDI-SAM. Participants with psychotic and bipolar disorders were excluded using the Structured Clinical Interview for DSM Disorders (SCID; First et al., 1997). Participants with severe current depression or anxiety were excluded using the Beck Depression Inventory (BDI; Beck et al., 1961; score>29) and the Beck Anxiety Inventory (BAI; Beck et al., 1988; score>29), respectively. Participants were excluded if they were cognitively impaired according to the Folstein Mini-Mental State Exam (MMSE; Folstein et al., 1975; score<24). Participants were excluded if they were intoxicated or were in moderate to severe alcohol withdrawal, based on breath alcohol test (estimated blood alcohol level > 0.05) and the Clinical Institute for Withdrawal from Alcohol Assessment-Revised (CIWA-Ar; Sullivan et al., 1989; score>9), respectively. Participants were also excluded if they were currently taking any psychoactive medications; had a history of severe medical complications of alcohol dependence (e.g. cirrhosis, withdrawal seizures or delirium tremens); had a history of violence or suicide attempts, psychiatric hospitalization within the last year; or were homeless.
Baseline Assessments
All participants were administered the 90-day Timeline Followback Interview (TLFB; Sobell et al., 1979), from which were derived the number of standard drinks per week (DPW) and the percent of heavy drinking days (%HDD, calculated as percent of days consuming > 5/4 drinks for men/ women over 90 days). They also completed the Alcohol Dependence Severity Scale (past 12 months) (ADS; Skinner and Allen, 1982); the Obsessive Compulsive Drinking Scale (previous 30 days) (OCDS; Anton et al., 1995); the Barratt Impulsiveness Scale (current state) (BIS; Patton et al., 1995); the Short Self-Regulation Questionnaire (current state) (SSRQ; Carey et al., 2004); and the Alcohol Urge Questionnaire (current state) (Bohn et al., 1995).
Regulation of Craving Task
All participants completed the Regulation of Craving (ROC) task. In this task, participants were presented 50 pictures of alcoholic beverages and 50 pictures of high calorie foods in pseudorandom order. All pictures were obtained from a high-quality stock photo website (123rf.com), and had previously been rated as eliciting moderate to high levels of craving in an earlier pilot experiment in AD (unpublished data). None of the pictures contained people, as the presence of people had been shown in this same pilot experiment to result in lower craving levels. Each trial began with a fixation cross (4.5 sec), followed by a regulation instruction (NOW or LATER; 2 sec), followed by the cue picture (ALCOHOL or FOOD; 6 sec), followed by a fixation cross (3.5 sec), followed by an instruction to rate their craving “How much do you want this item?” on the computer keyboard using a 1–5 Likert scale (rating period terminated by key press, maximum 6 sec duration). The order of the stimuli and the regulation conditions were randomized, with approximately equal numbers of 4 trial types: NOW/ALCOHOL; LATER/ALCOHOL; NOW/FOOD; LATER/FOOD. The task was programmed and presented on a laptop computer using E-Prime software (Psychology Software Tools, Sharpsburg, PA).
Prior to the task, participants were informed that the study was examining the way in which thinking about positive and negative consequences can change the desire to consume alcohol and food, respectively. They were then asked to describe some long-term, negative consequences and short-term, pleasurable consequences of consuming alcohol and high-calorie foods, respectively. They were then instructed on the ROC task, including being told what to do when they saw the “LATER” and “NOW” cues. Specifically, there were instructed that on trials when they received the LATER instruction, they were to recall long-term, negative consequences of repeatedly consuming the depicted item (alcohol or high-calorie food, respectively); on trials where they received the NOW instruction, they were to recall the immediate, pleasurable consequences of consuming the depicted item right now. They were not instructed to reduce or increase their craving for any of the conditions, i.e. regulation of craving was not an explicit goal of the task. They then completed 4 practice trials, during which they were asked to verbalize the LATER and NOW strategies for each trial to confirm that they understood the task instructions. The total time to complete the ROC task was approximately 20 minutes. Following the completion of the task, participants were debriefed to confirm that they continued to understand the task instructions throughout the entire task.
Data Analysis
Demographic variables (age, sex, education), measures of the heavy drinking (DPW and %HDD), and baseline self-report psychological measures (the MMSE, the BIS, the ADS, AUQ, OCDS and SSRQ) were collected. The OCDS subscales for Resistance, Obsession and Interference (Roberts et al., 1999) were also calculated. All of these variables were compared between AD and SD groups using t-tests for normally distributed variables, or Mann-Whitney U tests for non-normally distributed variables. In order to address the potential effects of using heavy drinking status for recruitment/matching purposes, we also calculated the number of participants in the AD and SD groups who were initially classified as light drinkers or heavy drinkers, respectively.
For each subject, the craving rating made after each trial was averaged across all of the trials of the same type (4 trial types: NOW/ALCOHOL; LATER/ALCOHOL; NOW/FOOD; LATER/FOOD). A mixed effects model was used to examine within-subject effects of cue type (ALCOHOL vs. FOOD) and regulation instruction (LATER vs. NOW), between-subject effects of group (AD vs. SD), and all 2- and 3-way interactions among within- and between-subject effects. Baseline BAI and BDI were included as control variables in the mixed effects model. The 3-way interaction provided a test of whether AD vs. SD differed in the contrast between how they regulated their alcohol cravings vs. how they regulated food cravings (i.e. the specificity of regulation deficits in AD vs. SD to alcohol craving). When the 3-way interaction was found not to be significant, a reduced model including only the 2-way group (AD vs. SD) by regulation (NOW vs. LATER) interaction in the model was re-run, which provided a test of whether AD vs. SD differed in how they regulated their cravings, regardless of cue-type. Post-hoc t-tests were used to test for significant differences in self-reported craving within each group between the NOW and LATER conditions, collapsed across FOOD and ALCOHOL cues. Similar post-hoc tests were conducted to test for significant differences in self-reported craving within each group between alcohol and food cues, collapsed across the NOW and LATER conditions. All significance levels were Bonferroni-adjusted for multiple comparisons. Effect sizes (ES) were also estimated using Cohen’s d, correcting for the correlation between repeated measures (Morris and DeShon, 2002).
For each subject, a measure of regulation success was calculated by subtracting the average rating for the LATER trials from the average rating for the NOW trials. This was done separately for ALCOHOL and FOOD cues. Correlations were performed to examine relationships between individual differences in regulation ability for ALCOHOL cues and individual differences in regulation ability for FOOD cues. This was done separately for AD and SD. A regression of regulation ability for food on regulation ability for alcohol, group, and their interaction was performed to test for differential associations between regulation abilities by group. Additionally, correlations between regulation ability and measures of heavy drinking (DPW, %HDD) and baseline self-report measures (BIS, ADS, AUQ, OCDS and SSRQ) were performed separately for ALCOHOL cues only. These correlations were done separately for AD and SD. Pearson correlation or Spearman correlation were used, depending on whether the variables were normally or non-normally distributed, respectively. Alpha levels were Bonferroni-adjusted for multiple correlations.
Results
Comparing AD and SD on demographic and baseline measures
Table 1 shows that AD and SD were not significantly different with respect to sex, age and level of education. Table 2 shows that AD had significantly higher DPW and %HDD, higher scores on the ADS and AUQ, as well as the BDI. There was a trend toward a higher BAI score in AD compared to SD, but its significance did not survive correction for multiple comparisons. There were no differences between AD and SD on the MMSE, BIS, the OCDS (including subscales) and the SSRQ Four out of 19 participants in the AD group were light drinkers according to the criteria used during recruitment/telephone screening (less than 15/25 drinks per week for women/men), while 1 out of the 21 participants in the SD group were heavy drinkers according to these criteria (more than 14/24 drinks per week for women/men).
Table 1.
Demographic variables, comparing alcohol dependent (AD) and social drinkers (SD).
| Variable | AD (N=19) | SD (N=21) | p-value |
|---|---|---|---|
| N male (%) | 12(63) | 16(76) | 0.49 (Χ2) |
| Age | 36.7±9.1 | 34.7±9.5 | 0.55 |
| Education completed | 0.73 (Χ2) | ||
| Grade school | 0 | 1 | |
| High school | 11 | 10 | |
| College | 5 | 7 | |
| Graduate school | 3 | 3 |
Age compared using Mann-Whitney U test. Frequencies compared using Χ2 test. No significant differences were found between groups. Significance levels not adjusted for multiple comparisons.
Table 2.
Baseline clinical and psychological variables in alcohol dependent (AD) and social drinkers (SD).
| Variable | AD (N=19) | SD (N=21) | p-value |
|---|---|---|---|
| DPW | 35.7±14.4 | 12.5±13.1 | <0.001* |
| %HDD | 35.8±20.2 | 13.81±22.3 | <0.001* |
| ADS | 10.7±6.1 | 4.2±2.8 | <0.001* |
| AUQ | 30.0±10.6 | 22.3±4.38 | <0.01* |
| OCDS (total) | 18.47±15.09 | 18.76±19.55 | 0.10 |
| Obsession factor | 4.42±2.92 | 4.04±3.77 | 0.73 |
| Interference factor | 1.89±4.35 | 3.00±4.34 | 0.37 |
| Resistance factor | 1.93±1.57 | 1.91±1.95 | 0.97 |
| BIS | 33.4±8.5 | 35.2±7.5 | 0.197 |
| SSRQ | 99.79±9.40 | 98.71±8.08 | 0.75 |
| BDI | 14.1±12.3 | 5.4±7.3 | <0.01* |
| BAI | 9.2±11.1 | 3.8±6.0 | 0.014 |
| MMSE | 28.7±1.4 | 29.2±1.0 | 0.254 |
Shown are group means ± standard deviations for AD and SD.
Significant difference between SD and AD, adjusting alpha-levels for multiple comparisons.
DPW=drinks per week; %HDD=percent heavy drinking days; ADS=Alcohol Dependence Scale; AUQ=Alcohol Urge Questionnaire; OCDS=Obsessive Compulsive Drinking Scale; BIS=Barratt Impulsivity Scale; SSRQ=Short Self-Regulation Questionnaire; BDI=Beck Depression Inventory; BAI=Beck Anxiety Inventory; MMSE=Folstein Mini Mental State Examination.
Effects of cue-type, regulation instruction, and group on cue-induced craving
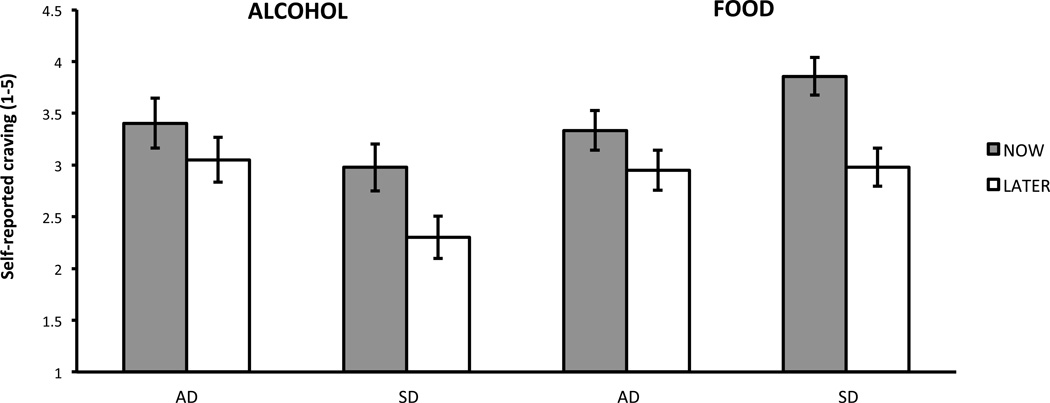
There was a significant main effect of regulation instruction, such that, collapsing across AD and SD groups as well as across alcohol and food cues, craving was significantly higher for NOW instructions than for LATER instructions [F(1,36)=26.0, p<0.0001, ES=0.72]. There was also a significant main effect of cue-type, such that, collapsing across AD and SD groups as well as across NOW and LATER instructions, craving was significantly higher for food cues than for alcohol cues [F(1,36)=5.51, p=0.025, ES=0.35]. There was no significant main effect of group [F(1,36)=0.14, p=0.71, ES=0.09]. There was not a significant 3-way interaction between group, instruction and cue-type [F(1,36)=0.71, p=0.404] and so a reduced model including only the significant 2-way interactions was run. The reduced model found a trend toward a significant interaction effect between instruction and group [F(1,36)=3.55, p=0.068)]. After collapsing across cue type, a post-hoc t-test showed that the significant effect of regulation instruction in SD [t=5.14, p<.0001, ES=1.00] was more than twice as large as the non-significant (after correcting for multiple comparisons) effect of regulation instruction in AD [t=2.29, p=0.028, ES=0.47]. There was a significant interaction effect between cue-type and group on craving [F(1,36)=7.81, p=0.008]. After collapsing across regulation instructions, a post-hoc t-test showed that craving was significantly higher for food than for alcohol in SD [t=3.60, p=0.001, ES=0.69], but did not differ significantly between food and alcohol for AD [t=0.48, p=0.64, ES=-0.10] (Figure 1).
Figure 1.
Correlations between regulation success and baseline measures
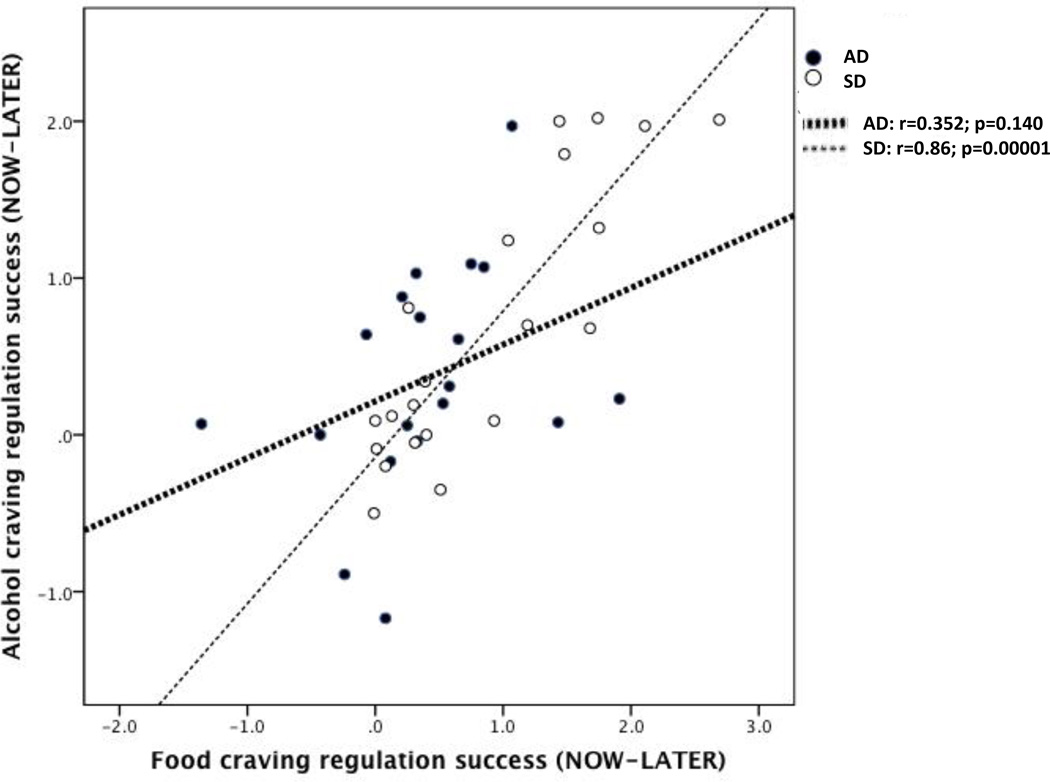
For SD, there was a significant and strong positive correlation between regulation success for ALCOHOL and regulation success for FOOD (r=0.86, p=0.00001). For AD, there was no significant correlation between regulation success for ALCOHOL and regulation success for FOOD (r=0.35, p=0.14) (Figure 2). There was a trend toward a significant difference between these correlations (t=1.88, p=0.069) based on the regression testing the interaction of cue type regulation success by group. There were no significant correlations found for either AD or SD between regulation success for alcohol cues and any of the baseline self-report measures, including DPW, %HDD, SSRQ, ADS, OCDS, AUQ and BIS (Table 3).
Figure 2.
Table 3.
Correlations between ability to regulate cue-induced alcohol craving (NOW-LATER) and baseline psychological variables in alcohol dependent (AD) and social drinkers (SD).
| AD | SD | |||
|---|---|---|---|---|
| Variable | Correlation | p-value | Correlation | p-value |
| DPW | r=−0.35 | 0.14 | ρ=0.03 | 0.88 |
| %HDD | r=−0.01 | 0.99 | ρ=−0.01 | 0.96 |
| SSRQ | ρ=0.31 | 0.20 | ρ=−0.03 | 0.90 |
| ADS | ρ=−0.23 | 0.35 | ρ=−0.16 | 0.50 |
| OCDS | ρ=0.10 | 0.68 | ρ=0.17 | 0.45 |
| AUQ | r=0.31 | 0.20 | ρ=−0.13 | 0.59 |
| BIS | ρ=0.23 | 0.34 | ρ=−0.26 | 0.26 |
Pearson’s r reported for parametric data; Spearman’s ρ reported for non-parametric data.
DPW=drinks per week; %HDD=percent heavy drinking days; SSRQ=Short Self-Regulation Questionnaire ADS=Alcohol Dependence Scale; OCDS=Obsessive Compulsive Drinking Scale; AUQ=Alcohol Urge Questionnaire; BIS=Barratt Impulsivity Scale.
No significant correlations were found.
Discussion
Here we found support for the hypothesis that the cognitive regulation strategy of focusing on long-term negative consequences reduces self-reported cue-induced alcohol and food craving, i.e. there was a significant main effect of regulation instruction, with craving during the LATER condition lower than during the NOW condition for both alcohol and food cues. While SD showed strong effects of this regulation strategy, AD showed relatively weak effects, with the difference in regulation ability between SD and AD, measured as the interaction between group and regulation instruction, trending toward statistical significance. This provides limited support for the hypothesis that AD are impaired in cognitively regulating craving, compared to SD. Contrary to our initial hypothesis, we found that these relative impairments in regulating craving in AD were not specific to alcohol cues, i.e. there was not a significant 3-way interaction between regulation instruction, group and cue-type. Furthermore, we failed to find any correlations between individual differences in the ability to regulate cue-induced alcohol craving and any of the baseline self-report measures of alcohol dependence severity or self-regulation ability, in either AD or SD.
Overall the results are consistent with the social learning theory that underlies CBT, which posits that alcohol dependent individuals possess deficits in a variety of coping/regulation abilities, including the ability to self-regulate craving (Larimer et al., 1999), although our results show only a strong trend-level effect to support this model. With a larger sample size, it is likely that this effect would be significant, and would support the existence of deficits in regulation of craving in AD vs. SD. Similar studies in cigarette smokers using the same regulation of craving paradigm (Kober et al., 2010a) have shown that heavy smokers were no less able to cognitively regulate cue-induced cigarette craving than occasional smokers (“chippers”). Our results suggest, at a trend level, that craving regulation deficits may be more severe in alcohol dependence than in nicotine dependence.
Contrary to our initial hypothesis, the deficits in cognitive regulation of craving in AD compared to SD were not specific to alcohol cues, but generalized to food cues as well. Deficits in reward-related self-regulatory processes that have been previously been shown in AD, such as conflict monitoring (Field et al., 2007), response inhibition (Lawrence et al., 2009, Bjork et al., 2004), reward learning (Sjoerds et al., 2013), delayed discounting (Field et al., 2007) and reward-based decision-making (Xiao et al., 2013), were mostly found using experimental tasks utilizing monetary rewards, not alcohol. This is consistent with AD being associated with a broad inability to regulate appetitive motivational states, as opposed to a specific inability to regulate alcohol-related motivational states. Another possibility is that, in the regulation of craving task, AD may have a tendency to generate negative consequences that are less salient overall, leading to less apparent regulation ability for both alcohol and food craving.
In SD, the high degree of correlation between the ability to cognitively regulate cue-induced alcohol craving and cue-induced food craving, respectively, suggests that, in this group, some common factor underlies both forms of regulation. It is plausible that this common factor functions to regulate all appetitive emotional states, including food craving as well as craving for alcohol. In AD, we failed to find a significant correlation between the ability to regulate alcohol and food craving, respectively. This suggests that, in AD, regulation ability for food cues and alcohol cues, respectively, are determined by a different set of factors. We set out to uncover these factors by examining the correlations between the ability to cognitively regulate cue-induced alcohol craving and a number of self-report measures of heavy drinking, severity of alcohol dependence, trait impulsivity, self-reported self-regulation ability, baseline alcohol craving, and obsessive thoughts about alcohol, but found no significant relationships between regulation ability and any of these measures. The strongest relationship was found between cognitive regulation ability and heavy drinking (DPW), with a negative correlation of −0.35 (p=0.14). With a larger sample size, it is possible that this would have been a significant negative correlation, which would suggest that heavier drinking is associated with greater impairments in cognitive regulation of cue-induced alcohol craving.
Interestingly, a number of these self-report measures that we collected at baseline (the BIS, SSRQ, OCDS) did not differ between AD and SD, even though these groups did differ in regulation ability in the current task. Therefore, it is not surprising that these self-report measures did not correlate with regulation ability. This could also explain the relatively low level of cue-induced craving in the AD group for the NOW condition; the AD patients in our sample may have attributed a relatively low level of incentive salience to alcohol cues, compared to a more severe population, for example. Furthermore, the absence of difference on the SSRQ (a self-report measure of general self-regulation ability) contrasts with our finding of impaired regulation of craving. This may be due to this particular self-report measure of self-regulation ability being less sensitive to deficits in AD, compared to the experimental measure that we utilized. Also, this task is a specific measure of regulation of craving, whereas the SSRQ addresses self-regulation more broadly, including domains that may not be impaired in AD.
This study possesses a number of limitations that may affect the interpretation of the results, as well as their generalizability and clinical applicability. The study was small, which reduced the power to detect some of the interaction effects, which were evident in large differences in regulation effect sizes between AD and SD that only trended toward significance. We included only non-treatment-seeking AD. This limits the ability to apply these results to treatment-seekers, whose level of drinking may be higher, and who are likely to have a higher motivation to regulate cravings at baseline. We also excluded participants with severe depression and anxiety and other substance use disorders, all of which may limit generalizability, as these disorders are highly comorbid with alcohol dependence. Since we did not measure participants’ body mass index (BMI), we were unable to account for the potential confound of differences in regulation ability between AD and SD for food cues that were due to differences in BMI.
The regulation of craving task used in this study has a number of limitations. While similar to a specific coping/regulation skill taught in CBT (“challenging and changing” thoughts about alcohol), the ROC task differs in that subjects were not instructed to have an explicit goal of reducing craving, as they are in CBT. This may limit the ability to use the results of this study to directly infer CBT mechanisms, since CBT teaches patients to have an explicit goal of regulating craving, in addition to providing them with strategies for going about this regulation. Additionally, the way the ROC task was designed did not allow us to determine whether the key cognitive factor that caused a reduction in cue-induced craving was thinking about negative consequences per se vs. thinking about long-term consequences. This could be addressed by experiments that include conditions where subjects are instructed to think about long-term, positive consequences and short-term, negative consequences. Furthermore, we only addressed one specific cognitive coping/regulation skill, limiting the ability to make inferences about broad deficits in coping ability. Additionally, the experimental task was only 20 minutes long, which may have limited the sensitivity to detect differences between AD and SD.
CBT and related treatments are believed to change drinking behavior by improving cognitive regulation of craving and other coping skills (Larimer et al., 1999), though this has been difficult to prove (Morgenstern and Longabaugh, 2000). To examine whether coping skills acquisition is an active ingredient in the therapeutic mechanism of CBT, future studies may use the ROC task to track changes in regulation ability from pre- to post- CBT, and to relate these to clinical outcomes of CBT. Furthermore, the ROC task could be adapted for functional neuroimaging experiments designed to uncover the neural systems that govern the regulation of cue-induced alcohol craving, how the functioning of these processes differ between AD and SD, and how treatments that are targeted at improving coping skills, such as CBT, can remediate the functioning of these neural systems.
Acknowledgments
This research was supported by NIAAA U13 A01779801 (J.M.), NIAAA K23 AA022771 (N.H.N.) and the Leon Levy Foundation (N.
Contributor Information
Nasir H. Naqvi, Columbia University, Department of Psychiatry
Kevin N. Ochsner, Columbia University, Department of Psychology
Hedy Kober, Yale University, Department of Psychiatry
Alexis Kuerbis, Columbia University, Department of Psychiatry
Tianshu Feng, Columbia University, Department of Psychiatry
Melanie Wall, Columbia University, Department of Psychiatry
Jon Morgenstern, Columbia University, Department of Psychiatry
References
- A.P.A. Diagnostic and Statistical Manual of Mental Disorders : DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism, clinical and experimental research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. Journal of consulting and clinical psychology. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism, clinical and experimental research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Carey KB, Neal DJ, Collins SE. A psychometric analysis of the self-regulation questionnaire. Addict Behav. 2004;29:253–260. doi: 10.1016/j.addbeh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Carter AC. Series Cue reactivity and the role of social alcohol expectancies in the college-aged drinking population. Scholar Commons: Department of Psychology Masters Thesis, University of South Florida; 2006. Cue reactivity and the role of social alcohol expectancies in the college-aged drinking population. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Clinician Version, User's Guide. Arlington, VA.: American Psychiatric Publishing; 1997. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I) [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Kadden R. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Rockville, Md.: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 2007. [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010a;106:52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010b;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt's cognitive-behavioral model. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R. Cognitive-behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95:1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Naqvi NH, Debellis R, Breiter HC. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2013;27:336–350. doi: 10.1037/a0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Niaura R. Cognitive social learning and related perspectives on drug craving. Addiction. 2000;95(Suppl 2):S155–S163. doi: 10.1080/09652140050111726. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biological psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcoholism, clinical and experimental research. 1999;23:1484–1491. [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of general psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, Veltman DJ. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Translational psychiatry. 2013;3:e337. doi: 10.1038/tp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour research and therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu ZL, Palmer P, Wei Y, Jia Y, Johnson CA. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol Addict Behav. 2013;27:443–454. doi: 10.1037/a0027892. [DOI] [PubMed] [Google Scholar]
- Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. International journal of obesity. 2013;37:1565–1570. doi: 10.1038/ijo.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]