Abstract
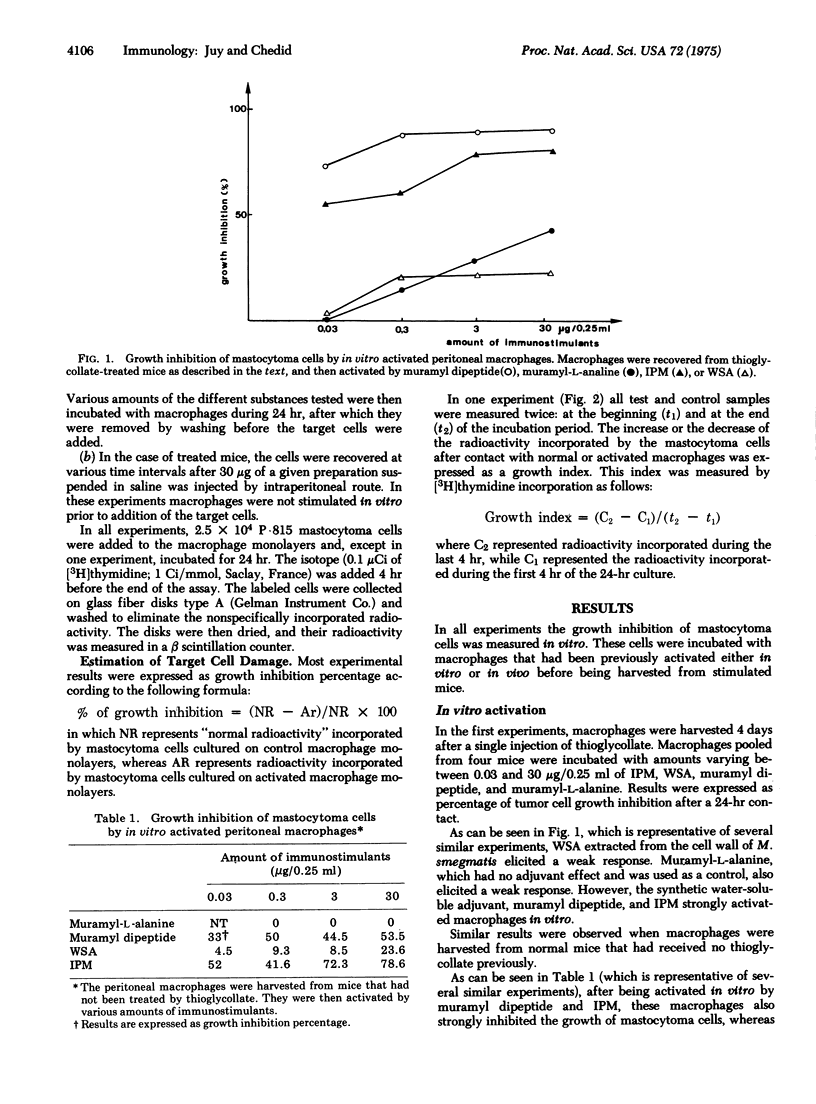
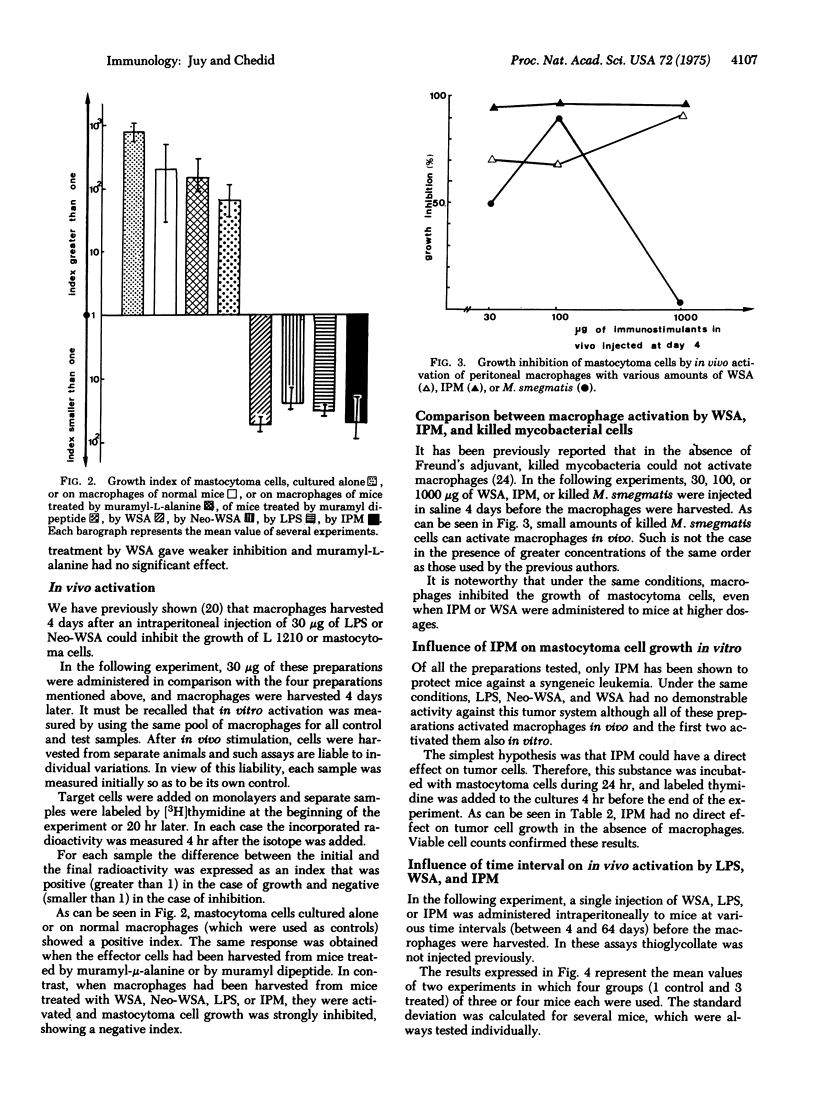
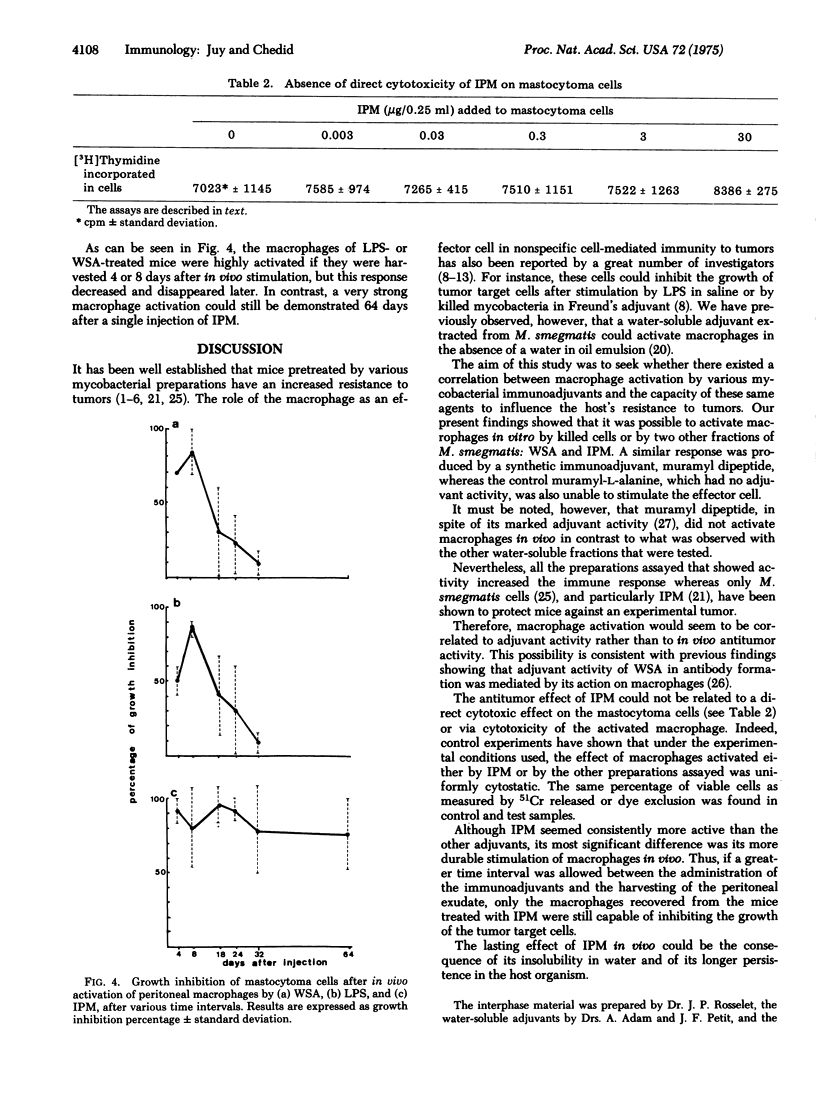
It has repeatedly been observed that various bacterial preparations could increase the host's resistance to tumors. It has also been shown that after nonspecific activation by BCG (bacillus Calmette-Guérin), peritoneal macrophages could inhibit in vitro the growth of neoplastic target cells. In the present study a fraction extracted from Myobacterium smegmatis and referred to as interphase material was tested in view of measuring its ability to activate macrophages in vitro and in vivo. This preparation was previously shown to protect mice against a syngeneic leukemia and to increase the immune response of the guinea pig. Other water-soluble adjuvants devoid of demonstrable antitumor activity in vivo were also assayed. The results argue in favor of a correlation between adjuvant activity and the capacity of activating macrophages. Moreover, interphase material administered in vivo consistently induced stronger and more persistent stimulations of macrophages than the other preparations assayed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E., Chedid L., Lamensans A., Parant F., Parant M., Rosselet J. P., Berger F. M. Preparation and biological properties of water-soluble adjuvant fractions from delipidated cells of Mycobacterium smegmatis and Nocardia opaca. Infect Immun. 1973 Jun;7(6):855–861. doi: 10.1128/iai.7.6.855-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- BIOZZI G., STIFFEL C., HALPERN B. N., MOUTON D. [Effect of inoculation with Calmette-Guerin bacillus on the development of Ehrlich ascites tumor in the mouse]. C R Seances Soc Biol Fil. 1959;153:987–989. [PubMed] [Google Scholar]
- Bennett B. Specific suppression of tumor growth by isolated peritoneal macrophages from immunized mice. J Immunol. 1965 Oct;95(4):656–664. [PubMed] [Google Scholar]
- Chedid L., Lamensans A., Parant F., Parant M., Adam A., Petit J. F., Lederer E. Protective effects of delipidated mycobacterial cells and purified cell walls against Ehrlich carcinoma and a syngeneic lymphoid leukemia in mice. Cancer Res. 1973 Sep;33(9):2187–2195. [PubMed] [Google Scholar]
- Cleveland R. P., Meltzer M. S., Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain BCG. J Natl Cancer Inst. 1974 Jun;52(6):1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970 Nov 14;228(5272):620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Gillette R. W., Swanson M. H. Kinetic studies of macrophages. V. Effect of antigen and adjuvant stimulation. J Reticuloendothel Soc. 1974 Jan;15(1):31–40. [PubMed] [Google Scholar]
- Hawrylko E., Mackaness G. B. Immunopotentiation with BCG. IV. Factors affecting the magnitude of an antitumor response. J Natl Cancer Inst. 1973 Nov;51(5):1683–1688. doi: 10.1093/jnci/51.5.1683. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Adjuvant induced resistance to tumor development in mice. Proc Soc Exp Biol Med. 1972 Mar;139(3):1053–1056. doi: 10.3181/00379727-139-36296. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Macrophage nonimmunologic recognition: target cell factors related to contact inhibition. Science. 1973 May 25;180(4088):868–870. doi: 10.1126/science.180.4088.868. [DOI] [PubMed] [Google Scholar]
- Juy D., Bona C., Chedid L. Effet antitumoral de macrophages péritonéaux activés par un adjuvant hydrosoluble d'origine mycobactérienne. C R Acad Sci Hebd Seances Acad Sci D. 1974 May 27;278(22):2859–2862. [PubMed] [Google Scholar]
- Kaplan A. M., Morahan P. S., Regelson W. Induction of macrophage-mediated tumor-cell cytotoxicity by pyran copolymer. J Natl Cancer Inst. 1974 Jun;52(6):1919–1923. doi: 10.1093/jnci/52.6.1919. [DOI] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Hess M. W. Tumour growth and non-specific immunity in rats: the mechanisms involved in inhibition of tumour growth. Br J Exp Pathol. 1972 Oct;53(5):570–577. [PMC free article] [PubMed] [Google Scholar]
- Laucius J. F., Bodurtha A. J., Mastrangelo M. J., Creech R. H. Bacillus Calmette-Guerin in the treatment of neoplastic disease. J Reticuloendothel Soc. 1974 Dec;16(6):347–373. [PubMed] [Google Scholar]
- Mathé G. Active immunotherapy. Adv Cancer Res. 1971;14:1–36. doi: 10.1016/s0065-230x(08)60517-5. [DOI] [PubMed] [Google Scholar]
- Modolell M., Luckenbach G. A., Parant M., Munder P. G. The adjuvant activity of a mycobacterial water soluble adjuvant (WSA) in vitro. I. The requirement of macrophages. J Immunol. 1974 Jul;113(1):395–403. [PubMed] [Google Scholar]
- OLD L. J., CLARKE D. A., BENACERRAF B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature. 1959 Jul 25;184(Suppl 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- WEISS D. W., BONHAG R. S., DEOME K. B. Protective activity of fractions of tubercle bacilli against isologous tumours in mice. Nature. 1961 Jun 3;190:889–891. doi: 10.1038/190889a0. [DOI] [PubMed] [Google Scholar]
- Zbar B., Bernstein I. D., Rapp H. J. Suppression of tumor growth at the site of infection with living Bacillus Calmette-Guérin. J Natl Cancer Inst. 1971 Apr;46(4):831–839. [PubMed] [Google Scholar]



