Abstract
Background
As measurement of arterial oxygen saturation (SpO2) is common in the delivery room, target SpO2 ranges allow clinicians to titrate oxygen therapy for preterm infants in order to achieve saturation levels similar to those seen in normal term infants in the first minutes of life. However, the influence of the onset of ventilation and the timing of cord clamping on systemic and cerebral oxygenation is not known.
Aim
We investigated whether the initiation of ventilation, prior to, or after umbilical cord clamping, altered systemic and cerebral oxygenation in preterm lambs.
Methods
Systemic and cerebral blood-flows, pressures and peripheral SpO2 and regional cerebral tissue oxygenation (SctO2) were measured continuously in apnoeic preterm lambs (126±1 day gestation). Positive pressure ventilation was initiated either 1) prior to umbilical cord clamping, or 2) after umbilical cord clamping. Lambs were monitored intensively prior to intervention, and for 10 minutes following umbilical cord clamping.
Results
Clamping the umbilical cord prior to ventilation resulted in a rapid decrease in SpO2 and SctO2, and an increase in arterial pressure, cerebral blood flow and cerebral oxygen extraction. Ventilation restored oxygenation and haemodynamics by 5–6 minutes. No such disturbances in peripheral or cerebral oxygenation and haemodynamics were observed when ventilation was initiated prior to cord clamping.
Conclusion
The establishment of ventilation prior to umbilical cord clamping facilitated a smooth transition to systemic and cerebral oxygenation following birth. SpO2 nomograms may need to be re-evaluated to reflect physiological management of preterm infants in the delivery room.
Introduction
Aeration of the lungs at birth is the primary trigger for the transition from fetal to newborn patterns of gas exchange and cardiopulmonary circulation[1–4]. While the majority of infants manage this transition without any difficulty, the most vulnerable infants, particularly those born preterm, often require assistance in the form of oxygen therapy or respiratory support to facilitate this transition. Indeed, 18.7% of all babies born in Australia in 2009 required respiratory support in the form of oxygen therapy or intermittent positive pressure ventilation to assist with the transition at birth[5]. Clinicians have used pulse oximetry measurements of heart rate and peripheral oxygen saturation to guide intervention.
Dawson et al., 2010 reported the normal range of SpO2 in preterm infants during the first 10 minutes of life[6]. These infants all had the umbilical cord clamped early, which was the standard practice at the time. These nomograms showed that the median SpO2 at 1 minute is 62% (47–72% IQR) for preterm infants and 68% (55–75%) for term infants not requiring respiratory support. Therefore, half of these infants have an SpO2 lower than normal fetal levels at 1 min, with infants delivered by caesarean section having significantly lower SpO2 levels than vaginally delivered infants[7–9]. Similar nomograms have been established for cerebral regional oxygenation (SctO2) in newborn infants; the median (10th-90th percentiles) SctO2 was 41% (23–64) at 2 minutes, and increasing to 79% (65–90) at 10 minutes[10]. An important determinate of the initial SpO2 and SctO2 may be whether adequate respiration has been established prior to umbilical cord clamping.
The timing of umbilical cord clamping during the fetal newborn transition has received renewed attention recently. Studies have shown that delayed cord clamping (DCC) has many immediate, short-term and long-term benefits in preterm infants, including improved cardiopulmonary adaptation[11,12], reduced need for blood transfusions, and decreased incidence of intraventricular haemorrhage and necrotising enterocolitis[7,13]. The major mechanism responsible for the benefits of DCC was thought to be increased placental transfusion resulting in greater blood volume[7,14]. However, we have recently shown that the initiation of ventilation prior to cord clamping may be more important than the delay in cord clamping itself[15]. This study demonstrated that the initiation of ventilation prior to cord clamping improved cardiac output by increasing pulmonary blood flow before the cord is clamped. This enables pulmonary venous return to immediately replace umbilical venous return as the primary source of left ventricular output, without any reduction in supply, thereby stabilising the cerebral haemodynamic transition. This effect is consistent with recent clinical trials showing that delayed cord clamping improves cardiac output after birth[16,17]. However, the interaction between the timing of ventilation onset relative to umbilical cord clamping on systemic and cerebral oxygenation is not known.
The establishment of adequate ventilation of preterm infants is not only critical to trigger the transition from fetal to newborn physiology, but it also ensures provision of adequate oxygen delivery to important organs, especially the brain. The aim of this study was to compare systemic and cerebral oxygenation in preterm lambs when ventilation was initiated prior to, or after umbilical cord clamping. We hypothesized that aerating the lung and therefore providing an alternative source of oxygenation prior to removal of the placental circulation with umbilical cord clamping, would improve systemic and cerebral oxygenation during the first 10 min after birth.
Methods
Ethics Statement
The experimental protocol was performed in accordance with guidelines established by the National Health and Medical Research Council of Australia and was approved by the relevant animal ethics committee at Monash University.
Experimental Design
At 126 ± 2 days gestation (term ∼ 148 days), Border-Leicester ewes were anaesthetised with an intravenous bolus of 5% sodium thiopentone (Pentothal; 1g in 20 ml) and, following intubation, maintained with inhalation of 1.5–3% halothane in air. The fetal head and neck were exposed via caesarean section and an ultrasonic flow probe (3 mm: Transonic Systems, Ithaca, NY, USA) was placed around a carotid artery. Heparinised saline-filled polyvinyl catheters were inserted into the other carotid artery and into a jugular vein. The fetal trachea was intubated with a 4.0 mm cuffed endotracheal tube and lung liquid was drained passively for ∼ 10 seconds or until liquid ceased exiting the airways. A transcutaneous arterial oxygen saturation (SpO2) probe (Masimo, Radical 4, CA, USA) was placed around the right forelimb and the output recorded continuously. A Near Infrared Spectroscopy optode (Casmed Foresight, CAS Medical Systems Inc, Branford, CT, USA) was placed over the left frontal cortex and used to continuously measure cerebral tissue oxygen saturation (SctO2). After completion of instrumentation, the ewe was rotated onto its side and the fetus was completely exteriorised from the uterus, still attached to the umbilical cord, dried, and placed on a delivery table immediately next to the ewe. Physiological parameters were allowed to stabilise prior to the birth procedures being initiated (see below)—the delay between exteriorisation and intervention was a mean (SD) of 181 ± 31 seconds for all lambs.
Each fetus was randomised to either umbilical cord clamping prior to initiation of ventilation (Clamp 1st; n = 10) or the initiation of ventilation prior to umbilical cord clamping (Vent 1st; n = 7) groups. In Clamp 1st lambs, the umbilical cord was immediately clamped and cut, the lamb transferred to an infant warmer (CosyCot, Fisher and Paykel, Auckland, New Zealand) and ventilation commenced as soon as possible. In Vent 1st lambs, ventilation commenced while the umbilical cord remained patent. Umbilical cord clamping was delayed until after PBF had increased, indicating cardiopulmonary transition, whereupon the cord was clamped and cut. In both groups ventilation was initiated using positive pressure ventilation in volume guarantee mode with a tidal volume of 7 mL/kg and a positive end-expiratory pressure of 5 cmH2O using warmed and humidified inspired gases, with an initial fraction of inspired oxygen (FiO2) of 21% (Babylog 8000+, Dräger, Lübeck, Germany). Peak inspiratory pressure was limited to 40 cmH2O to avoid pneumothoraces. FiO2 was adjusted to target SpO2 at 70–90% by 3 min, 80–90% by 5 min and 90–96% by 10 min.
Regular blood gas analysis (ABL30, Radiometer, Copenhagen, Denmark) and real-time SpO2 measurements were used to monitor the lambs. All lambs received sedation (Alfaxane i.v. 5–15 mg/kg/h; Jurox, East Tamaki, Auckland, New Zealand) in 5% dextrose via the jugular vein catheter to minimize spontaneous breathing during the experiment. The ewes were humanely euthanized using sodium pentobarbitone (100 mg/kg i.v) after caesarean section and the lambs were euthanized after completion of the ventilation study, at 30 min—2 h later, depending on the study.
Measurements
Instantaneous blood flow in the carotid artery, used as a proxy for cerebral blood flow [18] (CBF), SaO2 and SctO2 were recorded digitally using a data acquisition system (Powerlab; ADInstruments, Castle Hill, Australia). Arterial pressures were measured using pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) and also recorded digitally.
Calculations
SpO2 and SctO2 were used to calculate cerebral oxygen consumption and extraction as described previously[19]; Cerebral oxygen consumption (VO2) was calculated as: Mean CBF x (SaO2-SctO2) where CBF = cerebral blood flow, measured from the carotid artery.
Cerebral oxygen extraction was calculated as: SaO2-SctO2/SaO2.
Arterial blood gas samples prior to intervention (i.e. fetal sample) and at 5 and 10 minutes after cord clamping were used to calculate oxygenation index, arterial oxygen content and cerebral oxygen delivery: Oxygenation Index = (FiO2 x mean airway pressure)/PaO2, where FiO2 is the inspired oxygen concentration and PaO2 is the arterial oxygen tension.
Arterial oxygen content (CaO2) = [1.39•Hb•SaO2/100] + [0.003•PaO2])[20], where Hb is the haemoglobin concentration (g/dL).
Cerebral oxygen delivery (DO2) = (CBF•CaO2)[20].
Statistics
Fetal data collected prior to intervention were compared using Students t-test. Average values were obtained from recordings twenty seconds in duration obtained prior to the first intervention (either umbilical cord clamping or initiation of ventilation) and throughout the intervention and selected time points. All subsequent data were compared over time and between groups using a two-way repeated measures ANOVA for postnatal physiological data with post-hoc analysis (Holm-Sidak) determining the time that differences were evident (Sigmastat v3.0, SPSS Inc.). Data are presented as mean ± SEM unless otherwise stated. Statistical significance was accepted for p<0.05.
Results
Fetal characteristics
Fetal body weights were not different between groups (Mean (SD): Vent 1st: 3.6 ± 0.6 kg: Clamp 1st: 3.2 ± 0.4 kg;). There were more males in the Clamp 1st group (Table 1), but we have previously shown no sex-related differences to the cardiopulmonary haemodynamic transition at birth in preterm lambs[21]. Prior to any intervention, fetal pH tended higher, PaCO2 was significantly lower and PaO2 was significantly higher in Clamp 1st lambs compared to Vent 1st (Table 1); other fetal arterial blood gas parameters were not different between groups (Table 1). Fetal cerebral oxygen content (Table 1) was not different between groups prior to the intervention.
Table 1. Fetal Characteristics.
| Group | n | Male (%) | pH | PaCO2 (mmHg) | PaO2 (mmHg) | SaO2 (%) | Hb (g/dl) | CaO2 |
|---|---|---|---|---|---|---|---|---|
| Vent First | 10 | 20 | 7.20 ± 0.03 | 65.8 ± 3.0 | 20.4 ± 2.6 | 63.2 ± 4.6 | 12.4 ± 0.5 | 10.1 ± 0.9 |
| Clamp First | 7 | 71 | 7.31 ± 0.02 # | 49.2 ± 4.8* | 29.4 ± 2.4* | 68.2 ± 4.7 | 11.3 ± 0.4 | 10.9 ± 1.0 |
Fetal blood gas values of pH, Partial pressure of arterial (Pa) oxygen (O2), carbon dioxide (PaCO2), haemoglobin (Hb) and arterial oxygen content (CaO2).
*indicates significant difference (p<0.05)
# indicates trend (p<0.06).
Ventilation and respiratory values
In Clamp 1st lambs, ventilation commenced at a mean of 79 s (range 48–134 s) after umbilical cord clamping, which is greater than that recommended in international guidelines (< 60 s)[22]. In Vent 1st lambs, ventilation commenced at a mean of 198 s (range 149–240 s) prior to umbilical cord clamping, which was the time that PBF was observed to increase, which is indicative of the haemodynamic transition.
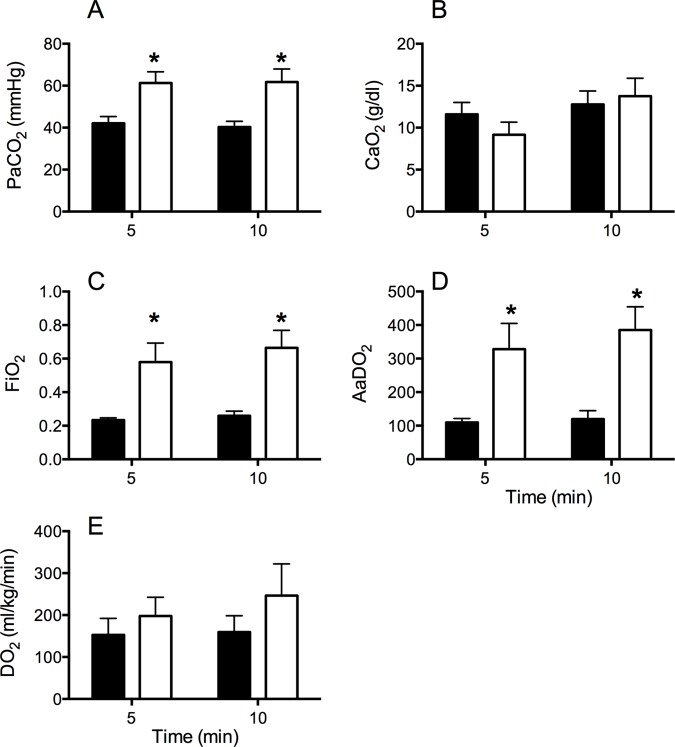
Tidal volume, peak inspiratory pressure, mean airway pressure and respiratory rate were not different between groups throughout the 10 min of ventilation (Data not shown). pH tended to be higher in Vent 1st lambs 5 and 10 minutes after initiation of ventilation (p = 0.053). PaCO2, oxygenation index and AaDO2 were significantly lower in Vent 1st lambs compared to Clamp 1st lambs (Fig. 1), indicative of better oxygenation. PaO2 was not different between groups at 5 or 10 min. Vent 1st lambs received significantly lower FiO2 at 5 min (Mean (range) Vent 1st: 0.23 (0.21–0.32) vs. Clamp 1st: 0.58 (0.21–1.0); p<0.005) and at 10 min (Vent 1st: 0.26 (0.21–0.48) vs. Clamp 1st: 0.66 (0.21–1.0); p<0.001) (Fig. 1).
Fig 1. Blood gas parameters during ventilation.
The partial pressure of (A) arterial carbon dioxide (PaCO2), (B) arterial oxygen content (CaO2), (C) the fraction of inspired oxygen (FiO2), (D) alveolar-arterial difference in oxygen (AaDO2) and (E) cerebral oxygen delivery (DO2) in Vent 1st (Black) and clamp 1st (white) preterm lambs. * indicates significant difference Vent 1st vs. Clamp 1st (p<0.05). Vent 1st and Clamp 1st lambs had similar arterial oxygen content and cerebral oxygen delivery, but Clamp 1st lambs had worse PaCO2 and AaDO2 and thus required higher FiO2 to achieve similar tissue levels 5 and 10 minutes after delivery.
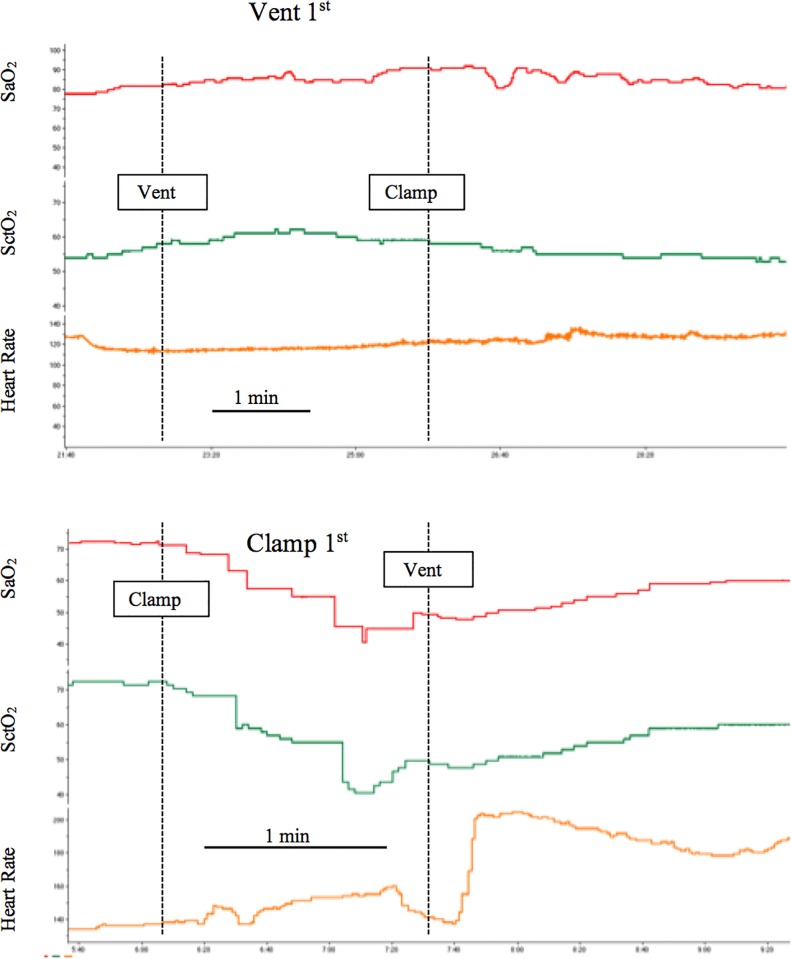
A representative real-time recording illustrating the effect of the timing of ventilation onset relative to umbilical cord clamping on arterial and cerebral oxygenation and cerebral blood flow is shown in Fig. 2. In Vent 1st lambs, there was a steady increase in SpO2 with no discernible effect on SctO2, CBF or blood pressure (Fig. 3). Subsequent clamping of the umbilical cord had little physiological effect.
Fig 2. Effect of the timing of ventilation onset relative to umbilical cord clamping.
Representative traces obtained from a lamb in which ventilation was initiated prior to umbilical cord clamping (Vent 1st), and a lamb in which umbilical cord clamping was conducted prior to the initiation of ventilation (clamp 1st). Dashed line indicates when an intervention occurred as labeled on the graphs. Note the difference in time scale. SpO2—arterial oxygen saturation, SctO2—cerebral oxygenation.
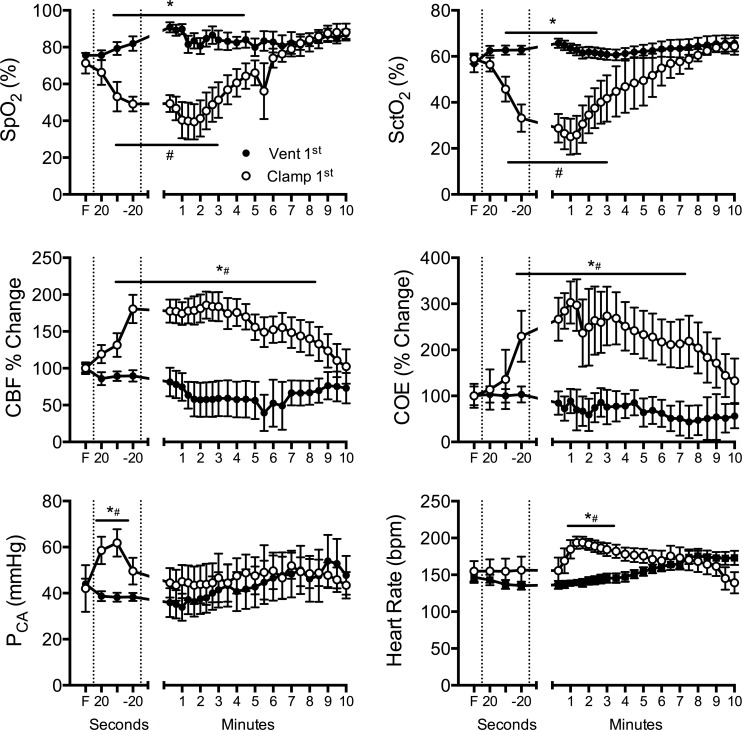
Fig 3. Arterial and cerebral oxygen saturation and haemodynamics.
(A) Arterial saturation of oxygen measured by pulse oximetry (SpO2), (B) cerebral oxygenation (SctO2), (C) cerebral blood flow (CBF), (D) cerebral oxygen extraction (COE), (E) arterial pressure measured in a carotid artery (PCA) and (F) heart rate measured in Vent 1st (closed circles) and Clamp 1st (open circles) preterm lambs. First dashed line indicates ventilation onset for Vent 1st lambs or umbilical cord clamping for Clamp 1st lambs; second dashed line indicates umbilical cord clamping for Vent 1st lambs and ventilation onset for Clamp 1st lambs. F = fetal value. * indicates significant difference Vent 1st vs. Clamp 1st (p<0.05). # indicates time difference from fetal (F) value (p<0.05). In the time between umbilical cord clamping and ventilation onset, Clamp 1st lambs significantly reduced arterial and cerebral oxygen saturation, and increased CBF and oxygen extraction to compensate. Ventilation onset increased arterial and cerebral oxygen saturation back to Vent 1st lambs values by 5 min. Vent 1st lambs maintained steady oxygenation and haemodynamics during the same time.
Clamping of the umbilical cord prior to ventilation resulted in a decrease in arterial saturation and cerebral oxygenation (Fig. 3). Prior to the initiation of ventilation and after clamping of the cord, Clamp 1st lambs also showed a rapid increase in systolic pressure, cerebral blood flow and cerebral oxygen extraction (Fig. 3). In the time taken between cord clamping and ventilation onset in the clamp 1st group, SpO2 fell from 68% to 29% (range of fall in SaO2 11–62%) and SctO2 fell from 52–31% (range of fall in SctO2 19–57%). Initiation of ventilation in Clamp 1st lambs resulted in a rapid increase in heart rate, which remained significantly higher than Vent 1st lambs until 5 minutes (Fig. 3). SaO2 and SctO2 increased, and reached values similar to Vent 1st lambs by 5–6 minutes (Fig. 3). CBF and cerebral oxygen extraction fell to similar levels as Vent 1st lambs by 9 minutes. Arterial oxygen content and oxygen delivery in the carotid artery were not different between groups at 5 and 10 min (Fig. 1). Blood pressure rapidly returned to baseline values by 1 min and was not different between groups thereafter.
Discussion
Aeration of the lung at birth is critical for the successful transition from a fetus to a newborn. Pulse oximetry is used extensively to assess the progress of the cardio-respiratory transition in preterm infants and guides interventions including supplemental oxygen delivery and respiratory support. We investigated whether the establishment of ventilation prior to umbilical cord clamping, improves arterial and cerebral oxygenation during the transition at preterm birth. Our findings demonstrate that ventilation before umbilical cord clamping improves arterial and cerebral oxygenation and haemodynamics, while cord clamping before adequate ventilation results in rapid arterial and cerebral desaturation. These findings suggest that the timing of ventilation onset relative to umbilical cord clamping may play a critical role in subsequent oxygenation of the preterm infant in the delivery room.
International, national and unit guidelines for the use of supplemental oxygen are informed by published SpO2 nomograms[6]. These allow clinicians to target specific SpO2 levels during the first minutes after birth. The use of SpO2 nomograms has led to numerous clinical trials investigating the best way to reach these targets in the delivery room, including the use of automated devices (e.g. [23]). An interesting finding of these nomograms is that the median SpO2 at 1 minute is similar to the normal fetal values, meaning that half of these otherwise healthy infants may be considered to be hypoxic at this time point. Our findings demonstrated that the period of time between cord clamping and onset of ventilation, during which time the apnoeic preterm lamb was being dried, warmed and positioned on the infant warmer, resulted in a more than halving of arterial and cerebral oxygen saturation (Figs. 2 & 3). This occurred despite having a higher PaO2 and lower PaCO2 than Vent 1st lambs immediately prior to umbilical cord clamping. These findings demonstrate the potential importance of adequate ventilation or breathing prior to umbilical cord clamping for maintenance of oxygenation.
Subsequent ventilation of Clamp 1st lambs resulted in a slow improvement in oxygenation, despite similar tidal volumes and pressures to the Vent 1st animals. It took 5–6 minutes before SpO2 was within “normal” ranges based on the Dawson nomogram. These findings are of concern given the known association between prolonged episodes of hypoxia and preterm brain injury[24]. As a consequence, Clamp 1st lambs required a significantly higher FiO2 to reach the target SpO2. The higher requirement for FiO2 in the Clamp 1st lambs directly parallel the transient requirement for FiO2 of preterm infants in clinical trials studying the effect of the initial FiO2 on systemic oxygenation[8,25,26]. In these trials, the mean FiO2 at 5 min for most preterm infants, irrespective of the starting FiO2, was between 50–60%, consistent with our observations. All infants in these trials underwent immediate cord clamping. The transient nature of the increased oxygen requirement in the delivery room has been a consistent observation in preterm infants after immediate cord clamping. Our study suggests that the cause of the increased requirement for FiO2 in preterm infants may be due to a poor cardiovascular transition resultant from inadequate aeration of the lung prior to umbilical cord clamping. Further, the level of FiO2 delivered in our study has been shown to produce local pulmonary and systemic changes indicative of oxidative stress in preterm infants[25,27], which can lead initiate lung injury resulting in longer/higher oxygen requirements in the NICU. Oxidative stress is also a major cause of preterm perinatal brain injury[24,28]. Although we did not measure markers of brain inflammation and oxidative stress in this study, ventilation prior to umbilical cord clamping may reduce these pathways.
The reference ranges for regional cerebral tissue oxygen saturation have recently been established in normal term and preterm infants, and, consistent with the Dawson nomogram, cerebral oxygen saturation was low at 2 minutes at normalised by 5–6 minutes[10]. In our study we observed a similar fall in SctO2 as SaO2 in our clamp first group, which took 5–6 minutes to normalise after ventilation onset. In our hands regional oxygen saturation is lower in preterm lambs compared to humans[29]. This difference likely relates to species differences, location of the probes or the different devices used (INVOS vs. CASMED). Irrespective, ventilation onset prior to cord clamping prevented this fall in cerebral oxygen saturation. Given that animal studies have shown that an average cerebral tissue oxygenation index of less than 55% results in cerebral injury [30], delaying cord clamping until ventilation onset may prevent prolong periods of time below this critical threshold.
CBF increased rapidly, by ∼80% within the first 60 seconds after cord clamping in Clamp 1st lambs and remained elevated throughout the entire ventilation strategy compared to Vent 1st lambs (see Fig. 3). The rapid increase in CBF we observed likely occurs through a combination of haemodynamic responses to umbilical cord clamping (removal of the capacitance placental circulation), and a subsequent increase due to an increasingly hypoxic environment. The later suggests intact cerebral autoregulation in lambs of this gestation, which allowed them to maintain cerebral oxygen delivery (See Fig. 1B). However, preterm infants are known to have immature cerebral vascular beds, which are prone to leakage and haemorrhage[31] and have impaired ability to autoregulate CBF, particularly during the first days of life[32]. Abnormal fluctuations in CBF (defined as prolonged swings in CBF, either high or low, for greater than 10 to 20s)[33] is a major mechanism of preterm brain injury[24,28,34]. The rapid rise in CBF we observed in this study, may translate to an increased the risk of cerebral vascular leakage and/or haemorrhage in preterm infants.
Past and current trials of delayed cord clamping use time after delivery (from 30 s up to 3 min) to determine when the umbilical cord is clamped[35]. The use of time is based on the idea that the main benefit of delaying cord clamping is to maximize blood volume within the infant (increase placental to fetal transfer). Our recent research suggests that an additive benefit of delaying cord clamping may be the establishment of breathing prior to clamping[15]. This supports the clinical findings of improved cardiovascular stability in preterm infants after delayed cord clamping[16,17,36], albeit at a time-point much later than we are studying (6–48 h), and the finding that respirations are an important determinant of the volume of placental transfusion[37].
Aeration of the lung, whether it occurs via spontaneous breathing or mechanical ventilation, is the critical factor causing the decrease in pulmonary vascular resistance and increase in pulmonary blood flow at birth, which initiates the cardiopulmonary transition[2,4,38]. During fetal life the majority of left ventricular preload and fetal oxygenation is provided by placental blood flow. Decreasing pulmonary vascular resistance at birth, increases pulmonary blood flow and allows for the pulmonary circulation to supply left ventricular preload and to replace the placental oxygenation supply with a pulmonary oxygen supply. This maintains cerebral circulatory stability throughout delivery[15]. For this reason we used the increase in pulmonary blood flow to indicate successful cardiopulmonary transition and to dictate the timing of cord clamping in Vent 1st lambs. Measurement of pulmonary blood flow during the transition is difficult to do in clinical practice, thus using aeration of the lung, as indicated by vigorous breathing of the baby, is the appropriate pseudo marker of transition. However, the influence of ventilation onset relative to the timing of umbilical cord clamping on systemic and cerebral oxygenation was not known. Our findings, and those of our previous study, suggest that the timing of umbilical cord clamping should not be based on elapsed time after birth, but rather focus on the physiology of the newborn, in particular on adequate breathing efforts. Indeed, a recent review stated that “rapid assessment of the newly born infant and the initial steps of drying, providing warmth, clearing the airway, and providing specific stimulation to breathe can be carried out with the umbilical circulation intact”, which would require a paradigm shift in our current clinical practice[36]. Our study also raises the potential issue that the current oxygen saturation nomograms with a dip in oxygenation below fetal levels in the first minutes of life are an artefact of immediate cord clamping, a potentially abnormal event, and are not evident when ventilation is established prior to cord clamping. Indeed, a recent study showed a higher SaO2 and lower heart rate in the first 3 minutes in infants who underwent delayed cord clamping and skin to skin contact[39]. Although no evaluation of the efficacy of breathing was undertaken, these were uncompromised term infants and started breathing within seconds after birth.
There are limitations to our study that may alter the clinical context. In our study, lambs were delivered via caesarean delivery while the ewe was receiving ventilation under anaesthesia. While this allowed us to demonstrate the importance of the timing of ventilation onset relative to umbilical cord clamping in a controlled situation, it does have limitations in transition to the delivery room. All lambs were completely exteriorised from the uterus for ∼ 181 s prior to either cord clamping or ventilation onset. This was conducted to allow for stabilisation of cardiopulmonary haemodynamics prior to intervention. While this could be interpreted as delayed cord clamping, the influence of maternal and fetal anaesthesia preventing fetal respiratory efforts, coupled with the absence of labour, prevents comparison with clinical delayed cord clamping. Indeed, we did not observe any change to haemodynamics or oxygenation during the EXIT procedure. In the clinical scenario, many factors including labour and/or oxytocin administration are likely to have significant effects on umbilical blood flow, cardiopulmonary haemodynamics and oxygenation[40] but these were not assessed in this study.
Vaginal delivery is known to result in a higher SaO2 in the first minutes of life [6]. Our studies have not investigated the influence of ventilation onset prior to cord clamping in vaginally delivered preterm lambs, which may alter the oxygenation response. However, we would hypothesise that delaying umbilical cord clamping until breathing is established has the most benefit in caesarean delivered neonates, due to the increased volume of lung liquid required to be removed[41,42], and the reduced likelihood of spontaneous breathing compared to vaginally delivered infants. More studies are required to investigate this.
It is important to note that the lambs in this study were not breathing. A significant proportion of preterm infants make some breathing efforts, gasping or crying, during delivery[43]. Thus the fall in systemic and cerebral oxygenation we observed might more closely mimic that seen clinically in an apnoeic infant, or an infant making poor respiratory efforts, compared to an infant making appropriate spontaneous breathing efforts. However, it is pertinent to note that ∼19% of all babies born in Australia require some respiratory support (oxygen or IPPV) to reach the target SpO2 nomogram[5], which indicates that a large proportion of otherwise normal babies are not transitioning smoothly at birth. The severity of the fall in systemic and cerebral oxygenation after birth is thus dependent upon the degree of aeration of the lung, which is best determined clinically by the degree of breathing.
The mean time between umbilical cord clamping and ventilation onset in the clamp 1st group of 79 s was longer than the recommended time for initiation of respiratory support (< 60 s)[22] and that reported in an audit of the time for initiation of respiratory support in preterm infants (70 (23) s)[44]. While this appears to be a long time, the time it takes to transfer the infant from the delivery table to the resuscitation area, perform initial assessment, begin warming, clear airways and initiate adequate respiratory support all takes significant time, and in some instances the initiation of respiratory support in preterm infants is occurring beyond this time-point[44].
Conclusions
In summary, the initiation of ventilation prior to umbilical cord clamping in preterm lambs resulted in smoother systemic and cerebral oxygenation during transition. Our study suggests that the onset of breathing/aeration of the lung is an important determinate of SpO2 and SctO2 within the first minutes of life. Physiological-based umbilical cord clamping (delaying until ventilation is established) may improve short and long term outcomes in preterm infants.
Acknowledgments
We would like to acknowledge the technical assistance of Valerie Zahra, Karyn Rodgers and Alison Moxham.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by National Institute of Health R01HD072848-01A1, National Health and Medical Research Council (NHMRC) Project Grant (1067615), NHMRC Research Fellowships (GRP: 1026890, JAD: APP1012686, PGD: APP1059111 and SBH: APP1058537), a Rebecca L. Cooper Medical Research Foundation Fellowship, Financial Markets for Children Research Grant (GRP) and the Victorian Government's Operational Infrastructure Support Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rudolph AM (1977) Fetal and neonatal pulmonary circulation. Am Rev Respir Dis 115: 11–18. [DOI] [PubMed] [Google Scholar]
- 2. Hooper SB, Harding R (2005) Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Current Respiratory Medicine Reviews 1: 185–195. [Google Scholar]
- 3. Polglase GR, Hooper SB (2006) Role of Intra-luminal Pressure in Regulating PBF in the Fetus and After Birth. Current Pediatric Reviews 2: 287–299. [Google Scholar]
- 4. Hooper SB, Kitchen MJ, Siew ML, Lewis RA, Fouras A, et al. (2009) Imaging lung aeration and lung liquid clearance at birth using phase contrast X-ray imaging. Clin Exp Pharmacol Physiol 36: 117–125. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Zeki R, Hilder L, Sullivan EA (2012) Australia's mothers and babies 2010. Perinatal statistics series no 27 Cat no PER 57 Canberra: AIHW National Perinatal Epidemiology and Statistical Unit.
- 6. Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, et al. (2010) Defining the reference range for oxygen saturation for infants after birth. Pediatrics 125: e1340–1347. 10.1542/peds.2009-1510 [DOI] [PubMed] [Google Scholar]
- 7. Rabe H, Diaz-Rossello JL, Duley L, Dowswell T (2012) Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 8:CD003248: 10.1002/14651858.CD14003248.pub14651853 [DOI] [PubMed] [Google Scholar]
- 8. Escrig R, Arruza L, Izquierdo I, Villar G, Saenz P, et al. (2008) Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics 121: 875–881. 10.1542/peds.2007-1984 [DOI] [PubMed] [Google Scholar]
- 9. Kamlin CO, Dawson JA, O'Donnell CP, Morley CJ, Donath SM, et al. (2008) Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J Pediatr 152: 756–760. 10.1016/j.jpeds.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Pichler G, Binder C, Avian A, Beckenbach E, Schmolzer GM, et al. (2013) Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr 163: 1558–1563. 10.1016/j.jpeds.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 11. Peltonen T (1981) Placental transfusion—advantage an disadvantage. Eur J Pediatr 137: 141–146. [DOI] [PubMed] [Google Scholar]
- 12. Tiisala R, Tahti E, Lind J (1966) Heart volume variations during first 24 hours of life of infants with early and late clamped umbilical cord. Ann Paediatr Fenn 12: 151–153. [PubMed] [Google Scholar]
- 13. March MI, Hacker MR, Parson AW, Modest AM, de Veciana M (2013) The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol 33: 763–767. 10.1038/jp.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabe H, Jewison A, Alvarez RF, Crook D, Stilton D, et al. (2011) Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol 117: 205–211. 10.1097/AOG.0b013e3181fe46ff [DOI] [PubMed] [Google Scholar]
- 15. Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, et al. (2013) Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol 591: 2113–2126. 10.1113/jphysiol.2012.250084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer MP, Mildenhall L (2012) Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study. Arch Dis Child Fetal Neonatal Ed Nov;97(6):F484–6. 10.1136/adc.2010.199703 [DOI] [PubMed] [Google Scholar]
- 17. Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, et al. (2012) Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics 129: e667–672. 10.1542/peds.2011-2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Bel F, Roman C, Klautz RJ, Teitel DF, Rudolph AM (1994) Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr Res 35: 329–333. [DOI] [PubMed] [Google Scholar]
- 19. Balegar KK, Stark MJ, Briggs N, Andersen CC (2014) Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants. J Pediatr 164: 475–480.e471. 10.1016/j.jpeds.2013.10.041 [DOI] [PubMed] [Google Scholar]
- 20. Nunn JF (1987) Applied Respiratory Physiology; F. NJ, editor. London: Butterworths. [Google Scholar]
- 21. Polglase GR, Hooper SB, Kluckow M, Gill AW, Harding R, et al. (2012) The cardiopulmonary haemodynamic transition at birth is not different between male and female preterm lambs. Reprod Fertil Dev 24: 510–516. 10.1071/RD11121 [DOI] [PubMed] [Google Scholar]
- 22. Wyllie J, Perlman JM, Kattwinkel J, Atkins DL, Chameides L, et al. (2010) Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations . Resuscitation 81 Suppl 1: e260–287. 10.1016/j.resuscitation.2010.08.029 [DOI] [PubMed] [Google Scholar]
- 23. Gandhi B, Rich W, Finer N (2013) Achieving targeted pulse oximetry values in preterm infants in the delivery room. J Pediatr 163: 412–415. 10.1016/j.jpeds.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 24. Khwaja O, Volpe JJ (2008) Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93: F153–161. 10.1136/adc.2006.108837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vento M, Moro M, Escrig R, Arruza L, Villar G, et al. (2009) Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124: e439–449. 10.1542/peds.2009-0434 [DOI] [PubMed] [Google Scholar]
- 26. Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, et al. (2008) Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics 121: 1083–1089. 10.1542/peds.2007-1460 [DOI] [PubMed] [Google Scholar]
- 27. Rook D, Schierbeek H, Vento M, Vlaardingerbroek H, van der Eijk AC, et al. (2014) Resuscitation of preterm infants with different inspired oxygen fractions. J Pediatr 164: 1322–1326.e1323. 10.1016/j.jpeds.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 28. Polglase GR, Miller SL, Barton SK, Kluckow M, Gill AW, et al. (2014) Respiratory support for premature neonates in the delivery room: Effects on cardiovascular function and the development of brain injury. Pediatr Res 10: 40. [DOI] [PubMed] [Google Scholar]
- 29. Barton SK, Moss TJ, Hooper SB, Crossley KJ, Gill AW, et al. (2014) Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation-induced lung or brain injury. PLoS One 9: e112402 10.1371/journal.pone.0112402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hagino I, Anttila V, Zurakowski D, Duebener LF, Lidov HG, et al. (2005) Tissue oxygenation index is a useful monitor of histologic and neurologic outcome after cardiopulmonary bypass in piglets. J Thorac Cardiovasc Surg 130: 384–392. [DOI] [PubMed] [Google Scholar]
- 31. Greisen G (2005) Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 81: 423–428. [DOI] [PubMed] [Google Scholar]
- 32. Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, et al. (2007) Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 61: 467–473. [DOI] [PubMed] [Google Scholar]
- 33. Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, et al. (2011) Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol 31: 722–729. 10.1038/jp.2011.17 [DOI] [PubMed] [Google Scholar]
- 34. Del Toro J, Louis PT, Goddard-Finegold J (1991) Cerebrovascular regulation and neonatal brain injury. Pediatr Neurol 7: 3–12. [DOI] [PubMed] [Google Scholar]
- 35. Hutton EK, Hassan ES (2007) Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA 297: 1241–1252. [DOI] [PubMed] [Google Scholar]
- 36. Niermeyer S, Velaphi S (2013) Promoting physiologic transition at birth: re-examining resuscitation and the timing of cord clamping. Semin Fetal Neonatal Med 18: 385–392. 10.1016/j.siny.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 37. Yao AC, Lind J (1974) Placental transfusion. Am J Dis Child 127: 128–141. [DOI] [PubMed] [Google Scholar]
- 38. Sobotka KS, Hooper SB, Allison BJ, Te Pas AB, Davis PG, et al. (2011) An initial sustained inflation improves the respiratory and cardiovascular transition at birth in preterm lambs. Pediatr Res 70: 56–60. 10.1038/pr.2011.281 [DOI] [PubMed] [Google Scholar]
- 39. Smit M, Dawson JA, Ganzeboom A, Hooper SB, van Roosmalen J, et al. (2014) Pulse oximetry in newborns with delayed cord clamping and immediate skin-to-skin contact. Arch Dis Child Fetal Neonatal Ed 31: 2013–305484. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt S, Polglase GR, Wallace EM, Te Pas AB, Hooper SB (2014) Ventilation before Umbilical Cord Clamping Improves the Physiological Transition at Birth. Front Pediatr 2:113: 10.3389/fped.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hooper SB, Siew ML, Kitchen MJ, te Pas AB (2013) Establishing functional residual capacity in the non-breathing infant. Semin Fetal Neonatal Med 18: 336–343. 10.1016/j.siny.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 42. Milner AD, Saunders RA, Hopkin IE (1978) Effects of delivery by caesarean section on lung mechanics and lung volume in the human neonate. Arch Dis Child 53: 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Donnell CP, Kamlin CO, Davis PG, Morley CJ (2010) Crying and breathing by extremely preterm infants immediately after birth. J Pediatr 156: 846–847. 10.1016/j.jpeds.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 44. Schilleman K, Siew ML, Lopriore E, Morley CJ, Walther FJ, et al. (2012) Auditing resuscitation of preterm infants at birth by recording video and physiological parameters. Resuscitation 83: 1135–1139. 10.1016/j.resuscitation.2012.01.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.