Abstract
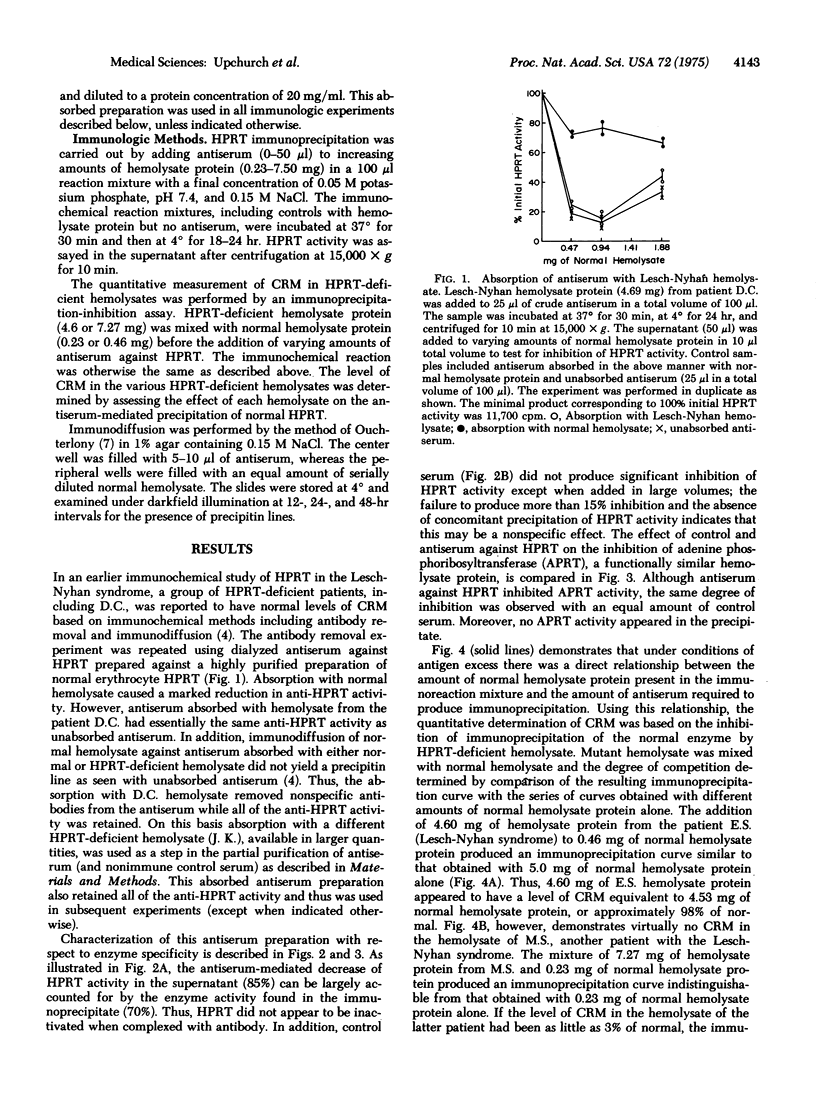
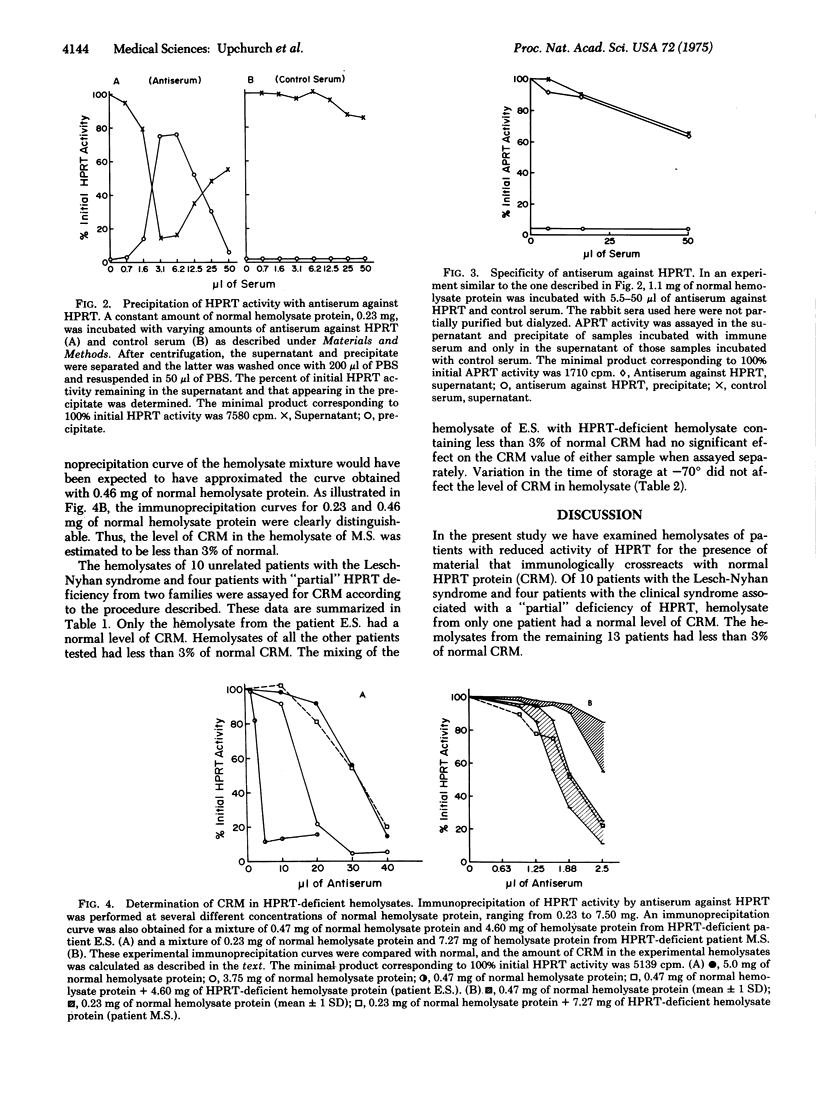
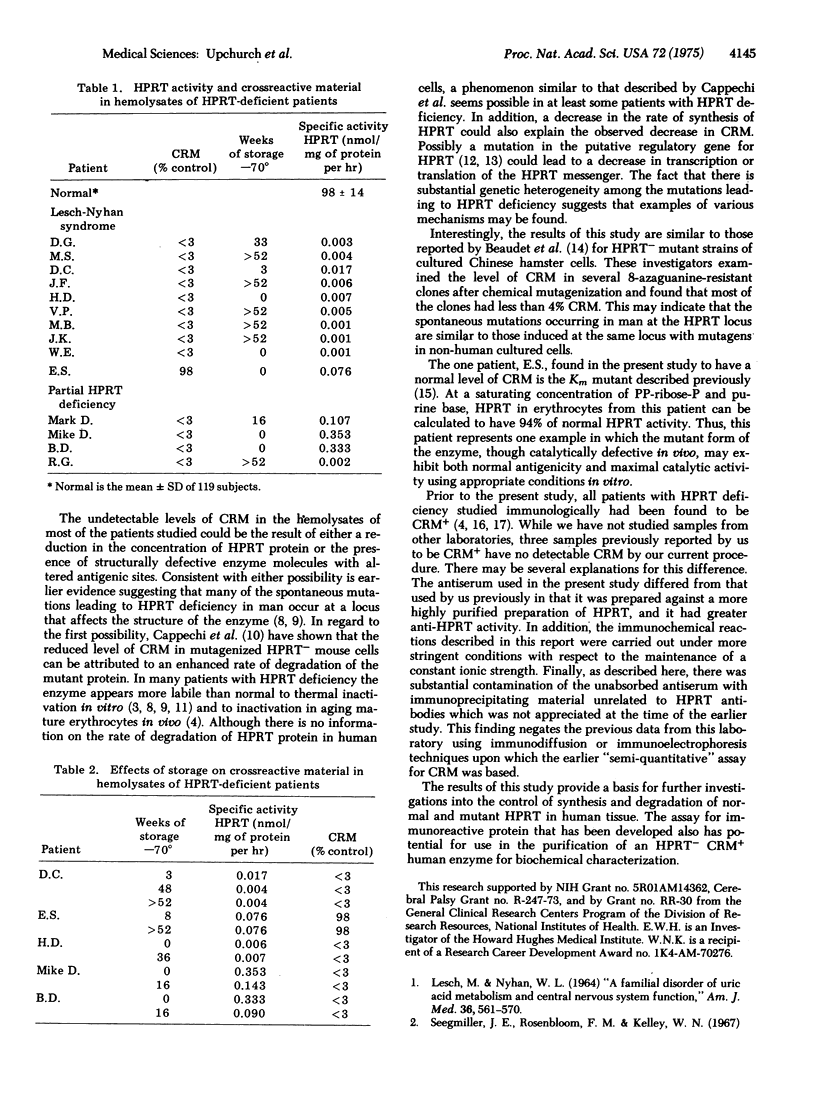
In the present study hemolysates from fourteen patients with a genetically determined deficiency of hypoxanthine phosphoribosyltransferase (EC 2.4.2.8; IMP:pyrophosphate phosphoribosyltransferase) activity were examined immunologically for the presence of material that crossreacts with the normal enzyme. A quantitative assay for crossreacting material in enzyme-deficient hemolysates was based on the inhibition of the immunoprecipitation of the normal enzyme. As little as 3% of normal crossreacting material could be detected. One patient in this series was found to have a normal amount of crossreacting material, whereas the remainder had no detectable crossreacting protein. The lack of detectable crossreacting material in these patients raises the possibility that a defect in synthesis or degradation of enzyme protein may be present in many patients deficient in hypoxanthine phosphoribosyltransferase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. J., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and subunit structure. J Biol Chem. 1971 Dec 10;246(23):7398–7404. [PubMed] [Google Scholar]
- Arnold W. J., Meade J. C., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase: characteristics of the mutant enzyme in erythrocytes from patients with the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jul;51(7):1805–1812. doi: 10.1172/JCI106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay B., Croce C. M., Koprowski H., Nyhan W. L. Restoration of hypoxanthine phosphoribosyl transferase activity in mouse 1R cells after fusion with chick-embryo fibroblasts. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1998–2002. doi: 10.1073/pnas.70.7.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet A. L., Roufa D. J., Caskey C. T. Mutations affecting the structure of hypoxanthine: guanine phosphoribosyltransferase in cultured Chinese hamster cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):320–324. doi: 10.1073/pnas.70.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto W. Y., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency: activity in normal, mutant, and heterozygote-cultured human skin fibroblasts. Proc Natl Acad Sci U S A. 1970 Mar;65(3):577–584. doi: 10.1073/pnas.65.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Arnold W. J. Human hypoxanthine-guanine phosphoribosyltransferase: studies on the normal and mutant forms of the enzyme. Fed Proc. 1973 Jun;32(6):1656–1659. [PubMed] [Google Scholar]
- Kelley W. N., Meade J. C. Studies on hypoxanthine-guanine phosphoribosyltransferase in fibroblasts from patients with the Lesch-Nyhan syndrome. Evidence for genetic heterogeneity. J Biol Chem. 1971 May 10;246(9):2953–2958. [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971 Feb 19;171(3972):689–691. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B., Gormley I. P., Gardiner S. E., Evans H. J., Harris H. Reappearance of murine hypoxanthine guanine phosphoribosyl transferase activity in mouse A9 cells after attempted hybridisation with human cell lines. Exp Cell Res. 1972 Dec;75(2):401–409. doi: 10.1016/0014-4827(72)90446-6. [DOI] [PubMed] [Google Scholar]



