Abstract
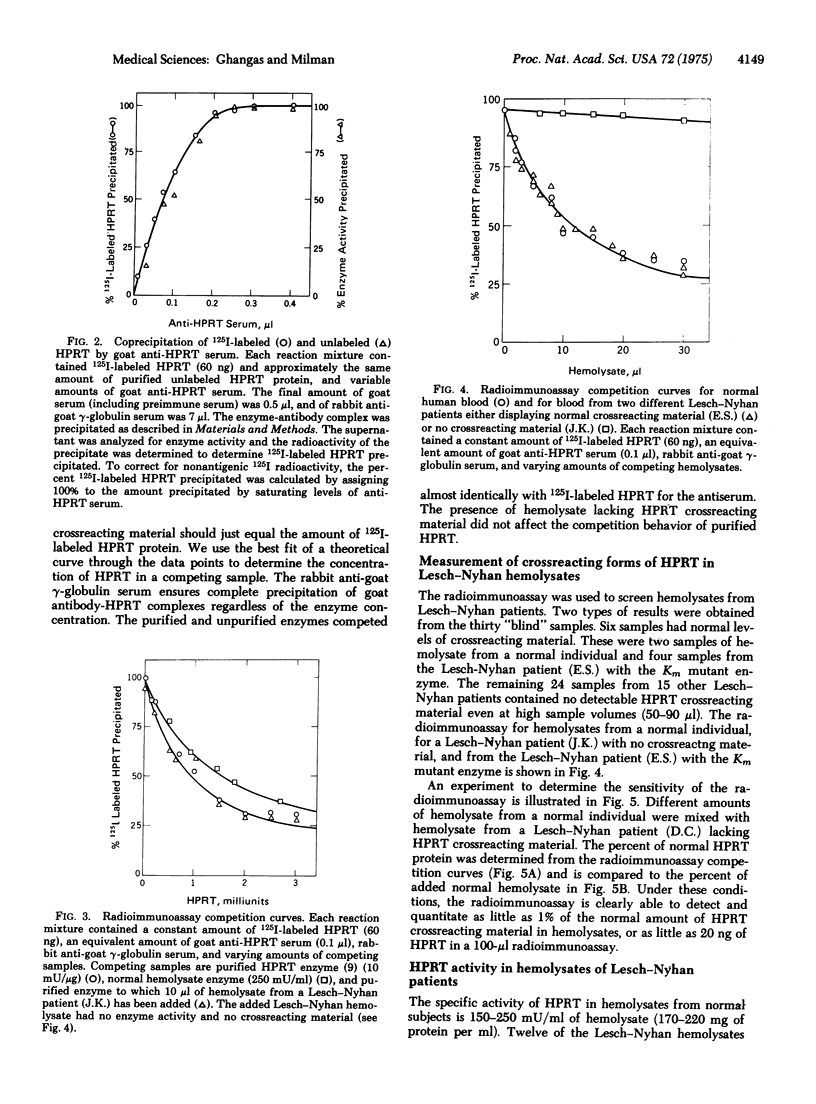
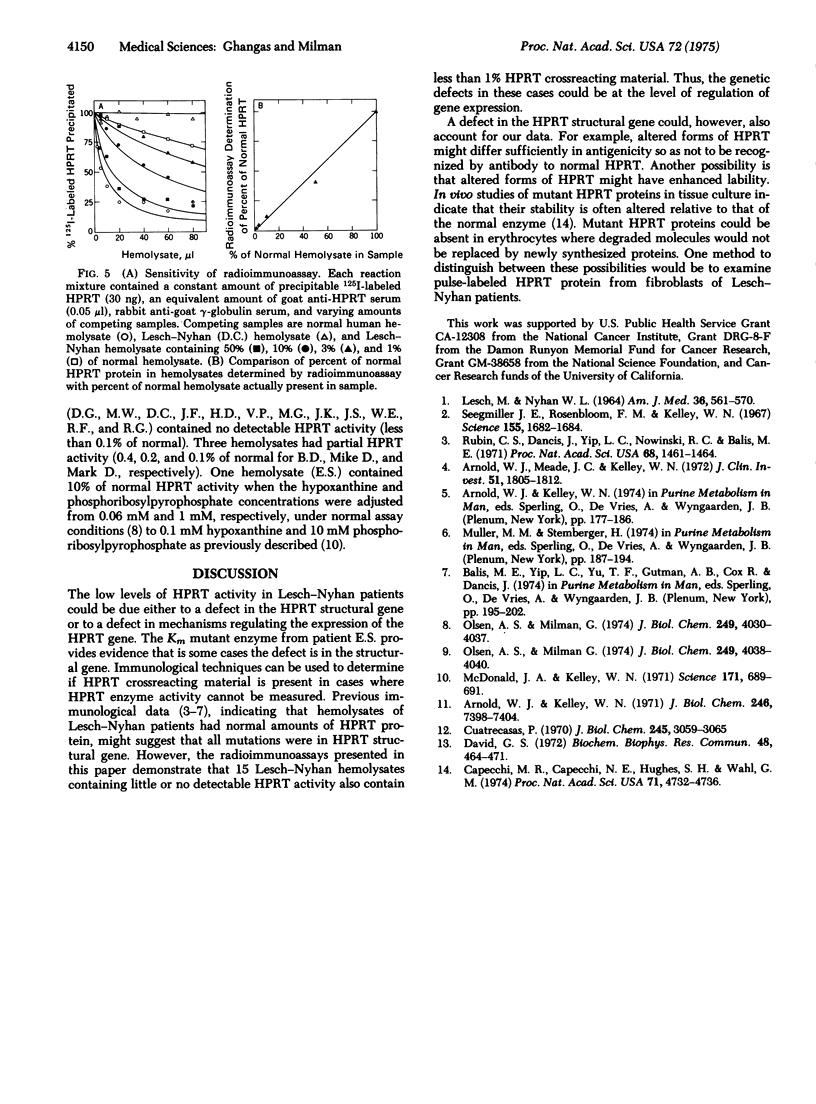
We have developed a sensitive radioimmunoassay capable of detecting and quantitating 20 ng of hypoxanthine phosphoribosyltransferase (EC 2.4.2.8; IMP:pyrophosphate phosphoribosyltransferase) protein. For this assay, hypoxanthine phosphoribosyltransferase from human erythrocytes was iodinated with 125I under mild conditions using hydrogen peroxide and lactoperoxidase attached to Sepharose-4B. Antisera prepared against homogeneous human hypoxanthine phosphoribosyltransferase precipitates the iodinated enzyme as effectively as the unlabeled enzyme. The radioimmunoassay has been used to look for hypoxanthine phosphoribosyltransferase crossreacting material in hemolysates from sixteen different patients with a marked genetic deficiency of this enzyme characteristic of the Lesch-Nyhan syndrome. Fifteen hemolysates contained no detectable (less than 1% of normal) crossreacting material. One hemolysate contained a normal amount of crossreacting material. Hypoxanthine phosphoribosyltransferase from this patient (E.S.) has been shown to be a Km mutant enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. J., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and subunit structure. J Biol Chem. 1971 Dec 10;246(23):7398–7404. [PubMed] [Google Scholar]
- Arnold W. J., Meade J. C., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase: characteristics of the mutant enzyme in erythrocytes from patients with the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jul;51(7):1805–1812. doi: 10.1172/JCI106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971 Feb 19;171(3972):689–691. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Chinese hamster hypoxanthine-guanine phosphoribosyltransferase. Purification, structural, and catalytic properties. J Biol Chem. 1974 Jul 10;249(13):4030–4037. [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Subunit molecular weight of human hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1974 Jul 10;249(13):4038–4040. [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]



