Abstract
Objectives: Recently, photodynamic therapy (PDT) has been introduced as a new modality in oral bacterial decontamination. Besides, the ability of laser irradiation in the presence of photosensitizing agent to lethal effect on oral bacteria is well documented. Current research aims to evaluate the effect of photodynamic killing of visible blue light in the presence of plaque disclosing agent erythrosine as photosensitizer on Porphyromonas gingivalis associated with periodontal bone loss and Fusobacterium nucleatum associated with soft tissue inflammation, comparing with the near-infrared diode laser.
Materials and methods: Standard suspension of P. gingivalis and F. nucleatum were exposed to Light Emitting Diode (LED) (440–480 nm) used to photopolymerize composite resine dental restoration in combination with erythrosine (22 µm) up to 5 minutes. Bacterial sample were also exposed to a near-infrared diode laser (wavelength, 830 nm), using identical irradiation parameters for comparison. Bacterial samples from each treatment groups (radiation-only group, erythrosine-only group and light or laser with erythrosine group) were subcultured onto the surface of agar plates. Survival of these bacteria was determined by counting the number of colony forming units (CFU) after incubation.
Results: Exposure to visible blue light and diode laser in conjugation with erythrosine significantly reduced both species examined viability, whereas erythrosine-treated samples exposed to visible light suggested a statically meaningful differences comparing to diode laser. In addition, bactericidal effect of visible light or diode laser alone on P. gingivalis as black-pigmented bacteria possess endogenous porphyrins was noticeably.
Conclusion: Our result suggested that visible blue light source in the presence of plaque disclosing agent erythrosine could can be consider as potential approach of PDT to kill the main gram-negative periodontal pathogens. From a clinical standpoint, this regimen could be established as an additional minimally invasive antibacterial treatment of plaque induced periodontal pathologies.
Keywords: Erythrosine, Fusobacterium nucleatum, Porphyromonas gingivalis, Diode laser, visible light
Introduction
Periodontal diseases are characterized by an inflammatory process in periodontal tissue caused by bacterial infection, resulting in the destruction soft tissue and alveolar bone. Porphyromonas gingivalis and Fusobacterium nucleatum are strongly believed as major pathogens in the etiology of adult periodontitis 1,2,3). Conventional strategies for reducing the bacterial load are first, mechanical removal includes scaling and root planning and brushing and second, antimicrobial chemotherapy. Mechanical debridement can achieve a temporary decrease in the subgingival levels of P. gingivalis and F. nucleatum together with other pathogens 4). However, organisms cannot be removed from the majority of periodontal pockets by mechanical therapy alone. Antimicrobial chemotherapy may further suppress the periodontal pathogens and increase the benefits obtained by conventional mechanical treatment. Numerous systemic and local antimicrobial chemotherapeutic agents have been evaluated for the treatment of periodontitis with various degrees of success 5, 6, 7). The effectiveness of these approaches are comprised by patient motivation, manual dexterity and the development of drug-resistant strains 8, 9). In addition, this methods have some limitations such as mechanical damage to the oral mucosa in patients with mechanoblistering disease caused by brushing or scraping, limited penetration of chemotherapeutic agents into bacterial biofilm and the difficulty to maintain therapeutic concentration of antimicrobials in the oral cavity which consequently results in reduced susceptibility of this kind of treatment 10, 11).
Obviously there is a growing need for innovative and alternative approaches leading to bacteria eradication. One potential alternative approach is Photodynamic Therapy (PDT), which is a therapeutic process, involving the combination of light and photosensitive agents called photosensitizers 12). The photodynamic process is a two-step protocol, in which target cells are exposed to a photosensitizer and irradiated with a harmless light in the maximum absorption of the sensitizer wavelength, leading to the production of singlet oxygen and free radicals that can damage essential components of the cells, such as plasma membrane and DNA, or modifying metabolic activities in an irreversible way, thus possibly resulting in cell death 13, 14). Antimicrobial PDT (a-PDT) is a localized, nonthermal and non-invasive antimicrobial method to decrease bacterial contamination in oral infections 15, 16, 17). Several studies have illustrated that PDT has a strong effect on a large number of oral gram positive and negative bacteria, using different photosensitizers and light sources 18, 19).
Traditionally, lasers as coherent light sources were considered to be superior to the conventional light sources. On the other hand, the usage of lasers also has some essential drawbacks. First of all, they are very expensive. Second, they require specially trained personnel to work with them 20). As a result, the alternative conventional light sources were developed. For instance, in treatment of surface lesions non-coherent light sources are more suitable, because they can evenly irradiate an entire lesion's field in order to ensure equal light portions for the whole surface 21). Recently there are several reports on the bactericidal effect of visible light, most of them claiming the blue part (wavelength, 400–500nm) to be responsible for killing various pathogens. Feuerstein et al. showed that broadband blue light sources such as light emitting diode (LED) used in dentistry for curing resin-composite materials at 400–500nm exert a phototoxic effect on P. gingivalis and F. nucleatum 22).
In addition to the light sources, Antibacterial photosensitizers currently under investigation for use in the mouth include toluidine blue O (TBO) 23) and chlorin e6 24). These agents show great promise, but will be subject to lengthy clinical and legislative assessment. More immediate benefit could be attained from photosensitizers already available for use in the mouth. One such photosensitizer is erythrosine. Dental practitioners currently use erythrosine to stain and visualize dental plaque in the form of disclosing solution or tablets. Erythrosine has some reported antimicrobial activity against Gram-positive and Gram-negative oral bacteria 25–27). However, erythrosine also belongs to a class of cyclic compounds called xanthenes, which absorb light in the visible region, and the ability of erythrosine to initiate photochemical reactions is well documented 28, 29). Moreover, the results reported by Wood et al. pointed out that erythrosine-mediated PDT is 5 – 10 times more effective than methylene blue -mediated PDT at killing Streptococcus mutans biofilm bacteria 30). This is extremely encouraging, as methylene blue is an established and effective tumour 31, 32) and antimicrobial photosensitizer 33, 34–36).
There are rare works attempting to explore the antimicrobial activity exerted by blue-band visible light in conjugation with erythrosine against periodontal pathogenic species. The purpose of this study was to carry out a preliminary assessment to test the effect of our novel therapeutic and supplementary regimen of visible light with erythrosine as an exogenous photosensitizer on the viability of P. gingivalis associated with periodontal bone loss and F. nucleatum associated with soft tissue inflammation. Besides, the near-infrared diode laser (wavelength, 830 nm), using identical irradiation parameters was applied because clinical reports showing a beneficial effect of diode laser on periodontal pockets hypothesized that this effect is attributable to its bactericidal effect 37).
Materials and Methods
2.1. Bacteria and growth conditions
Fresh lyophilized Porphyromonas gingivalis (33277), Fusobacterium nucleatum (25586) from the American Type Culture Collection (Rayen Biotechnology Co. Ltd., Tehran, Iran) were used. P. gingivalis and F. nucleatum were rehydrated in brain heart infusion (BHI) broth (Merck KGaA, Darmstadt, Germany) and incubated in an anaerobic jar at <1% O2 and 9–13% CO2 at 37°C. All the strains were subcultured twice before exposure to light. The bacterial concentration after 24 h incubation was standardized by dilution with sterile broth to OD650nm = 0.45, equivalent to ∼ 5×106 colony forming units (CFU).
2.2. Light source and Photosensitizer
We applied two sources for light energy: a commercially available visible light source, usually used in dental office, was Light Emitting Diode (LED) (440–480 nm with peak at 460 nm) (Starlight pro, Mectron, Italy). For comparison, irradiation was performed at a wavelength of 830 nm, using a diode laser (DLT-101, Behsaz Gostar Co. Ltd., Tehran, Iran). The laser beam was coupled with an optical fiber and was defocused by an expanding lens at its distal end. The distance between the light source tip and the exposed sample was fixed to obtain a constant power density. An average light power of 570 mW/cm2 and 400 mW/cm2 was measured for LED and diode laser respectively using a power meter (Puyesh Tajhiz Sanat Pasargad Co., Tehran, Iran) over a spot of 0.7 cm diameter. To calculate power density, the average power was divided by the area of light spot. Besides, a 1% (w/v) erythrosine (Sigma Ltd, Poole, UK) powder as photosensitizer was used and dissolved in distilled water to reach the final concentration of 22 µm, where the filter was sterilized to obtain clear and homogenous solution.
2.3. Lethal photosensitization of bacteria
Colonies of P. gingivalis and F. nucleatum from Mueller-Hinton (MH) Agar plates were suspended in BHI broth, and bacterial density was visually adjusted to a turbidity of 0.5 McFarland standard reagents. The exact density (CFU/mL) of each suspension was verified on MH agar plates. P. gingivalis and F. nucleatum solutions were prepared for five 96-well (7mm diameter) flat-bottom plates with lids (Orange Scientific, Belgium) as follow: visible light + erythrosine (LED+ ER+), laser + erythrosine (L+ ER+), laser (L+ ER−), visible light (LED+ ER−) and erythrosine (L− or LED− ER+). In each study well of plates, 175 µL of P. gingivalis or F. nucleatum suspension plus 175 µL of erythrosine were added. In the groups of laser (LED+ ER−), visible light (LED+ ER−), 175 µL of the sterile phosphate-buffered saline (PBS) was added to equalize the level of the walls. Samples were then kept in the dark for 5 minutes before irradiation. Samples of bacteria in suspension were exposed in a laminar flow hood (Besat, Tehran, Iran) under dark aseptic and aerobic conditions to the maximum output of each light source. The treatment was performed under aerobic condition since the result of a study strongly recommended that the mechanism of phototoxicity of blue light on periopathogenic bacteria is oxygen dependent, which might result mainly in the formation of hydroxyl radicals 38). Light devices were fixed in vertical positions at the level of the wells. To prevent light transmission into neighboring wells, 15 wells of each plate, with 2-well distance between them, were selected and plates were covered with a black shield with an orifice corresponding to the diameter of the wells. Every sample was exposed 1, 2, 3, 4 and 5 min to each light source, bacterial strain and medium combinations, equivalent to flounce of 34-172 J/cm2 using LED. Similar bacterial samples were exposed to the near-infrared diode laser using light exposure parameters similar to those used for blue visible light.
2.4. Determination of bacterial survival
After exposure of the bacteria in suspension to light, samples were diluted 1:10 for six executive times in sterile broth. Then, triplicates of 10 µL were applied to the agar plates. Survival of these bacteria was determined by counting the number of colony forming units (CFU) after incubation. P. gingivalis and F. nucleatum were cultured under anaerobic condition at 37°C until bacteria colonies were visible (1–5 days). The percentage of surviving bacteria was calculated in relation to the control nonexposed samples under similar experimental conditions. All the experiments in which the results of the treated samples differed from those of the control were repeated at least five times.
2.5. Temperature changes in the medium after exposure to the light
A rise in temperature could be secondary factor affecting bacterial survival. For each combination of light source and medium, the temperature was measured in triplicate inside the exposed suspension using thermocouple electrodes (Almemo, Holzkirchen, Germany), before and immediately after a 5 min exposure to the light.
2.6. Statistical methods
To assess the effect of bacterial strains, light source, photosensitizer and the length of exposure to light on bacterial survival, multiway analysis of variance (ANOVA) was applied. The one-sample t-test was used to determine whether the change in bacterial count was significant. All the applied tests were two-tailed, and a P value of ≤ 0.05 was considered statistically significant.
3. Results
3.1. Effect of different treatments and exposure time on bacterial growth
Viability was assessed after different treatments were applied to bacteria under same conditions and is expressed by percent survival of bacteria in suspension. To assess the effect of exposure time and different treatments on bacterial survival, multiway analysis of variance (ANOVA) was applied which its results (P=0.00 for both bacteria species) suggested that the both factors including the exposure time and treatment were significantly effective to reduce the viability of bacteria. Besides, to achieve the optimal treatment and exposure time for each bacteria species, the t-test was used when multiple pairwise comparisons were made. Exposure to visible blue light and diode laser in conjugation with erythrosine resulted in the reduced of P. gingivalis and F. nucleatum noticeably, which was positively affected by exposure time. In addition, we found the same pattern of bactericidal effect of different light sources alone on P. gingivalis as black-pigmented bacteria possesses endogenous porphyrins.
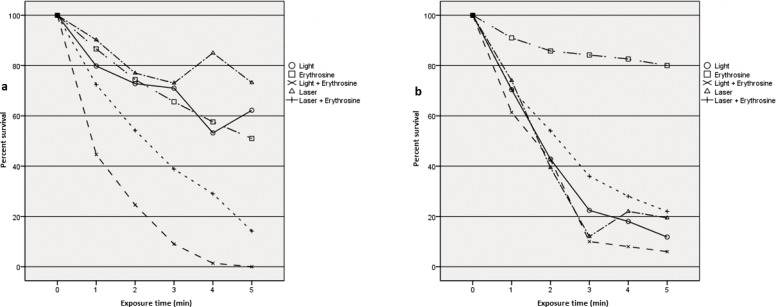
The reduced viability of P. gingivalis after three minutes exposure to visible light or laser without photosensitizer was significantly higher in comparison with F. nucleatum (Fig. 1a, b). The survival rate was moderately lower when the F. nucleatum bacteria treated with erythrosine alone in comparison with the P. gingivalis at the end of whole process which may indicates the probable susceptibility of F. nucleatum to erythrosine as photosensitizer. For example, F. nucleatum in suspension exposed to visible light in the presence of erythrosine for 4 minutes resulted in nearly zero survival, compared with approximately 60% survival when F. nucleatum expose to blue light alone which may points out a possible synergic phototoxic effect of visible blue light and erythrosine as a photosensitizer on this bacteria species (P=0.00, pairwise comparison, t-test) (table 1). Interestingly, the viability of F. nucleatum was reduced remarkably when exposed to diode laser in conjugation with erythrosine comparing with diode laser alone which the difference is statistically meaningful (P=0.00, t-test). Therefore, the result presented here indicates that erythrosine-mediated PDT is a potential treatment to reduce the F. nucleatum as one of the main periopathogenic bacteria. In addition, the number of survived P. gingivalis when exposed to diode laser in the presence of erythrosine was similar to that after treated with diode laser alone at the end of process (Fig. 1b).
Fig. 1:
Effect of different treatments on viability of bacteria in suspension of F. nucleatum (a) and P. gingivalis (b), with exposure time up to 5 minutes.
Our results suggested that 3 minutes exposure of visible blue light with erythrosine exerted a remarkable phototoxic effect on both bacteria species compared with diode laser in the presence of erythrosine which the differences were statistically significant [(P. gingivalis. P=0.013)(F. nucleatum, P=0.066 as a borderline difference), pairwise comparison, t-test) (table 1,2). Perhaps, our suggested treatment (LED+ ER+) can be consider as potential approach of PDT to kill the main periopathogenic species particularly F. nucleatum.
3.2. Temperature change following exposure to light and its effect on bacterial growth
The bacterial medium temperature was measured before and immediately after exposure to diode laser and blue light for up to 5 minutes. The maximum temperature change recorded was 26.5°C and 25.0°C for visible light and diode laser respectively, were measured when compared with the control at 23.0°C. There was no difference in bacterial growth between samples incubated at 27.0 °C for five minutes and the control samples (data not shown).
4. Discussion
Currently, there is considerable interest in the use of locally applied antimicrobial agents in the treatment of periodontitis 39). A major advantage of this approach over the systemic administration of such agents is that it minimizes disruption of the normal microflora at other body sites, so helping to avoid opportunistic infections at these sites. However, one problem with this approach is the difficulty in maintaining therapeutic levels of the agent for a sufficient period of time due to elution of the agent by gingival crevicular fluid 40). The use of PDT, however, is not beset by such problems, as the photosensitizer needs to be retained in the periodontal pocket for only a short time. This is extremely encouraging, as the results of our study show a significant bactericidal effect of blue visible light in the presence of erythrosine on two main periopathogenic species during exposure time of 3 minutes.
In the current study, we applied LED or noncoherent blue light for activating erythrosine which are commonly used in dentistry for photopolymerization of tooth-colored restorative material. LED is a non-monochromatic light that has become a practical technology for PDT in the last few years, especially for irradiation of easily accessible tissue surfaces. The main advantages of LED over laser are their low cost and ease configuration of LED arrays into different geometries 41). In this investigation, we use moderate power light sources since a stimulatory effect of low energy visible light irradiation on various cells proliferation have been largely demonstrated in vitro in a variety of cell lines 42, 43). Besides, higher exposure doses were required to kill bacteria in suspension and this is probably attributable to the scattering and absorption of the light beams in the suspension. Our results indicated that the bactericidal effects of visible light and infrared diode laser were similar at the end of exposure time on both species examined. This is, however, partly in contrast with a study where the authors recommended that using near-infrared laser had no effect on the survival rate of P. gingivalis and F. nucleatum 22). In addition, there are some authors claiming bacteria killing with red and near IR light. For example Nussbaum et al. reported a bactericidal effect at 630 nm for Pseudomonas aeruginosa and Escherichia coli 44). Therefore, the lethal exposure dose of diode laser probably was not dependent not only bacteria species but also on the experimental conditions.
Bacteria species such as Porphyromonas and Prevotella endogenously synthetize porphyrines which absorb at wavelength similar to visible blue light used in this study 45). Soukos et al. claimed that broadband light (380 to 520 nm) rapidly and selectively kills oral black-pigmented bacteria (BPB) in pure cultures and in dental plaque samples obtained from human subjects with chronic periodontitis and they hypothesize that this killing effect is a result of light excitation of their endogenous porphyrins 46). Besides, the results of a study pointed out those bacteria which possess high amounts of endogenous photosensitizers can easily be destroyed with visible light 47) and are in agreement completely with our findings that the exposure visible light or diode laser alone after 3 minutes resulted in significant reduction of viability of P. gingivalis comparing with F. nucleatum. However, it was beyond the scope of the present study to test the role of this photosensitizer in phototoxicity of blue light on bacteria.
The photosensitizer that was used in this study was oral plaque disclosing agent or erythrosine. However, despite the main medical application of erythrosine being its use in staining the aetiological agent of common oral diseases (dental plaque), to our knowledge there are rare reports of the use of erythrosine as a photosensitizer in the mouth. Clearly, erythrosine has an advantage over other photosensitizers in development, as it already targets dental plaque and has full approval for use in the mouth. To determine the phototoxic effect of erythrosine as bacterial sensitizer, we have observed that when the bacteria species exposed to both light source particularly visible blue light in conjugation with erythrosine, the survival rate decreased noticeably. To illustrate, 4 minutes exposure of P. gingivalis and F. nucleatum to the visible light with erythrosine led to nearly zero percent survival. These results completely are in agreement other findings demonstrated well the efficacy of erythrosine in sensitizing of non-oral microbes to killing by light 48–50) which probably highlight the excellence clinical potential of erythrosine-mediated PDT in the control and treatment of periopathogenic and dental plaque biofilm bacteria.
The result of our study confirmed that the bactericidal effects of both light sources with erythrosine decreased moderately in fourth and fifth minute comparing with the first three minute of exposure time. This fact can be explained not only by the limited numbers of photosensitizer's molecules but also by the limited reactive oxygen species (ROS) generating capacity. Moreover, the photodynamic process also leads to diminish erythrosine levels due to the photobleaching 30). Metcalf et al. observed that the fractionation of white light during the erythrosine-mediated PDT of S. mutans biofilm grown in vitro results in increased cell killing compared with continuous irradiation. This may be due to the replenishment, during dark periods, of target molecules (such as oxygen) for the excited photosensitizer and any photosensitizer concentration gradient might be equilibrated during dark periods (51). Therefore, we concluded that the maximum bactericidal effect of our suggested treatment (LED+ ER+) for both species examined could be achieved by optimal exposure time of 3 minutes. However, for the longer exposure duration, we suggest to increase the concentration of the photosensitizer or consider a dark period in which the general replenishment of target molecules (such as oxygen) or redistribution of the photosensitizer would be happened.
The argument that the mechanism of killing of P. gingivalis by blue light is not photochemical but heat induced 52) is not inline with the result of a study where the authors indicated that toxic ROS are generated. In the present investigation, we found that when using lethal light doses (up to 172 J/cm2) an increase in the temperature of the bacteria suspension was recorded but did not reached 27 °C under the experimental conditions. Thus, this result probably may not support a rise in temperature as the killing mechanism involved; however, the possibility that under certain conditions oxygen synergize with temperature in reducing bacterial viability should not be rule out. Perhaps, in clinical condition, the increased temperature duo to the light exposure may be reduced in the presence of some factors such as saliva.
On the other hand, the results of some in vivo studies indicated positive potential effects of photodynamic therapy on reduction of inflammatory signs and main periopathogenic species in animal model 53–55). To illustrate, Moritz et al. studied the efficacy of diode laser on treatment of periodontal pockets and interestingly, they observed that the exposure of diode laser revealed a bactericidal effect and help to reduce the periodontal signs of redness and bleeding on probing in addition to scaling 37). However, there is a lack of clinical or animal researches to determine the efficacy of visible blue light-mediated PDT in the presence of erythrosine particularly on periopathogenic species and periodontal inflammatory signs that merits further investigations.
Conclusion
We conclude that the blue light source, which is used to photopolymerize dental composite material, in conjugation with plaque disclosing agent erythrosine could also serve for the significant reduction of main periopathogenic bacteria. It is likely that the phototoxic effect would be greater under clinical conditions where bacteria are under stress than under ideal in vitro conditions. The encouraging results of this preliminary study suggest that an in vivo investigation of this novel approach are worth undertaken to establish as an additional minimally invasive antibacterial treatment of plaque induced periodontal pathologies such as periodontitis.
Acknowledgment:
This experiment is supported by the Dental Research Center of the Mashhad University of Medical Sciences, Mashhad, Iran. We also wish to thanks Dr. Yasaman Maleki for her invaluable input in statistical analysis of the date.
Conflict of interest:
The authors report no conflicts of interest.
References
- 1: Holt SC, Bramanti TE. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev oral Biol Med 1991;2:177-281. [DOI] [PubMed] [Google Scholar]
- 2: Consensus report periodontal diseases: pathogenesis and microbial factors. Ann Periodontol 1996;1:926-932. [DOI] [PubMed] [Google Scholar]
- 3: Haffajee AD, Socransky SS. Microbial etiologic agents of destructive periodontal diseases. Periodontol 2000, 1994;5:78-111. [DOI] [PubMed] [Google Scholar]
- 4: Sbordone L, Ramaglia I, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planning in human periodontitis. J Periodontol 1990;61:579-584. [DOI] [PubMed] [Google Scholar]
- 5: Chaves ES, Jeffcoat MK, Ryerson CC, Snyder B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J Clin Periodontol 2000;27:897-903. [DOI] [PubMed] [Google Scholar]
- 6: Loesche WJ, Syed SA, Morrison EC, Kerry GA, Higgins T, Stoll J. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. J. Periodontol. 1984;55:325-335. [DOI] [PubMed] [Google Scholar]
- 7: Stabholz A, Nicholas AA, Zimmerman GJ, Wikesjo UM. Clinical and antimicrobial effects of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine in deep periodontal pockets. J Clin Periodontol 1998;25:794-800. [DOI] [PubMed] [Google Scholar]
- 8: Ferez M, Haffajee AD, Goncalves C, Allard KA, Som S, Smith J, Goodson M, Socransky SS. Systemic doxycycline administration in the treatment of periodontal infections. II. Effect on antibiotic resistance of subgingival species. J Clin periodontal 1999;26:784-792. [DOI] [PubMed] [Google Scholar]
- 9: Olsvik B, Tenover FC. Tetracycline resistance in periodontal pathogens. Clin Infect Dis 1993;16:310-313. [DOI] [PubMed] [Google Scholar]
- 10: Marsh PD, Bradshaw DJ. Microbial community aspects of dental plaque. In Dental Plaque Revisited: Oral Biofilm in Health and Disease (Edited by Newman H, Wilson M.), Bioline; Cardiff UK. 1999;pp:237-253. [Google Scholar]
- 11: Pereira CA, Romeiro RL, Costa AC, Machado AK, Junqueira JC, Jorge AO. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Laser Med Sci 2011;26(3):341-348. [DOI] [PubMed] [Google Scholar]
- 12: Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 1998;42:13-28. [DOI] [PubMed] [Google Scholar]
- 13: Gursoy H, Ozcakir-Tomruk C, Tanalp J, Yilmaz S. Photodynamic therapy in dentistry: a literature review. Clin Oral Investig 2013;17(4):1113-1125. [DOI] [PubMed] [Google Scholar]
- 14: Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre dos Santos M, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur J Oral Sci. 2009; 117(5):568-574. [DOI] [PubMed] [Google Scholar]
- 15: Baptista A, Kato IT, Prates RA, Suzuki LC, Raele MP, Freitas AZ, et al. Antimicrobial photodynamic therapy as a strategy to arrest enamel demineralization: a short-term study on incipient caries in a rat model. Photochem Photobiol 2012;88(3):584-589. [DOI] [PubMed] [Google Scholar]
- 16: Longo JP, Leal SC, Simioni AR, Fátima Menezes Almeida-Santos M, Tedesco AC, Azevedo RB. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: an in vitro and clinical study. Lasers Med Sci. 2012;27(3):575-584. [DOI] [PubMed] [Google Scholar]
- 17: Suci P, Kang S, Gmür R, Douglas T, Young M. Targeted delivery of a photosensitizer to Aggregatibacter actinomycetemcomitans. Antimicrob Agents Chemother 2010;54(6):2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18: George S, Hamblin MR, Kishen A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem Photobiol Sci 2009;8(6):788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19: Mang TS, Tayal DP, Baier R. Photodynamic therapy as an alternative treatment for disinfection of bacteria in oral biofilm. Lasers Surg Med 2012; 44(7):588-596. [DOI] [PubMed] [Google Scholar]
- 20: Zivile L. New approach to Inactivation of Harmful and Pathogenic Microorganisms by Photosensitization. Food Technol. Biotechnol 2005;43 (4):411-418. [Google Scholar]
- 21: Luksiene Z, Rutkovskiene L, Griciute L, Vaicaitis V, Sirutkaitis V. New non-coherent light source for photodynamic treatment of cancer. Acta Medica Lithuanica 2002;9:58-61. [Google Scholar]
- 22: Feuerstein O, Persman N, Weiss EI. Phototoxic Effect of Visible Light on Porphyromonas gingivalis and Fusobacterium nucleatum: An In Vitro Study. Photochem and Photobiol 2004;80(3):412-415. [DOI] [PubMed] [Google Scholar]
- 23: Bhatti M, MacRobert A, Meghji S, et al. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol 1998;68:370-376. [PubMed] [Google Scholar]
- 24: Rovaldi CR, Pievsky A, Sole NA, et al. Photoactive porphyrin derivative with broad-spectrum activity against oral pathogens in vitro. Antimicrob Agents Chemother 2000;44:3364-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25: Begue WJ, Bard RC, Koehne GW. Microbial inhibition by erythrosine. J Dent Res 1966;45:1464-1467. [DOI] [PubMed] [Google Scholar]
- 26: Baab DA, Broadwell AH, Williams BL. A comparison of antimicrobial activity of four disclosant dyes. J Dent Res 1983;62:837-841. [DOI] [PubMed] [Google Scholar]
- 27: Marsh PD, Bevis RA, Newman HN, et al. Antibacterial activity of some plaque-disclosing agents and dyes. Caries Res 1989;23:348-350. [DOI] [PubMed] [Google Scholar]
- 28: Tran J, Olmsted J., III Intramolecular triplet-triplet energy transfer from xanthene dyes to an anthyrl substituent. J Photochem Photobiol A Chem 1993;71:45-49. [Google Scholar]
- 29: Conlon KA, Berrios M. Light-induced proteolysis of myosin heavy chain by Rose Bengal-conjugated antibody complexes. J Photochem Photobiol B 2001;65(1):22-28. [DOI] [PubMed] [Google Scholar]
- 30: Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 2006;57:680-684. [DOI] [PubMed] [Google Scholar]
- 31: Williams JL, Stamp J, Devonshire R, Fowler GJ. Methylene blue and the photodynamic therapy of superficial bladder cancer. J Photochem Photobiol B 1989;4:229-232. [DOI] [PubMed] [Google Scholar]
- 32: Orth K, Beck, Genze F, Rück A. Methylene blue mediated photodynamic therapy in experimental colorectal tumours in mice. J Photochem Photobiol B 2000;57(22-3):186-192. [DOI] [PubMed] [Google Scholar]
- 33: Wilson M, Dobson J, Sarkar S. Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 1993;8:182-187. [DOI] [PubMed] [Google Scholar]
- 34: Gutter B, Speck WT, Rosenkranz HS. A study of the photoinduced mutagenicity of methylene blue. Mutation Res 1997;44:177-182. [DOI] [PubMed] [Google Scholar]
- 35: Capella MAM, Menzies S. Synergism between electrolysis and methylene blue photodynamic action in Escherichia coli. Int J Radiat Biol 1992;62:321-326. [DOI] [PubMed] [Google Scholar]
- 36: Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photo-bactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Med Surg 2001;29(2):165-173. [DOI] [PubMed] [Google Scholar]
- 37: Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W. Treatment of periodontal pockets with a diode laser. Lasers Sur Med 1998;22(5):302-311. [DOI] [PubMed] [Google Scholar]
- 38: Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss E. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol 2005;81:1186-1189. [DOI] [PubMed] [Google Scholar]
- 39: Slots J, Jorgensen MJ. Effective, safe, practical and affordable periodontal antimicrobial therapy: where are we going, and are we there yet? Periodontol. 2000, 2002;28:298-312. [DOI] [PubMed] [Google Scholar]
- 40: Oosterwaal PJ, Mikx FH, Renggli HH. Clearance of a topically applied fluorescein gel from periodontal pockets. J. Clin. Periodontol 1990;17:613-615. [PubMed] [Google Scholar]
- 41: Nagata JY, Hioka N, Kimura E, Batistela VR, Terada RS, Graciano AX, Baesso ML, Hayacibara MF. Antibacterial photodynamic therapy for dental caries: evaluation of the photosensitizers used and light source properties. Photodiagnosis Photodyn Ther 2012;9(2):122-131. [DOI] [PubMed] [Google Scholar]
- 42: Peplow PV, Chung TY, Baxter GD. Laser photobio-modulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 2010. ;28 (Suppl 1):3-40. [DOI] [PubMed] [Google Scholar]
- 43: Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photodestroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 2008;40(10):734-737. [DOI] [PubMed] [Google Scholar]
- 44: Nussbaum EL, Lilge L, Mazzulli T. Effects of 630, 660, 810, and 905nm laser irradiation delivering radiant exposure of 1-50 J/cm2 on three species of bacteria in vitro. J Clin Laser Med Surg 2002; 20(6):325-333. [DOI] [PubMed] [Google Scholar]
- 45: Shah HN, Bonnett R, Mateen B, Williams RAD. The porphyrin pigmentation of subspecies of Bacteroides melaninogenicus. Biochem J 1979; 180,45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46: Soukos NS, Som S, Abernethy AD, Ruggiero K, dunham J, Lee C, Doukas AG, Goodson JM. Phototargeting oral black-pigmented bacteria. Antimicrob agents Chemother 2005;49:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47: Lubart R, Lipovski A, Nitzan Y, Friedmann H. a possible mechanism for the bactericidal effect of visible light. Laser Ther 2011;20(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48: Jodlbauer A, von Tappeiner H. Uber die wirkung photodynamischer (fluoreszierender) stoffe auf bakterien. Munch Med Wochenschr 1904;51:1096-1097. [Google Scholar]
- 49: Huber H. Weitere verusche mit photodynamischen, sensibilisierenden farbstoffen (eosin, erythrosin). Pufund der wirkung des tageslichtes auf lebensfahigkeit und virulenz von bakterian, auf toxine und antitoxine und auf das labferment. Archiv Hygeine 1905;54:53-88. [Google Scholar]
- 50: Krasnoff SB, Faloon D, Williams J, et al. Toxicity of xanthene dyes to entomopathogenic fungi. Biocontrol Science and Technology 1999;9:215-225. [Google Scholar]
- 51: Metcalf D, Robinson C, devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother 2006;58:190-192. [DOI] [PubMed] [Google Scholar]
- 52: Izzo AD, Walsh JT. Light induced modulation of Porphyromonas gingivalis growth. J Photochem Photobiol 2004;77:63-69. [DOI] [PubMed] [Google Scholar]
- 53: Sigusch BW, Pfitzner A, Albrecht V, Glockmann E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog. J Periodontol 2005; 76(7):1100-1105. [DOI] [PubMed] [Google Scholar]
- 54: Moritz A, Gutknecht N, Doertbudack O, Goharkhay K, Schoop U, Schauer P, Sperr W. Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg 1997;15(1):33-37. [DOI] [PubMed] [Google Scholar]
- 55: Hayek RR, Araujo NS, Gioso MA, Ferreira J, Baptista-sobrinho CA, Yamada AM, Ribeiro MS. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dog. J Periodontol 2005;76(8);1275-1281. [DOI] [PubMed] [Google Scholar]