Abstract
During penicillin treatment of an autolysin defective mutant pneumococcus we have observed three novel phenomena: (i) Growth of the mutant cultures is inhibited by the same concentrations of penicillin that induce lysis in the wild type. (ii) Mutant bacteria treated with the minimum growth inhibitory concentration of penicillin will lyse upon the addition of wild-type autolysin to the growth medium. Chloramphenicol and other inhibitors of protein synthesis protect the cells against lysis by exogenous enzyme. Sensitivity of the cells to exogenous autolysin requires treatment with penicillin or other inhibitors of cell wall synthesis (e.g., D-cycloserine or fosfonomycin) since exogenous autolysin alone has no effect on bacterial growth. (iii) Treatment with penicillin (or other inhibitors of cell wall synthesis) causes the escape into the medium of a choline-containing macromolecule that has properties suggesting that it contains pneumococcal lipoteichoic acid (Forssman antigen). Each one of these three phenomena (growth inhibition, sensitization to exogenous autolysin, and leakage of lipoteichoic acid) shows the same dose response as that of the penicillin-induced lysis of wild-type pneumococci. On the basis of these findings we propose a new hypothesis for the mechanism of penicillin-induced lysis of bacteria. It is suggested that inhibition of cell wall synthesis by any means triggers bacterial autolytic enzymes by destabilizing the endogenous complex of an autolysin inhibitor (lipoteichoic acid) and autolytic enzyme. Escape of lipoteichoic acid-like material to the growth medium is a consequence of this labilization. Chloramphenicol protects bacteria against penicillin-induced lysis by interfering with the activity of the autolytic enzyme, rather than by depleting the concentration of the enzyme at the cell surface.
Full text
PDF
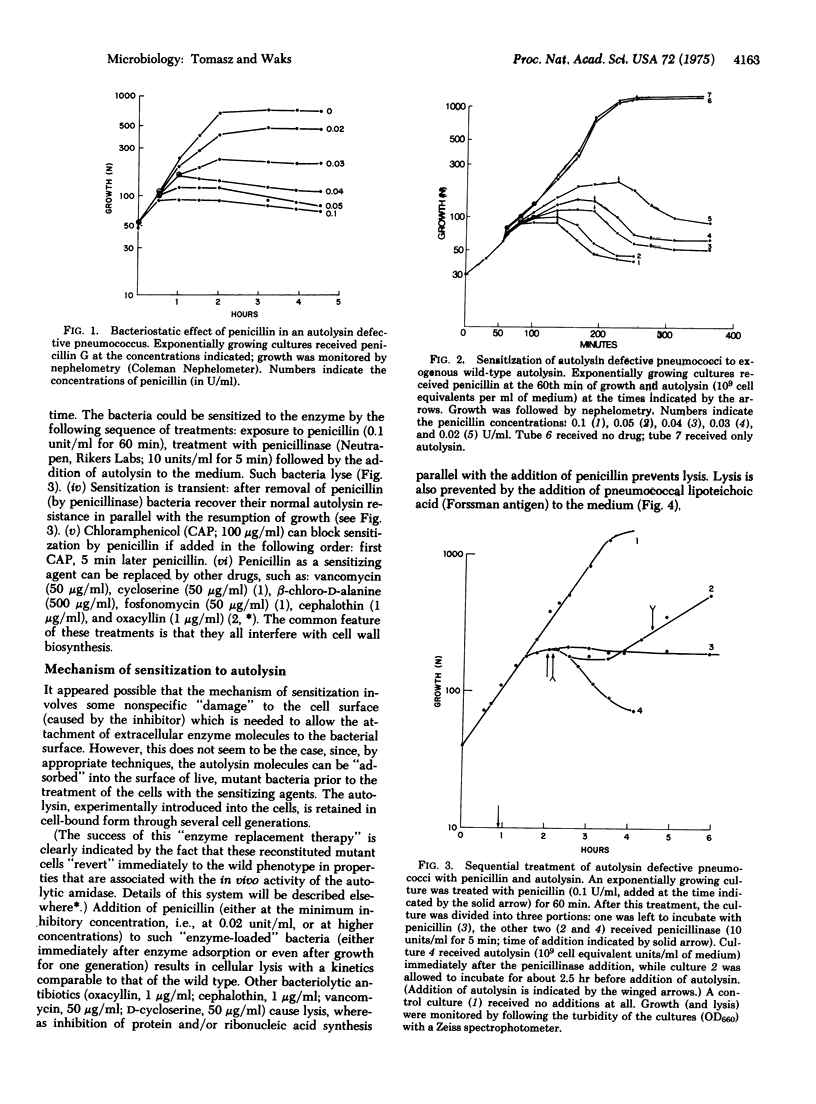
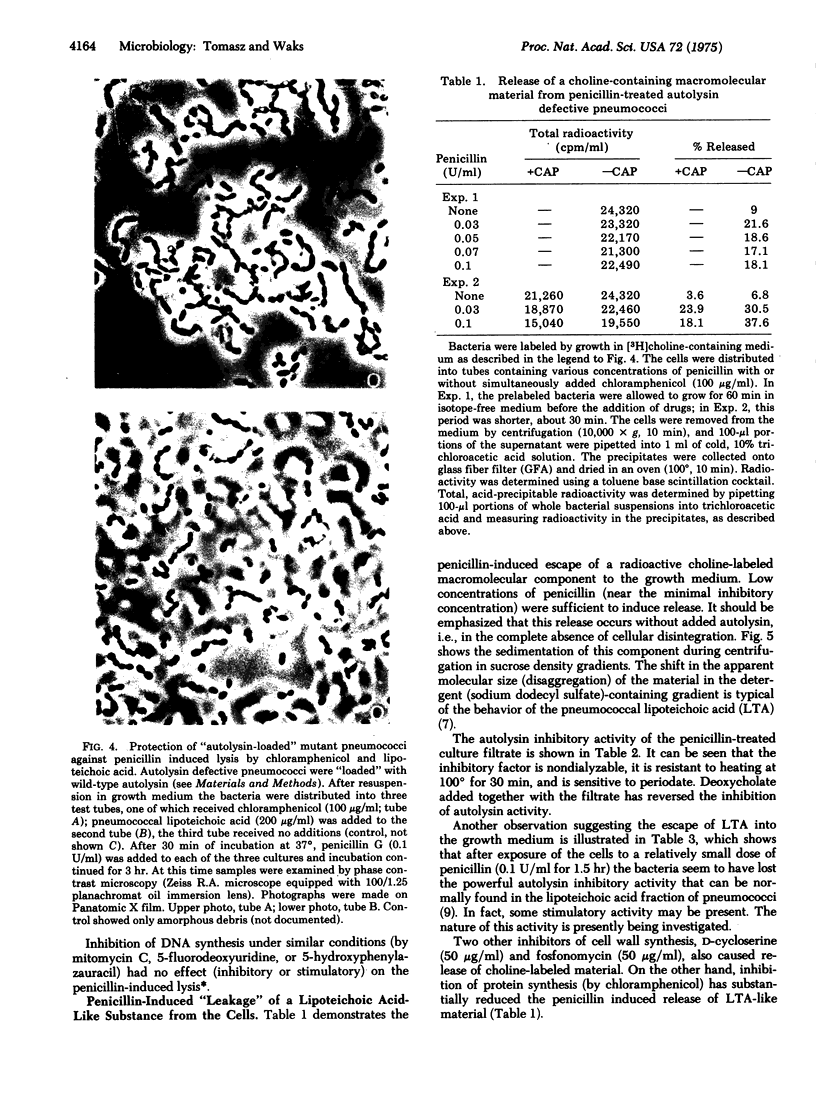
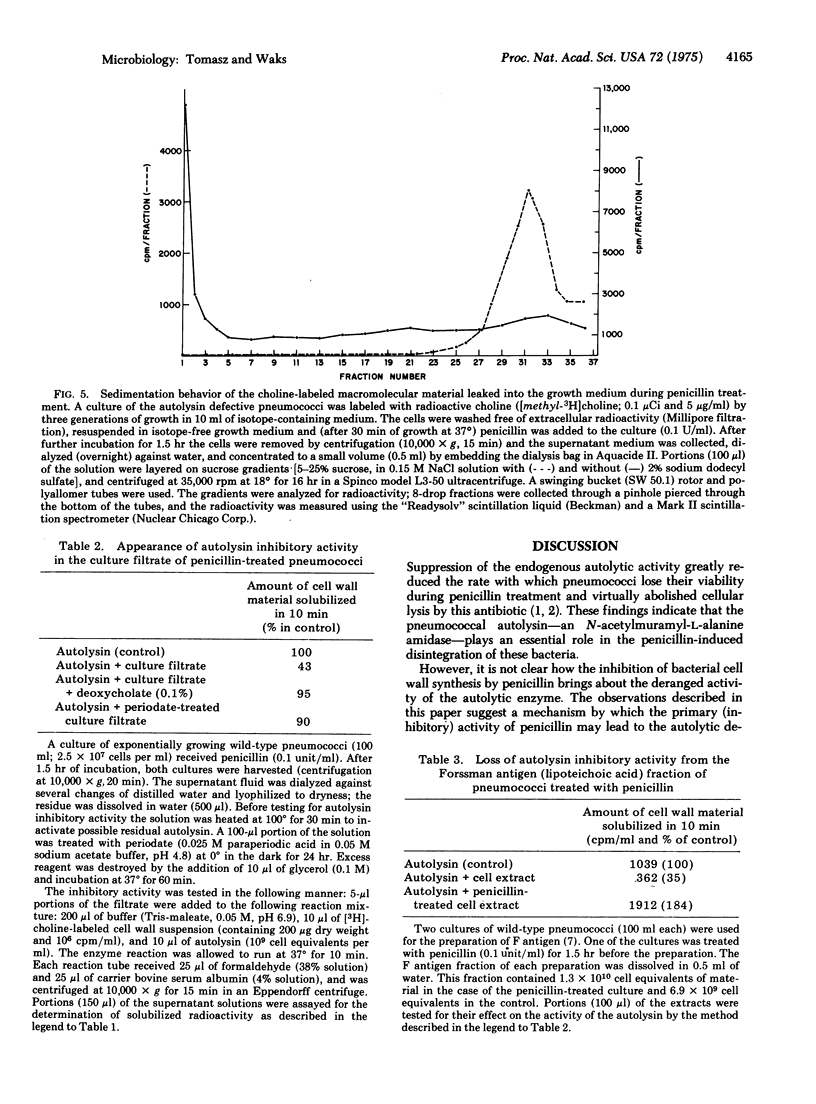

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. I. Purification of polyribitol phosphate polymerase and lipoteichoic acid carrier. J Biol Chem. 1974 May 10;249(9):2684–2689. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Teichoic acid phosphorylcholine esterase. A novel enzyme activity in pneumococcus. J Biol Chem. 1974 Nov 10;249(21):7032–7034. [PubMed] [Google Scholar]
- JAWETZ E., GUNNISON J. B., SPECK R. S., COLEMAN V. R. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. AMA Arch Intern Med. 1951 Mar;87(3):349–359. doi: 10.1001/archinte.1951.03810030022002. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Dietrich C. P., Strominger J. L. Incorporation of glycine into the cell wall glycopeptide in Staphylococcus aureus: role of sRNA and lipid intermediates. Proc Natl Acad Sci U S A. 1965 Aug;54(2):587–594. doi: 10.1073/pnas.54.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]