Abstract
Background
The ACOSOG Z0011 trial concluded that axillary lymph node dissection (ALND) may not be necessary for all patients with sentinel lymph node (SLN) metastasis undergoing breast-conserving therapy (BCT). The aim of this study was to assess applicability of Z0011 results to our patient population and determine what percentage may be affected by these results.
Study Design
Patients with clinical T1-2,N0 breast cancer treated with surgery first between 1994 and 2009 who had 1–2 positive SLNs were included in this study. Kaplan-Meier survival curves were calculated and log-rank used to compare overall survival (OS) and disease-free survival (DFS) for ALND vs. SLN dissection (SLND) alone in two patient populations: patients undergoing BCT or total mastectomy (TM) and patients undergoing BCT only.
Results
Of 861 patients, 188 (21.8%) underwent SLND alone. Of 488 (56.7%) patients who underwent BCT, 125 (25.6%) had SLND alone. Of 412 patients undergoing TM, 67 (16.3%) had SLND alone. Patients undergoing ALND were significantly younger, had larger tumors, macrometastasis and extranodal extension in both populations. Compared with the Z0011 cohort, our BCT patients had more T1 tumors (76.0% vs 69.3%, P = .01) and more grade II–III tumors (87.3% vs 76.2%, P < .0001). After adjusting for T-stage, there were no significant differences in DFS and OS between patients undergoing SLND alone or ALND in both populations.
Conclusions
Examination of our breast cancer patients with Z0011 trial criteria suggests that almost 75% of SLN-positive patients would be candidates to avoid ALND if they undergo BCT.
Keywords: ACOSOG Z0011, node-positive breast cancer, axillary lymph node dissection, sentinel lymph node dissection, survival
INTRODUCTION
Sentinel lymph node (SLN) dissection (SLND) has become a widely accepted method of nodal staging for patients with clinically lymph node-negative breast cancer.1,2 Because complete axillary lymph node dissection (ALND) often requires a second surgery and is potentially associated with significant morbidity, the need for ALND in all patients with SLN metastases has been questioned.3–7 Two recent studies using data from population-based databases reported that compared with SLND alone, SLND with complete ALND did not appear to be associated with significantly improved survival for breast cancer patients with micrometastases in the SLNs; one of these studies used data from the Surveillance Epidemiology and End Results (SEER) database,8 and the other used data from the National Cancer Data Base (NCDB).9 These 2 studies suggested that surgeons have been omitting ALND in patients with what would be perceived as favorable characteristics.
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial was a randomized trial designed to compare overall survival (OS) and disease-free survival (DFS) in SLN-positive patients with and without ALND (Figure 1).10 Last year, the study investigators reported that they found no differences in local or regional recurrence rates and no differences in OS between the 2 study arms at a median follow-up of 6.3 years and they concluded that ALND may not be necessary for patients with limited SLN metastasis.11 Based on their findings, current guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend considering no further surgery for patients who meet ACOSOG Z0011 eligibility criteria.12 The aim of our study was to assess the applicability of the Z0011 trial results to our patient population and to determine what percentage may be affected by these recent results.
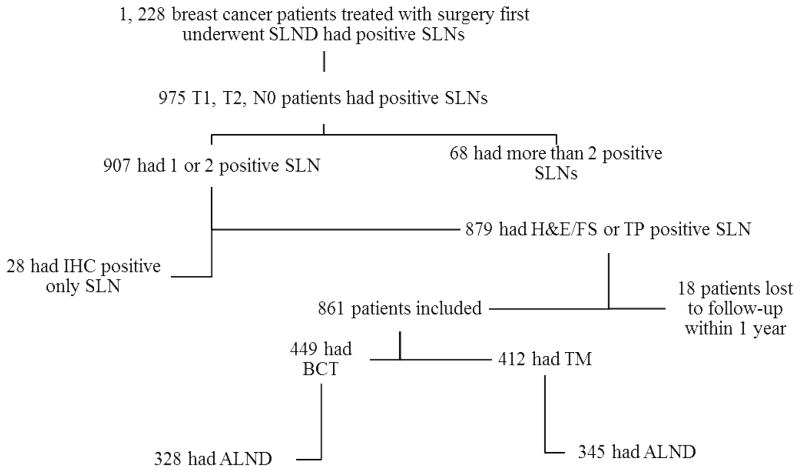
Figure 1.
Nodal management of breast cancer patients treated with surgery as the first intervention who underwent SLND between January 1994 and December 2009 and had positive SLNs. ALND, axillary lymph node dissection; BCT, breast-conserving therapy; FS, frozen section; H&E, hematoxylin and eosin; IHC, immunohistochemistry; SLN, sentinel lymph node; SLND, sentinel lymph node dissection; TM, total mastectomy; TP, touch preparation.
METHODS
Patient Selection and Data Collection
We used the Surgical Breast Oncology Research database at The University of Texas MD Anderson Cancer Center to retrospectively identify patients with invasive breast cancer treated with surgery as the first intervention who had SLND between January 1994 and December 2009 and had positive SLNs. We included only clinical T1/T2,N0 patients with 1 or 2 positive SLNs identified by frozen section, touch preparation, or hematoxylin and eosin (H&E) staining of permanent sections. Because our goal was to determine the number of patients who would be eligible for omission of ALND based on tumor biology, we included patients who underwent breast conserving therapy (BCT) and mastectomy realizing that only patients who elect to undergo BCT with adjuvant whole breast radiotherapy would be eligible for a SLND-only approach given the Z0011 criteria. Patients with positive SLNs identified by immunohistochemistry (IHC) only were excluded from this study. SLN metastases were defined as micrometastases if the H&E-stained tumor deposits were >0.2–2.0 mm in diameter and macrometastases if the tumor deposits were >2.0 mm in diameter. We excluded patients who were lost to follow-up within 1 year after surgery. The MD Anderson Institutional Review Board approved this study.
For each patient, we extracted demographic, pathologic, clinical, and follow-up data from the database. Pathologic data included nuclear grade, histology, lymph node status, presence of lymphovascular invasion (LVI), presence of extranodal extension, lymph node metastasis diameter, estrogen receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor 2 (HER-2) status. Clinical data included clinical T stage, clinical tumor size, surgery type, and use of adjuvant chemotherapy, adjuvant hormonal therapy, and adjuvant radiation therapy. Follow-up data included evaluation for the occurrence and timing of local, regional, and systemic recurrence and vital status at last follow-up.
Study Endpoints
The primary endpoints were DFS time, defined as the time from SLND to death due to breast cancer or first documented recurrence of breast cancer; OS time, defined as the time from SLND to death from any cause. First sites of recurrence were classified as local, regional, or systemic. Isolated skin, chest wall, and/or subcutaneous recurrences were considered local, and isolated regional lymph node metastases were classified as regional.
Statistical Analysis
Patient, tumor, and treatment characteristics were evaluated and compared between the ALND group and the SLND-alone group in two patient populations: patients treated with BCT or total mastectomy (TM) and patients undergoing BCT only. The Wilcoxon rank-sum test or the Student t test was used to compare the means of continuous variables. The χ2 test or Fisher’s exact test was used for univariate comparison of categorical variables. Kaplan–Meier survival curves were calculated for the 2 groups, and the log-rank test was used to compare the OS, DFS, and alive with no evidence of disease (ANED) survival between groups in two patient populations: patients undergoing BCT or total mastectomy (TM) and patients undergoing BCT only. The stratified log-rank test was used for equality of survivor functions between the ALND and SLND-alone groups stratified by clinical T stage in two patient populations: patients undergoing BCT or total mastectomy (TM) and patients undergoing BCT only. A multivariate stratified Cox proportional hazards model was used to identify significant predictors of OS and DFS survival. Estimated risks of death were calculated with hazard ratios (HRs) with 95% confidence intervals (CIs). Stata statistical software (SE 10.1, StataCorp, College Station, TX) was used for statistical analyses. All P values were 2-tailed, and P ≤ .05 was considered significant.
RESULTS
Patients
We identified 1228 breast cancer patients treated with surgery as the first intervention who had undergone SLND and had 1 or 2 positive SLNs. Of those, 975 (79.4%) patients had clinical T1/T2,N0 tumors (Fig. 1); 907 (93.0%) of these patients had 1 or 2 positive SLNs, and among those, 879 (96.9%) had positive SLNs identified by frozen section, touch preparation, or H&E staining of permanent sections. We excluded 18 patients who were lost to follow-up within 1 year, leaving 861 patients in the study population. Of these, 673 (78.2%) patients underwent ALND after SLND. Of the 861 patients in the study population, 488 (56.7%) underwent breast-conserving therapy (BCT).
Patient, Tumor, and Treatment Characteristics in Patients who Underwent BCT or Mastectomy
We compared patient and tumor characteristics between patients who underwent ALND and those who underwent SLND alone in patients who underwent BCT or total mastectomy (TM) (Table 1). Patients in the ALND group were significantly younger, had larger tumors, and were more likely to have extranodal extension in the SLNs. Patients in the ALND group were more likely to have LVI and more likely to undergo mastectomy. There were no significant differences with respect to nuclear grade, ER status, PR status, or tumor histologic subtype between the ALND and SLND-alone groups in patients who underwent BCT or mastectomy.
Table 1.
Comparison of Patient, Tumor, and Treatment Characteristics between Sentinel Lymph Node Dissection Alone and Axillary Lymph Node Dissection Groups in Patients who Underwent Breast-Conserving Therapy or Total Mastectomy and had Positive Sentinel Lymph Nodes
| Characteristic | n (%)*
|
||
|---|---|---|---|
| ALND (n = 673) | SLND Alone (n = 188) | p Value | |
| Age, y | 0.0005 | ||
| Mean | 55.0 | 58.3 | |
| Median (range) | 54 (22–91) | 57 (29–89) | |
| Clinical T stage | <0.0001 | ||
| T1 | 445 (66.1) | 152 (80.9) | |
| T2 | 228 (33.9) | 36 (19.1) | |
| Clinical tumor size, cm | <0.0001† | ||
| Mean | 1.8 | 1.5 | |
| Median (range) | 1.6 (0.09–5) | 1.3 (0.1–5) | |
| Nuclear grade | 0.1 | ||
| I | 68 (10.1) | 29 (15.4) | |
| II | 371 (55.1) | 103 (54.8) | |
| III | 234 (34.8) | 56 (29.8) | |
| Presence of LVI | 0.007 | ||
| No | 419 (62.3) | 137 (72.9) | |
| Yes | 254 (37.7) | 51 (27.1) | |
| Surgery type | 0.002 | ||
| BCT | 328 (48.7) | 121 (64.4) | |
| Total mastectomy | 345 (51.3) | 67 (35.6) | |
| SLN extranodal extension | <0.0001 | ||
| Yes | 142 (21.1) | 10 (5.3) | |
| No | 531 (78.9) | 178 (94.7) | |
| Lymph node metastasis diameter | <0.001 | ||
| Micrometastasis (>0.2–2.0 mm) | 158 (23.5) | 136 (72.3) | |
| Macrometastasis (>2.0 mm) | 515 (76.5) | 52 (27.7) | |
| Adjuvant chemotherapy | <0.001 | ||
| Yes | 545 (81.0) | 126 (67.0) | |
| No | 128 (19.0) | 62 (33.0) | |
| Adjuvant hormonal therapy | 0.4 | ||
| Yes | 535 (79.5) | 144 (76.6) | |
| No | 138 (20.5) | 44 (23.4) | |
| Adjuvant radiation therapy | 0.08 | ||
| Yes | 418 (62.5) | 129 (69.4) | |
| No | 251 (37.5) | 57 (30.6) | |
| Unknown | 4 | 2 | |
| Time from surgery to adjuvant chemotherapy, d | 0.1† | ||
| Mean | 47.9 | 46.0 | |
| Median (range) | 43 (2–179) | 41.5 (1–159) | |
| Follow-up time, y | 0.01† | ||
| Mean | 5.4 | 5.8 | |
| Median (range) | 4.9 (1–17.1) | 5.5 (1.2–11.2) | |
| Local recurrence | 0.7‡ | ||
| Yes | 12 (1.8) | 2 (1.1) | |
| No | 661 (98.2) | 186 (98.9) | |
| Regional recurrence | 0.1‡ | ||
| Yes | 11 (1.6) | 0 | |
| No | 662 (98.4) | 188 | |
| Distant recurrence | 0.2‡ | ||
| Yes | 36 (5.4) | 5 (2.7) | |
| No | 637 (94.6) | 183 (97.3) | |
| Overall survival rate, % | |||
| 5-Year | 94.3 | 95.5 | |
| 10-Year | 81.9 | 92.5 | |
| Disease-free survival rate, % | |||
| 5-Year | 95.7 | 98.0 | |
| 10-Year | 80.2 | 82.5 | |
Unless otherwise stated.
Wilcoxon rank-sum test.
Fisher’s exact test.
ALND, axillary lymph node dissection; BCT, breast-conserving therapy; LVI, lymphovascular invasion; SLN, sentinel lymph node; SLND, sentinel lymph node dissection; TM, total mastectomy.
The median total number of nodes removed (including SLNs) was 19 (range, 1–55) in the ALND group and 4 (range, 1–15) in the SLND-alone group. There was no significant difference between these groups in the median total number of nodes with histologically demonstrated tumor involvement (including SLNs). Micrometastases were identified in SLNs of a significantly smaller percentage of patients in the ALND group than in the SLND-alone group (P < .001). In the ALND group, 183 of 673 (27.2%) patients had additional metastasis in lymph nodes removed by ALND, including 7 (3.8%) patients with SLN micrometastasis who had macroscopically involved non-SLNs removed.
Adjuvant systemic therapy was delivered to 649 (96.4%) patients in the ALND group and 183 (97.3%) patients in the SLND-alone group (table 1). There were no significant differences in the percentage of patients who received hormonal therapy or radiation therapy. More patients in the ALND group received adjuvant chemotherapy. There was no difference between the groups in time from primary surgery to adjuvant chemotherapy, whereas there was a significant difference in time from primary surgery to adjuvant radiation therapy: 225 days in the ALND group vs 196 days in the SLND-alone group (P < .001).
Patient, Tumor, and Treatment Characteristics in Patients who Underwent Breast-Conserving Therapy
We compared patient and tumor characteristics between patients who underwent ALND and those who underwent SLND alone in patients treated with BCT (Table 2). Similar to patients in the entire cohort, patients in the ALND group treated with BCT were significantly younger, had larger tumors, and were more likely to have extranodal extension in the SLNs. There were no significant differences with respect to nuclear grade, presence of LVI, ER status, PR status, or tumor histologic subtype between the ALND and SLND-alone groups in patients treated with BCT.
Table 2.
Comparison of Patient, Tumor, and Treatment Characteristics between Sentinel Lymph Node Dissection Alone and Axillary Lymph Node Dissection Groups in Patients who Underwent Breast-Conserving Therapy and had Positive Sentinel Lymph Nodes
| Characteristic | n (%)*
|
||
|---|---|---|---|
| ALND (n = 328) | SLND Alone (n = 121) | p Value | |
| Age, y | 0.002 | ||
| Mean | 55.1 | 58.8 | |
| Median (range) | 55 (22–82) | 57 (37–88) | |
| Clinical T stage | 0.1† | ||
| T1 | 242 (73.8) | 98 (81.0) | |
| T2 | 86 (26.2) | 23 (19.0) | |
| Clinical tumor size, cm | 0.009‡ | ||
| Mean | 1.7 | 1.4 | |
| Median (range) | 1.5 (0.1–5) | 1.3 (0.5–3.5) | |
| SLN extranodal extension | <0.0001 | ||
| Yes | 80 (24.4) | 6 (5.0) | |
| No | 248 (75.6) | 115 (95.0) | |
| Lymph node metastasis diameter | <0.0001 | ||
| Micrometastasis (>0.2–2.0 mm) | 69 (21.0) | 87 (71.9) | |
| Macrometastasis (>2.0 mm) | 259 (78.5) | 34 (28.1) | |
| Adjuvant chemotherapy | <0.001 | ||
| Yes | 271 (82.6) | 79 (65.3) | |
| No | 57 (17.4) | 42 (34.7) | |
| Adjuvant hormonal therapy | 0.6 | ||
| Yes | 263 (80.2) | 94 (77.7) | |
| No | 65 (19.8) | 27 (22.3) | |
| Adjuvant radiation therapy | 1.0† | ||
| Yes | 317 (97.5) | 117 (98.3) | |
| No | 8 (2.5) | 2 (1.7) | |
| Unknown | 3 | 2 | |
| Time from surgery to adjuvant chemotherapy, d | 0.002‡ | ||
| Mean | 50.4 | 44.8 | |
| Median (range) | 46 (2–179) | 36 (1–159) | |
| Follow-up time, y | 0.3‡ | ||
| Mean | 5.7 | 5.8 | |
| Median (range) | 5.2 (1–17.1) | 5.4 (1.2–11.2) | |
| Local recurrence | 0.5† | ||
| Yes | 8 (2.4) | 1 (0.9) | |
| No | 320 (97.6) | 120 (99.1) | |
| Regional recurrence | 0.2† | ||
| Yes | 7 (2.1) | 0 | |
| No | 321 (97.9) | 121 | |
| Distant recurrence | 0.2† | ||
| Yes | 16 (4.9) | 2 (1.6) | |
| No | 312 (95.1) | 119 (98.4) | |
| Overall survival rate, % | |||
| 5-Year | 95.2 | 95.9 | |
| 10-Year | 81.7 | 93.8 | |
| Disease-free survival rate, % | |||
| 5-Year | 96.8 | 99.0 | |
| 10-Year | 88.6 | 94.0 | |
Unless otherwise stated.
Fisher’s exact test.
Wilcoxon rank-sum test.
ALND, axillary lymph node dissection; BCT, breast-conserving therapy; LVI, lymphovascular invasion; SLN, sentinel lymph node; SLND, sentinel lymph node dissection.
The median total number of nodes removed (including SLNs) was 19 (range, 1–50) in the ALND group and 3 (range, 1–13) in the SLND-alone group. Micrometastases were identified in SLNs of a significantly smaller percentage of patients in the ALND group than in the SLND-alone group (P < .0001). In the ALND group, 87 (24.0%) of 363 patients had additional metastasis in lymph nodes removed by ALND, including 5 (5.7%) patients with SLN micrometastasis who had macroscopically involved non-SLNs removed.
There were no significant differences in the percentage of patients who received hormonal therapy (table 2). More patients in the ALND group received adjuvant chemotherapy. For 10 patients without radiation therapy in the BCT group: 2 patients had M1 disease, 1 patient had metastatic peritoneal disease, 1 with recurrent tumor had radiation therapy for primary tumor before, 6 patients declined radiation therapy. There were significant differences in time from surgery to adjuvant chemotherapy between the ALND and SLND-alone groups: median time from surgery to adjuvant radiation therapy was 227 days in the ALND group vs 187 days in the SLND-alone group (P < .001), and median time from surgery to adjuvant chemotherapy was 47 days in the ALND group vs. 38 days in the SLND-alone group (P = .008).
Comparisons between the Z0011 Cohort and the MDACC BCT Cohort
Table 3 shows the comparisons between the Z0011 cohort and the MD Anderson Cancer Center (MDACC) BCT cohort. There were no significant differences in the adjuvant treatment, hormone receptor status, presence of LVI, or lymph node metastasis diameter. Patients in the Z0011 cohort had larger tumors (T2, 30.7% vs 24.0% in MDACC BCT cohort, P = .01), whereas a greater percentage of patients in the MDACC BCT cohort had higher nuclear grade (grade II/III, 87.3% vs 76.2% in the Z0011 cohort, P < .0001).
Table 3.
Comparisons between the Z0011 Cohort and the MD Anderson Cancer Center Breast-Conserving Therapy Cohort
| Characteristic | n (%)
|
p Value | |
|---|---|---|---|
| Z0011 Cohort (n = 856) | MDACC BCT cohort (n = 449) | ||
| Clinical T stage | 0.02* | ||
| T1 | 587 (69.3) | 340 (75.7) | |
| T2 | 260 (30.7) | 109 (24.3) | |
| No. of adjuvant treatments | 0.05* | ||
| ≥1 | 826 (96.5) | 442 (98.4) | |
| 0 | 30 (3.5) | 7 (1.6) | |
| Nuclear grade | <0.0001 | ||
| I | 152 (23.8) | 58 (12.9) | |
| II/III | 487 (76.2) | 391 (87.1) | |
| Presence of LVI | 0.5* | ||
| No | 397 (62.1) | 303 (67.5) | |
| Yes | 242 (37.9) | 146 (32.5) | |
| Hormone receptor status | 0.2* | ||
| ER+/PR+ | 526 (67.9) | 319 (71.2) | |
| ER+/PR− | 115 (14.8) | 73 (16.3) | |
| ER−/PR+ | 7 (0.9) | 3 (0.7) | |
| ER−/PR− | 127 (16.4) | 53 (11.8) | |
| Lymph node metastasis diameter | 0.8 | ||
| Micrometastasis (>0.2–2.0 mm) | 301 (33.8) | 156 (34.7) | |
| Macrometastasis (>2.0 mm) | 590 (66.2) | 293 (65.3) | |
Fisher’s exact test.
ER, estrogen receptor; LVI, lymphovascular invasion; MDACC, MD Anderson Cancer Center; PR, progesterone receptor; BCT, breast-conserving therapy.
Survival Outcomes
DFS rates did not differ significantly between patients undergoing ALND versus SLND-alone when all patients were evaluated regardless of surgical approach or when evaluating just those undergoing BCT. For patients who underwent BCT, the 5-year DFS rate was 94.3% (95% CI, 91.1–98.0%) for the SLND-alone group and 93.8% (95% CI, 91.4–95.5%) for the ALND group. The unadjusted HR comparing the SLND-alone group with the ALND group for patients who underwent BCT was 0.3 (95% CI, 0.1–1.01, P = .052), and the HR adjusted for clinical T stage, age, and adjuvant treatment was 0.3 (95% CI, 0.1–1.1, P = .06). Multivariate analysis showed that lack of chemotherapy and larger tumors were significantly associated with worse DFS (table 4).
Table 4.
Multivariate Analysis for Factors Associated with Survival Outcomes
| Characteristic | Patients who Underwent BCT or TM | Patients who Underwent BCT | ||||
|---|---|---|---|---|---|---|
| HR | p Value | 95% CI | HR | p Value | 95% CI | |
| Disease-free survival | ||||||
| SLND alone | NS | NS | ||||
| Clinical stage T2 | 3.1 | <0.001 | 1.8–5.4 | 3.5 | 0.001 | 1.7–7.5 |
| Adjuvant chemotherapy | ||||||
| Yes | Referent | Referent | ||||
| No | NS | 2.9 | 0.03 | 1.1–7.2 | ||
| Adjuvant hormonal therapy | ||||||
| Yes | Referent | |||||
| No | 2.1 | 0.009 | 1.2–3.6 | NS | ||
| Presence of LVI | 2.0 | 0.01 | 1.1–3.3 | NS | ||
| Age, y* | NS | 0.9 | <0.001 | 0.9–0.97 | ||
| Overall survival | ||||||
| SLND alone | NS | |||||
| Clinical stage T2 | 2.2 | 0.003 | 1.3–1.5 | 2.3 | 0.03 | 1.1–4.9 |
| Adjuvant chemotherapy | ||||||
| Yes | Referent | Referent | ||||
| No | 2.6 | <0.0001 | 1.5–4.3 | 3.7 | <0.0001 | 1.8–7.5 |
Treated as a continuous variable.
BCT, breast-conserving therapy; CI indicates confidence interval; ER, estrogen receptor; HR, hazard ratio; LVI, lymphovascular invasion; NS, not significant; SLND, sentinel lymph node dissection; TM, total mastectomy.
At a median follow-up of 5.2 years (range, 2–10.1) for the entire cohort, there were 63 deaths (SLND-alone group, 9; ALND group, 54). When evaluating patients that underwent mastectomy or BCT or including only those undergoing BCT, the use of SLND alone compared with ALND did not appear to result in statistically inferior OS rates. When we assessed patients by clinical T stage, no significant differences in OS rates were found between treatment groups in patients who underwent BCT or mastectomy.
DISCUSSION
Similar to the ACOSOG Z0011 trial, 11 we found no significant differences in local, regional, or systemic recurrence rates between patients who underwent SLND alone and those who underwent ALND in patients who underwent BCT or in the entire patient cohort, including those who underwent mastectomy. Importantly, there were no significant differences in DFS, ANED survival, and OS outcomes between the SLND-alone and ALND groups when we controlled for clinical T stage.
A meta-analysis showed that 53% of patients with a positive SLN had additional axillary nodes with metastatic disease on complete ALND.13 In patients with micrometastatic disease in the SLN(s), the rate of non-SLN involvement has been reported to be 20%; and in patients with isolated tumor cells, the rate decreases to 12%.14,15 These findings have prompted a trend toward omitting ALND in selected patients. ALND, as a means for achieving regional disease control, carries an indisputable and often unacceptable risk of complications such as seroma, infection, and lymphedema.16–18 The well-documented morbidities from ALND have led many clinicians to explore alternative methods of axillary treatment in patients with clinically negative nodes, including radiation and systemic therapy. Reports with these alternative methods have consistently demonstrated low axillary failure rates, with no significant differences in survival.19,20
Because the decision to administer systemic chemotherapy or hormonal therapy is dictated largely by tumor biology, and generally not by the absolute numbers of positive lymph nodes, the results of the Z0011 trial have not changed the adjuvant systemic therapy recommendations by our Breast Medical Oncology group.21 Both the 11th St. Gallen (Switzerland) Expert Consensus Meeting on the primary treatment of early breast cancer (in March 2009) and the 12th St. Gallen International Breast Cancer Conference (in March 2011) maintained an emphasis on targeting adjuvant systemic therapies according to subgroups defined by predictive markers.22,23 The 2011 expert panel agreed that factors arguing for the inclusion of chemotherapy were high histologic grade, high proliferation as measured by Ki-67, low hormone receptor status, positive HER2 status, and triple-negative status in invasive ductal carcinoma of usual forms.23 The decision to use systemic therapy is now most often decided by breast cancer subtypes, and the regional nodes have less importance in this decision making. The use of axillary staging still plays an important role in clinical decision making regarding use of systemic therapy, however, the role of completion node dissection in this decision making process is less clear The number of positive lymph nodes is still used in determining the need for post-mastectomy radiation therapy in women undergoing total mastectomy. This may be one area where completion axillary node dissection still plays an important role in adjuvant therapy decisions.
Our study has some limitations. First, it was performed using retrospectively collected data, and treatment was not assigned in a randomized fashion. There were 21.8% of patients in whom ALND was omitted which may due to the fact that the patients had more favorable clinicopathologic characteristics (as evidenced by table 1). It is possible that differences in the use of adjuvant radiation therapy might bias the result. It is unlikely that our physicians chose more intensive chemotherapy regimens for those receiving SLND alone to compensate for the less extensive surgical procedure, since we utilize the same adjuvant chemotherapy regimens for node negative and node positive patients. The extent of surgery, however, may have also impacted the delivery of adjuvant radiation therapy. We cannot compare the survival difference between patients treated with radiation therapy and without radiation therapy because of the small sample size.
Conclusions
In the current era, breast cancer is more likely to be detected on screening mammography resulting in smaller tumors with fewer involved lymph nodes. Multimodality treatment is the standard, and use of systemic therapy has been shown to improve local-regional control and survival. As a result, ALND is less often necessary in the current management of early stage breast cancer patients. The low event rate in both the Z0011 trial and a contemporary population of patients treated at our institution indicates that any survival advantage of ALND would be quite modest. The 12th St. Gallen International Breast Cancer Conference (held March 16–19, 2011, in St. Gallen, Switzerland) and current breast cancer treatment recommendation have accepted the option of omitting ALND for SLN metastases in the context of lumpectomy and radiation therapy for patients with clinically node-negative disease and 1–2 positive SLNs as reported from the ACOSOG Z0011 trial.11,23 However, this practice, based on specific eligibility criteria in a clinical trial, should not be extended more generally, such as to patients undergoing mastectomy, those who will not receive whole-breast tangential-field radiation therapy, those with involvement of >2 SLNs, and those receiving neoadjuvant therapy.23
Examination of our breast cancer patient population with the Z0011 study criteria suggests that up to 75% of SLN-positive patients would be candidates to avoid ALND if they underwent BCT. Up to 83% of SLN-positive patients in our cohort who underwent TM would also be candidates to avoid ALND if they chose BCT since omitting ALND has not been validated in TM patients in prospective trials. The use of SLND alone compared with ALND did not result in inferior survival.
Acknowledgments
The authors wish to acknowledge Karen R Muller, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editorial assistance.
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. Am J Ophthalmol. 2000;130:688. doi: 10.1016/s0002-9394(00)00754-6. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. doi: 10.1245/aso.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. doi: 10.1001/archsurg.138.1.52. [DOI] [PubMed] [Google Scholar]
- 5.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–730. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 6.Jeruss JS, Winchester DJ, Sener SF, et al. Axillary recurrence after sentinel node biopsy. Ann Surg Oncol. 2005;12:34–40. doi: 10.1007/s10434-004-1164-2. [DOI] [PubMed] [Google Scholar]
- 7.Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2011;255:109–115. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17:343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast, version 1.2012. 2012 http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 13.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 14.Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245–1252. doi: 10.1002/bjs.4725. [DOI] [PubMed] [Google Scholar]
- 15.van Deurzen CH, de Boer M, Monninkhof EM, et al. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst. 2008;100:1574–1580. doi: 10.1093/jnci/djn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivens D, Hoe AL, Podd TJ, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotze MT, Duncan MA, Gerber LH, et al. Early versus delayed shoulder motion following axillary dissection: a randomized prospective study. Ann Surg. 1981;193:288–295. doi: 10.1097/00000658-198103000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeoh EK, Denham JW, Davies SA, et al. Primary breast cancer. Complications of axillary management. Acta Radiol Oncol. 1986;25:105–108. doi: 10.3109/02841868609136386. [DOI] [PubMed] [Google Scholar]
- 19.Martelli G, Boracchi P, De Palo M, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T1N0 breast cancer: results after 5 years of follow-up. Ann Surg. 2005;242:1–6. doi: 10.1097/01.sla.0000167759.15670.14. discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronesi U, Orecchia R, Zurrida S, et al. Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16:383–388. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 21.Caudle AS, Hunt KK, Kuerer HM, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011;18:2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]