Abstract
Microvillous cells of the main olfactory epithelium have been described variously as primary olfactory neurons, secondary chemosensory cells, or non-sensory cells. Here we generated an IP3R3tm1(tauGFP) mouse in which the coding region for a fusion protein of tau and green fluorescent protein (tauGFP) replaces the first exon of the Itpr3 gene. We provide immunohistochemical and functional characterization of the cells expressing IP3 receptor type 3 in the olfactory epithelium. Since we determined that these cells bear microvilli at their apex, we call these cells IP3R3 MV cells. The cell body of these IP3R3 MV cells lies in the upper third of the main olfactory epithelium; a long thick basal process projects towards the base of the epithelium without penetrating the basal lamina. Retrograde labeling and unilateral bulbectomy corroborated that these IP3R3 MV cells do not extend axons to the olfactory bulb and therefore are not olfactory sensory neurons. The immunohistochemical features of the IP3R3 MV cell varied suggesting either developmental stages or the existence of subsets of these cells. Thus, for example, subsets of the IP3R3 MV cells make contact with substance P fibers or express the purinergic receptor P2X3. In addition, in recordings of intracellular calcium, these cells respond to ATP and substance P as well as to a variety of odors. The characterization of IP3R3 MV cells as non-neuronal chemoresponsive cells helps explain the differing descriptions of microvillous cells in the literature.
Keywords: calcium imaging, rodents, immunohistochemistry
Introduction
The main olfactory epithelium (MOE) of rodents comprises several cell types: ciliated olfactory sensory neurons (OSNs) sending odorant information to the olfactory bulb, supporting cells that control the local ionic environment, and basal cells that proliferate to give rise to the different epithelial cells (Finger et al., 2000). Furthermore, multiple microvillous cells whose function is not well understood have been observed in the MOE of rodents and humans: 1) Microvillous OSNs with an axon projecting to the olfactory bulb (Moran et al., 1982; Mombaerts, 2008) displaying a morphology similar to that of the microvillous sensory neurons of the vomeronasal organ (VNO) express the transient receptor channel C2 (TRP C2) (Liman et al., 1999). 2) Microvillous cells that are not OSNs and lack a basal process/axon (Carr et al., 1991; Braun and Zimmermann, 1998). In a recent study, two morphologically different types of the non-neuronal microvillous cell were identified that express the TRP M5 channel (Lin et al., 2007; Hansen & Finger, 2008). In addition, several microvillous cells were observed that were TRP M5-negative (Hansen & Finger, 2008). Elsaesser and coworkers reported microvillous cells in the rodent MOE that expressed phospholipase C beta-2 (PLCβ2), TRP C6, and the type 3 inositol trisphosphate receptor (IP3R3) (Elsaesser et al., 2005). These PLCβ2-immunoreactive cells responded to odors suggesting they were sensory cells. However, unlike OSNs, these cells did not degenerate after olfactory bulbectomy indicating that they may not send a process to the olfactory bulb. Elsaesser and co-workers concluded that these cells are “secondary chemosensory cells” of unknown function. Thus, the function of microvillous cells in the MOE remains enigmatic.
Here we provide new information on cells expressing IP3R3 in the MOE by immunohistochemical and functional characterization of IP3R3-tauGFP-expressing cells in an IP3R3-knockout/tauGFP knockin mouse. IP3 is a second messenger that is formed in response to stimuli and released into the cytoplasm where it can activate IP3-receptors, intracellular calcium channels that release calcium from the endoplasmic reticulum. This release of calcium regulates a wide variety of physiological processes including cell growth, development, sensory perception, neuronal signaling, endocrine processes and exocrine secretion (Nucifora, Jr. et al., 1996; Patterson et al., 2004; Futatsugi et al., 2005; Berridge, 2009). Differential expression of the 3 subtypes of mammalian IP3 receptors (types 1–3) is critical for the vast array of calcium signaling patterns (Hattori et al., 2004). In the CNS, Type 1 and 3 IP3Rs are expressed in neurons while type 2 IP3R is expressed predominantly in glia (Sharp et al., 1999). IP3 receptors mediate responses in chemosensory cells from catfish olfactory epithelium (Restrepo et al., 1990). We find a large number of IP3R3-tauGFP-positive microvillous cells in the MOE that lack axons and therefore are not receptor neurons (we call them IP3R3 MV cells). A subset of these IP3R3 MV cells may be the microvillous cells characterized by Elsaesser and co-workers (Elsaesser et al., 2005).
Materials and Methods
IP3R3-tauGFP mouse
We generated a mouse in which the coding region for a fusion protein of tau and green fluorescent protein (tauGFP) followed by three poly adenylation sites (3xpA) and a floxed neomycin gene replaces the first exon of the Itpr3 gene (Fig. 1, The Jackson Laboratory, Bar Harbor, ME, MMRRC Stock Number 32884: STOCK Itpr3<tm1.1(Mapt/GFP)Rmnc/Mmjax). In the rest of the manuscript the mouse is denoted as the IP3R3-tauGFP mouse. Fig. 1 shows the targeting construct that includes flanking DNA with 3′ and 5′ regions complementary to the sequence in the Itpr3 gene as well as a cassette encoding for tauGFP (amplified from the IRES-tauGFP-LNL plasmid from the Mombaerts laboratory) (Rodriguez et al., 1999). We use tau fusion to GFP so that if the construct were expressed in neurons the tauGFP would be transported down the axons. Modification of the tauGFP cassette included the addition of three polyadenylation sites at the end to ensure termination of the transcript (Maxwell et al., 1989). LNL is a floxed neomycin gene for selection of embryonic stem cells. Neo was flanked by loxP because this gene is known to decrease expression of the GFP insert in some gene-targeted mice. Indeed, in immunohistochemical studies we found vastly increased expression of GFP after crossing of IP3R3-tauGFP with actin-cre mice (cre recombinase excises the loxP-flanked neo insert). IP3R3-tauGFP mice were generated by targeted incorporation of the construct in F1 hybrid embryonic stem cells (c7.1, B6129XF1) (Chick et al., 2005). After crossing mice with actin-cre mice (in C57BL/6 background), all subsequent crosses were with C57BL/6. Importantly, the Itpr3 gene undergoes biallelic expression (Gimelbrant et al., 2007) which is relevant because if expression were monoallelic, then IP3R3 MV cells in IP3R3+ / IP3R3− tauGFP+ mice would be split into two populations, one expressing IP3R3 and the other expressing GFP, but not expressing IP3R3. Because IP3R3 undergoes biallelic expression, IP3R3 MV in the IP3R3+ / IP3R3−tauGFP+ mice will express both IP3R3 and GFP, in accordance with our preliminary results making the GFP marker useful in the study of the physiology and cell biology of this unique microvillous cell type. The IP3R3-tauGFP mice (IP3R3+ / IP3R3− tauGFP+) as well as IP3R3-knockout (IP3R3− tauGFP+ / IP3R3− tauGFP+) and wild type mice (both littermates and WT inbreds) (C57BL/6) used for control experiments were bred and housed in the animal facilities of the University of Colorado Denver School of Medicine and at Michigan State University. Animals of both sexes were 1 – 5 days (physiological experiments) or 1 to 6 months old (anatomical experiments). All procedures were in compliance with the University of Colorado Denver and Michigan State University’s Animal Care and Use Committees.
Fig. 1.
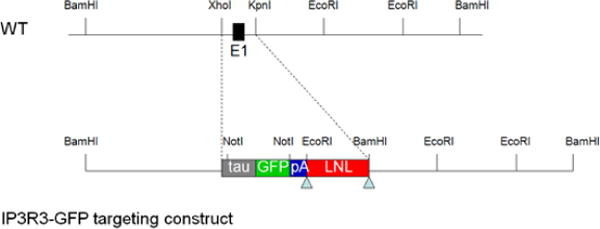
IP3R3-tauGFP targeting construct used to generate the IP3R3tm1(tauGFP) mouse. The targeting construct includes flanking DNA with 3′ and 5′ regions complementary to the sequence in the Itpr3 gene as well as a cassette encoding for tauGFP (amplified from the IRES-tauGFP-LNL plasmid).
Genotyping
PCR was performed in the Rocky Mountain Taste and Smell Center at the University of Colorado Denver or by Michigan State University’s Genomics Technology Support Facility to identify the presence of the transgene in mice. Primers (Invitrogen, Carlsbad, CA) for detection of IP3R3 transcripts were forward (5′-GGTGAGTGAGCCTAGGGCAAAGAGA-3′) and reverse (5′-TCTTCTCCAAGCATCCTCCAGGC-3′), primers for GFP were forward (5′-TTCA AGGACGACGGCAACT-3′) and reverse (5′-ACTTGTACAGCTCGTC CATGC-3′) and primers for CRE were forward (5′-GCTGGTTAGCACCGCAGGTGTAGAG-3′) and reverse (5′-CGCCATCTTCCAGCAGGCGCACC-3′) oligonucleotides.
DiI
Wild-type and IP3R3 MV mice were anesthetized with 20% chloral hydrate (2 mg/g body weight) or Nembutal (100 mg/kg), perfused transcardially with 0.9 % saline followed by 4% paraformaldehyde (PFA). The heads were collected and fixed in 4% PFA overnight. Then the skull above the olfactory bulbs was opened. A small crystal of DiI (Molecular Probes; Eugene, OR) was placed into each olfactory bulb close to the cribriform plate by means of an insect pin. The skull was closed with a layer of 2% agar. Then the heads were placed in 4% PFA at room temperature for 3 to 4 weeks. After incubation, the olfactory organs were dissected and embedded in 15% gelatin (Sigma; St. Louis, MO). 40 to 50 μm sections were cut on a vibratome (Ted Pella, Inc.; Redding, CA) and viewed under a fluorescence microscope or a confocal laser microscope (Olympus; Center Valley, PA). The DiI had traveled into most areas of the olfactory epithelium.
Unilateral olfactory bulb ablation
To perform unilateral olfactory bulb ablation, mice (IP3R3+ / IP3R3− tauGFP+) were anesthetized (4 % isoflurane to acquire anesthesia and then 2 % isoflurane to maintain anesthesia), the scalp was incised, and a hole drilled through bone directly above the right olfactory bulb. The olfactory bulb was aspirated using a small diameter glass pipette, the hole packed with gelfoam, and the skin closed with surgical staples. The exposed bulb was left undamaged in sham animals. To collect tissue, 2, 4, 6, and 8 days later mice were anesthetized (65mg/kg ketamine + 5mg/kg xylazine, ip), transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 4% PFA, and decapitated. The lower jaw and skin was removed and tissue was postfixed overnight in 4% PFA. Tissue was placed in 0.5 M EDTA (pH 8.0) for 4–5 days. After decalcification, the tissues were cryoprotected with 20% sucrose and embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA). Frozen coronal sections (20 μm) were collected from levels 2–6 of the mouse MOE (Young, 1981). Tissue was always compared from equivalent rostrocaudal levels between treatment groups. GFP was visualized at the level where the endoturbinate 4 is first present. An Olympus FV1000 confocal microscope (Olympus, Center Valley, PA) equipped with a krypton-argon ion laser was used for fluorescence excitation at 488 nm and fluorescence emissions were filtered at 510 nm. Image stacks were acquired using a 0.80 NA 40X water objective at a voxel size of 0.497 μm with 25–48 confocal planes of 1.5 μm/slice.
Immuno-light microscopy
Wildtype (C57BL/6) and IP3R3-tauGFP mice of both sexes aged between 2 weeks and 6 months were anesthetized with 20% chloral hydrate (2 mg/g body weight) or Nembutal (100 mg/kg), perfused transcardially with 0.9 % saline followed by PLP fixation (75 mM lysine, 1.6% paraformaldehyde, 10 mM sodium periodate, pH 7.2). The olfactory organs were dissected and postfixed in the same fixative for 15 min to overnight. Cryoprotection was carried out in 20% sucrose overnight. The tissue was embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA). Cryosections (12 – 14 μm) were mounted on Superfrost Plus slides (VWR, West Chester, PA) and frozen at −80°C until further use. Standard immunohistochemical procedures were used. Briefly, cryosections were rinsed in 0.1 M phosphate buffered saline (PBS), blocked in blocking solution containing 1% BSA, 3% normal donkey serum, and 0.3% Triton X-100 in PBS for 2 hours, and then incubated in the primary antisera overnight to 3 days. For details of antibodies see Table 1. After 3 washes, 20 min each, the sections were incubated in the appropriate secondary antibodies (Alexa 488, Alexa 568, 1:400; Invitrogen, Carlsbad, CA, DL549, DL649, Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. After incubation, sections were washed 3 times 20 min and coverslipped with Fluormount-G (Fisher Biotech, Birmingham, AL). Control slides were treated either without the primary antibody or with normal rabbit serum replacing the primary antiserum. When the appropriate peptides were available, the antisera were adsorbed with the blocking peptides. Control sections showed no labeling. The antibodies against IP3R3 were tested in IP3R3− tauGFP+ / IP3R3− tauGFP+ mice (IP3R3 knockout) and also showed no label in those tissues confirming the specificity of the reactivity reported below. Sections were viewed under a fluorescence microscope or a confocal laser microscope (Olympus; Center Valley, PA). The voxel size for x and y ranged from 1.32 μm to 11.70 μm depending on the magnification, step size for z was 0.75 μm. Stack size ranged from single confocal plane to 20.
Table 1.
Antisera used to characterize IP3R3 MV cells
| Antisera against | Marker for | Company | Lot | |
|---|---|---|---|---|
| Acetylated tubulin | olfactory cilia | Sigma T6793 | 041K4817 | − |
| AC III | component of olfactory transduction pathway | Santa Cruz sc-588 | J3009 | − |
| CGRP | trigeminal nerve fibers (contacting IP3R3 cells) | Peninsula Lab. T-4032 | 040826-4 | +/− |
| ChAT | enzyme that synthesises acetylcholine | Abcam AB65097 Chemicon AB144P |
634985 LV1443701 LV1580976 |
− |
| CK18 | supporting cells | Chemicon MAB3234 Bioworld Techn.,Inc. BS1204 |
0507004430 380704 |
− − |
| CNG2A | component of olfactory transduction pathway | Santa Cruz sc-13700 | – | − |
| Espin | actin-binding protein in microvilli | Dr. J. Bartles, Northwestern University, Chicago | – | − |
| Gαolf | component of olfactory transduction pathway | Santa Cruz sc-383 | L0707 | − |
| Gαq/11 | G-protein subunit q/11/14 – transduction component | Santa Cruz Sc-392 | F1107 | + |
| GFP | green fluorescent protein | Abcam AB290 | 207431 | + |
| Gustducin | G-protein subunit in taste and solitary chemosensory cells | Santa Cruz sc-395 | D0808, D181, E1704 | − |
| Hu-C | neuronal cells | Molecular Probes A21271 | A21271 | − |
| IP3R3 | IP3 receptor 3 – transduction component | Chemicon AB9076 | 25041643 | + |
| OMP | olfactory sensory neurons | Dr. F. Margolis, University of Maryland | – | − |
| P2X2 | purinergic receptor – transduction component | Alomone Labs APR003 | AN-06 | − |
| P2X3 | purinergic receptor – transduction component | Chemicon AB5895 | 0602021455 | +/− |
| PDE2A | transduction component | Santa Cruz Sc-17227 | L0402 | − |
| PGP 9.5 | neuronal and neuroendocrine cells | AbD Serotec 7663-0504 | 071207 | − |
| PLCβ2 | transduction component | Santa Cruz Sc-206 | I181, A1204, B0907 | − |
| Substance P | trigeminal nerve fibers (contacting IP3R3 MV cells) | Accurate YMC1021 | E9381 | +/− |
| SUS-1 | supporting cells in the MOE | Dr. James Morgan Roche Res. Ctr., Nutley, N.J. | 17S-4-III-83 | − |
| TH | Tyrosine hydroxylase | PhosphoSolutions 2025THRAB | ajo209 | − |
| TrpC6 | transient receptor potential channel C6 – transduction component | Abcam AB12249 | 342108 | +/− |
| VAChT | membrane transport protein | Chemicon AB1578 | 24080681 | − |
| Villin | actin-binding protein in microvilli | Beckman-Coulter (ImmunoTech) 0258 | 1D2C3 | − |
= expressed in all IP3R3 MV cells
= expressed in some IP3R3 MV cells or nerve fibers
= not expressed in IP3R3 MV cells)
Immuno-electron microscopy
For immunolabeling at the electron microscopic level, the animals were perfused, cryoprotected in a series of solutions containing sucrose and glycerin and processed as described for Immuno-light microscopy with the following exceptions: 30 to 50 μm floating sections were cut on a LEICA cryostat (LEICA, Bannockburn, IL), collected in 0.1 M PBS and treated with 3% H2O2 for 15 min. Triton X-100 was replaced with 1% saponin. Incubation times were extended to 3 days for primary antibodies and overnight for secondary antibodies. Secondary antibodies were biotinylated and processed with standard ABC/DAB methods (Vectastain kit, Vector Lab., Burlingame, CA) to produce a permanent reaction product. Sections containing labeled cells were then postfixed in 4% glutaraldehyde and 1% osmium tetroxide and embedded in Epon/Araldite between two Aclar sheets (Ted Pella; Redding, CA) for transmission electron microscopy. Silver to gold sections were cut with an ultramicrotome (LEICA, Bannockburn, IL) and examined with a G12 FEI TEM (FEI, Hillsboro, OR). Some of the ultrathin sections were viewed after staining with uranyl acetate and lead citrate.
Figures were created in Adobe Photoshop, Version CS2 (Adobe Systems Inc., San Jose, CA). In some micrographs dirt spots were removed from areas where no tissue was involved with the clone stamp tool.
Confocal Calcium Imaging
To prepare olfactory epithelial slices, neonatal mice (postnatal day 0–6) were quickly decapitated, and the skin and lower jaw were removed. Tissue was embedded in a carrot, mounted on a vibratome-cutting block in ice cold Ringer’s solution and 250–300 μm slices were made. Slices were loaded with 50 μg/ml X-Rhod 1 AM (Molecular Probes, Eugene, OR) made in Ringer’s solution with 0.00005% pluronic F-127 (Invitrogen, Carlsbad, CA) and 0.0001% DMSO for 60 minutes at 25 °C. Slices were placed in a laminar flow chamber (Warner Instruments, Hamden, CT) and perfused continuously with Ringer’s solution at a flow rate of 1.5–2.0 ml/min. Test solutions were applied using bath exchange and a small volume loop injector (200 μl). Our perfusion system exchanges the bath in ca. 7–10 s and traces were not corrected for this delay.
An Olympus FV1000 confocal microscope (Olympus, Center Valley, PA) was used for data collection and analysis. A krypton-argon ion laser was used for fluorescence excitation of GFP at 488 nm and a helium-neon laser was used to excite X-Rhod-1 AM at 543 nm. Fluorescence emissions were filtered at 505–525 and 560–660 nm, respectively. Using this configuration, we could visualize the IP3R3 MV cells and measure changes in calcium levels simultaneously, however, to prevent cross talk of fluorescence emission, the 488 laser line was turned off after the 2nd frame captured. The focal plane was 50–100 μm below the surface of the slice to avoid damaged cells. Time series experiments using a 0.80 NA water objective were performed collecting images of a voxel size of 0.3–0.4 μm at 0.2 – 1 Hz. The fluorometric signals obtained are expressed as relative fluorescence (F) change, ΔF/F = (F−F0)/F0, where F0 is the basal fluorescence level (mean F of first 10 frames). Data was collected from a minimum of 3 slices for each experiment.
To determine the response profiles of IP3R3 MV cells to bioactive compounds, odorants, ATP and substance P were superfused individually and, in the case of odorants, in combination. We limited the number of applications performed on any slice to 5 due to the observation of calcium rundown and photobleaching following more than 5 applications. Thus, with this limitation, not every bioactive compound was superfused on every slice. Some experiments were performed using calcium-free solution. Due to the limited number of transgenic pups, we performed the calcium-free experiments as follows. In half of the experiments, the slices were superfused in calcium-free solution for 5 minutes. Following separate applications of ATP and odorants, the bath was changed to calcium-containing solution for 5 minutes. Separate applications of ATP and then odorants were then performed. In the other half of the experiments, ATP and odorants were separately applied and then the bath was switched to calcium-free solution for 5 minutes. Odorants and ATP were then superfused in the maintained absence of calcium. There was no difference in the peak heights of ATP or odorants in calcium (t22 = 0.86, P = 0.40, t6 = 0.33, P = 0.75) or calcium free solutions (t22 = 1.34 P = 0.19, t6 = 0.05 P = 0.96). Hence, we combined the data and reported the combined averages. Some experiments were performed using a purinergic receptor antagonist PPADS. Following application of odorants or ATP, antagonist was bath perfused for 5 minutes and odorants and ATP were applied in the maintained presence of antagonist. The bath was perfused back to Ringer’s solution for 5 minutes and a recovery application of ATP or odorants was applied. Only cells in which the third application induced a calcium transient amplitude ± 20% of the first application were included in the data set.
Calcium imaging solutions
Ringer’s solution contained (mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose; pH 7.4, 290–320 mOsms. Probenecid (500 μM), an inhibitor of the organic anion transporter, was included to aid in the loading and retention of X-Rhod 1 AM (Di et al., 1990; Manzini et al., 2008). Zero calcium Ringer’s solution contained (mM) 130 NaCl, 5.5 KCl, 10 HEPES, 10 glucose; 4 glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 7.4, 290–320 mOsms. Concentrated stock solutions were made in water (Substance P) or Ringer’s solution (ATP, PPADS), stored at −20 ºC and reconstituted on the day of the experiment. The odorants were added directly to Ringer’s solution and were presented singly (50 μM isoamyl acetate, octanol, heptanol, R-carvone, cineole, vanillin, acetyl pyrazine, or isovaleric acid) or as a mixture (10 μM each of cineole, octanol, heptanol, isoamyl acetate and R-carvone).
If not listed differently, chemicals were purchased from SigmaAldrich (St. Louis, MO).
Statistical Analysis
Statistical comparisons were performed with either a Student’s t-test or with a Chi-Squared test. All statistical analyses were performed with GraphPad Software (La Jolla, CA) and a P-value below 0.05 was considered a statistically significant difference.
Results
Whole mounts and sections from IP3R3+ / IP3R3− tauGFP+ mice showed GFP-positive cells in both the main olfactory epithelium (MOE) and in the respiratory epithelium (RE) (Fig. 2A,B). However, the morphology of the cells in the two areas was different. The cells in the MOE spanned the whole depth of the MOE although the cell body was located in the uppermost portion of the epithelium among the nuclei of the supporting cells. Beneath the nuclei of the GFP-positive cells a long process reaches towards the basal lamina. This process is thicker than the axons of the olfactory sensory neurons (OSNs) (compare Figs. 2B and 2C) and often shows varicosities, sometimes even branches (Fig. 2B). Surprisingly, a few GFP-positive fibers are visible among the fila olfactoria beneath the basal lamina (Fig. 2D) but none were detected in the glomeruli of the olfactory bulb (Fig. 2E). These data suggested that these GFP-positive cells do not possess an axon and therefore are not olfactory receptor neurons. To further corroborate that this cell type does not project an axon to the olfactory bulb we retrogradely labeled OSNs with DiI applied to the cranial surface of the cribriform plate. Cells of the morphology seen in the GFP-positive cells were not labeled by DiI (Fig. 2C) although ciliated OSNs were.
Fig. 2.
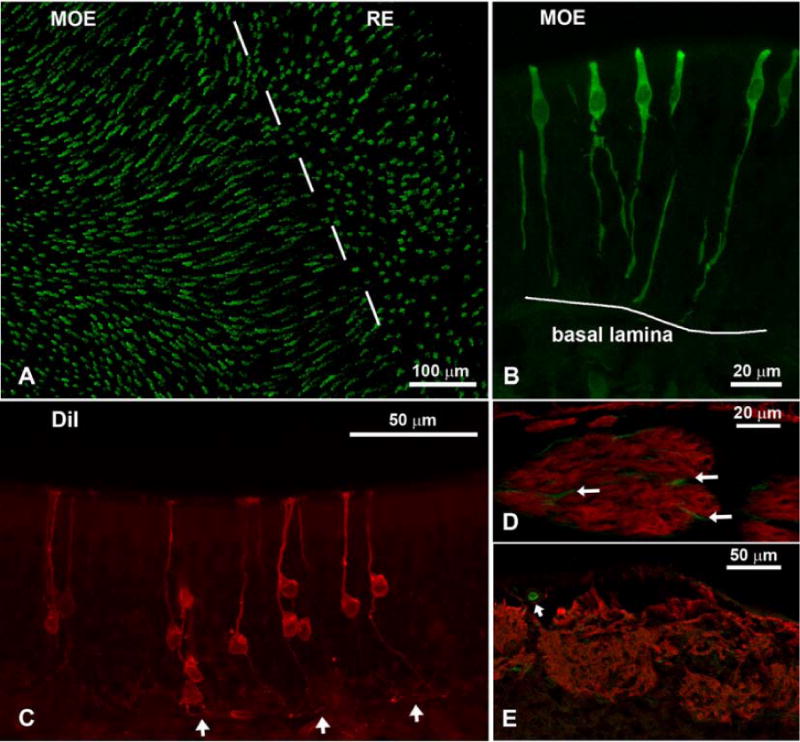
Confocal micrographs of IP3R3-tauGFP mice showing the olfactory epithelium (A-C), fila olfactoria (D), and the olfactory bulb (E). A) Whole-mount en face view of IP3R3 MV cells in an area where the main olfactory epithelium (MOE) and the respiratory epithelium (RE) meet. The IP3R3 MV cells are longer and more abundant in the MOE than in the RE. B) Higher magnification of the IP3R3 MV cells in a cross-section through the MOE. The cells stretch the whole height of the epithelium but the basal processes do not pass the basal lamina. The cell body lies in the upper third of the epithelium. C Cells retrogradely labeled with DiI from the olfactory bulb show the typical morphology of olfactory neurons (OSNs): a long thin dendrite; a cell body located in the lower portion of the epithelium; a thin axon projecting to and passing the basal lamina (arrows). D Antibodies against OMP show fila olfactoria beneath the MOE (red). Within the bundles, few IP3R3-positive fibers (green) of unknown origin are visible that do not double-label with OMP (arrows). E Section of the olfactory bulb where glomeruli are labeled with OMP. Note that the fibers of the glomeruli do not express IP3R3. However, small blood vessels show GFP labeling (arrow).
The GFP-positive cells in the RE are short and broad and usually have 2 basal processes. In addition to the differential morphology and abundance, this cell type also has different molecular features and will be described in a separate paper. Here we name the GFP-positive cells in the MOE of IP3R3+ / IP3R3− tauGFP+ mice “IP3R3 MV cells” (MV stands for “microvillous” and we use this term because as shown below (Fig. 3) these are cells bearing microvilli at their apical end).
Fig. 3.
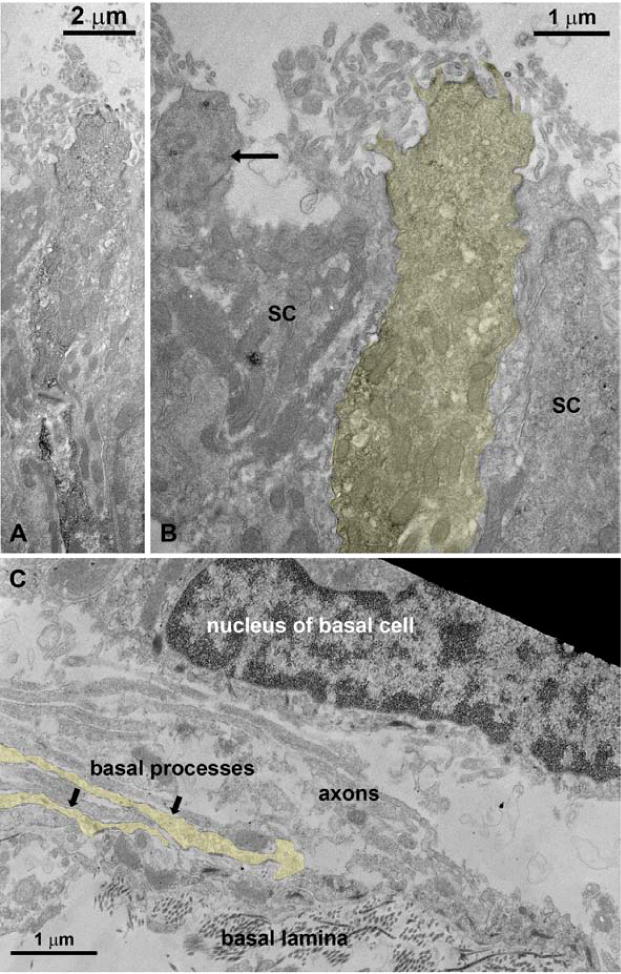
Transmission electron micrographs of IP3R3 MV cells. The tissue was reacted with an antibody against GFP. The IP3R3 MV cell (B) and their basal processes (C) have been artificially tinted yellow for visibility. (A) The permanent label visualized by ABC/DAB shows a long slender cell that bears microvilli at its apex. (B) Higher magnification of the apex. Note the unlabeled ciliated OSN (arrow). SC = supporting cell. (C) Axons bundles of OSNs traveling in the olfactory epithelium above the basal lamina contain also two basal processes of IP3R3 MV cells. These processes were never observed to penetrate the basal lamina.
IP3R3 MV cells are microvillous cells that do not penetrate the basal lamina
The IP3R3 MV cells in the MOE do not exhibit cilia and so we tested whether the apical ending of this cell type bears microvilli. Surprisingly, villin and espin, markers for microvilli did not immunoreact with these cells (not shown). We therefore incubated floating sections with an antibody against GFP combined with a biotinylated secondary antibody and embedded the sections for electron microscopy. Ultrathin sections confirmed that the IP3R3 MV cell is a microvillous cell (Fig. 3A–B). Furthermore, we examined the basal portion of the olfactory epithelium and did not observe the basal processes of the IP3R3 MV cells to penetrate the basal lamina (Fig. 3C). To further corroborate that this cell type does not project an axon towards the olfactory bulb we retrogradely labeled OSNs with DiI applied on the cranial side of the cribriform plate. Cells of the morphology seen in the IP3R3 MV cells were not labeled by DiI (Fig. 2C). Finally, we performed a unilateral bulbectomy which severs all cell processes traveling from the olfactory epithelium to the olfactory bulb, leading to cell death. Thus, if the IP3R3 MV cells extended a process to the bulb, the cells would die following bulbectomy. The presence of IP3R3 MV cells was observed at 2, 4, 6, and 8 days post-bulbectomy in the lesioned side of the epithelium, suggesting that IP3R3 MV cells do not extend processes to the olfactory bulb (Fig. 4). However, the depth of the olfactory epithelium decreased on the lesioned side compared to the unlesioned side due to the degenerating/dying OSNs, and the morphology of the IP3R3 MV cells was altered following bulbectomy. The basal process of the IP3R3 MV cells became thicker as well as shorter (see Fig. 4, 8 days). Collectively, the data indicate that these IP3R3 MV cells do not have an axon, i.e. are not OSNs.
Fig. 4.
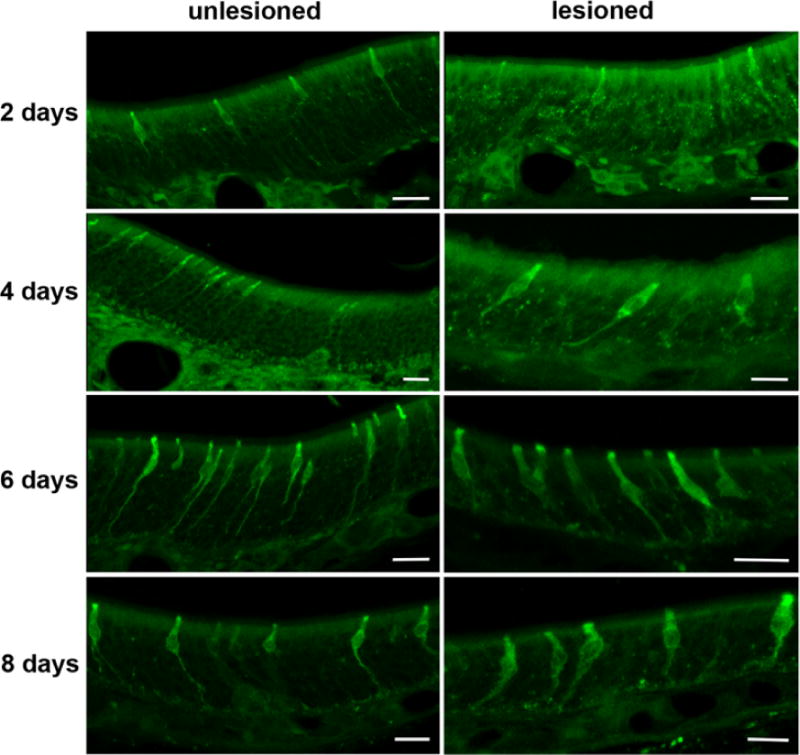
Unilateral bulbectomy revealed that the IP3R3 MV cells do not degenerate. Representative confocal z-stack images of the MOE 2 – 8 days post-bulbectomy are shown. The lesioned and the unlesioned control side contained a comparable amount of IP3R3 MV cells during the entire experiment as seen in these micrographs for days 2 to 8 after surgery. All scale bars = 20 μm.
Fig. 8.
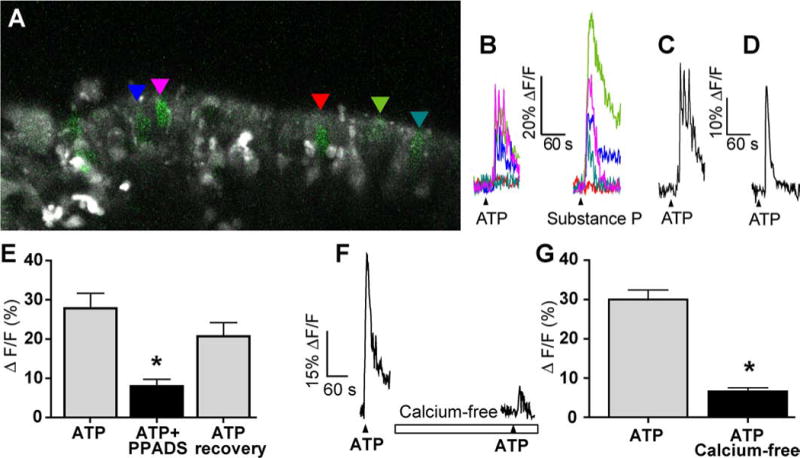
IP3R3 MV cells respond to bioactive compounds as measured by confocal calcium imaging. (A) Representative X-Rhod 1 AM loaded MOE slices containing IP3R3 MV cells (green). The GFP and X-Rhod 1 channel settings have been modified from data acquisition settings to improve visibility of the IP3R3 MV cells. Colored triangles indicate the IP3R3 MV cells analyzed and shown in B. (B) Time course of Ca2+ transients in response to ATP (10 μM) and substance P (500 nM) from outlined IP3R3 MV cells shown in A. Black triangles correspond to time of drug superfusion. Breaks in traces correspond to ~5 minutes when images were not collected. (C-D) Examples of the ATP-induced changes in intracellular calcium from two individual IP3R3 MV cells exhibiting a calcium transient with fused calcium oscillations or (C) or a single calcium transient (D). (E) Average peak calcium transient amplitudes induced by ATP, ATP in the presence of P2 receptor antagonist PPADS, or following washout of PPADS (recovery) are shown (mean ± s.e.m.; n = 9 cells from 4 slices). Asterisk indicates a significant decrease in [Ca2+]i compared to ATP (F2,8 = 16.4, P = 0.0001, 1 way ANOVA). (F-G) Representative ATP-induced calcium transient from an IP3R3 MV cell in normal and calcium-free Ringer’s solution (superfusion indicated by open rectangle). Black triangles correspond to time of drug superfusion (F). Average peak calcium transient amplitudes are shown in (G) (mean ± s.e.m. n = 24 cells from 3 slices).
IP3R3 MV cells do not express neuronal or glial-like markers
We then applied a large panel of antibodies (Table 1) to elucidate the molecular features of the IP3R3 MV cell. First, utilizing an antibody against IP3R3, we confirmed that the IP3R3 MV cells express IP3R3 protein (Fig. 5A). The same antibody did not label any cells in IP3R3-knockout mice (IP3R3− tauGFP+ / IP3R3− tauGFP+) (not shown) showing specificity of the antiserum. We then tested various markers for OSNs. None of the classical markers of OSNs, OMP, PGP9.5, or Hu-C, immunoreacted with the IP3R3 MV cells (Fig. 5B–D). We then applied antibodies against known members of the transduction pathways in the olfactory system of rodents. None of CNG2A, ACIII, or Gαolf were seen in IP3R3 MV cells (Fig. 6A and C). Also, acetylated tubulin, a marker for cilia, did not label IP3R3 MV cells (Fig. 6B). However, an antibody against Gαq/11 (Tizzano et al., 2008) labeled the apical endings of IP3R3 MV cells (Fig. 6D). Since the typical markers for OSNs did not show any label, we conclude that the IP3R3 MV cells are not conventional OSNs. Antibodies against SUS-1 and CK18, markers for olfactory supporting cells, did not label any of the IP3R3 cells (Fig. 5E-E″, F-F″) suggesting that they are not classical supporting cells.
Fig. 5.
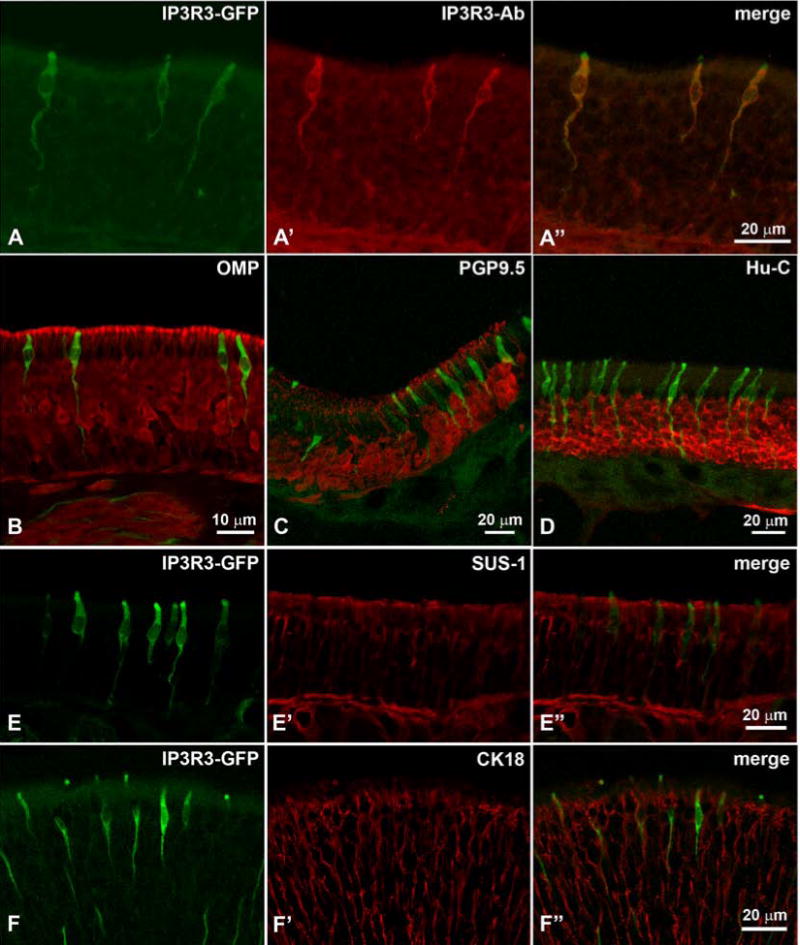
Confocal z-stacks of cryosections through the MOE reacted with an antibody against IP3R3 show total overlap between the IP3R3-tauGFP (green) and the antibody (red) (A – A″). Markers for OSNs (OMP in B, PGP9.5 in C, Hu-C in D) do not label any of the green IP3R3 MV cells. Note how high the cell bodies of the IP3R3 MV cells are located in the epithelium. Markers for supporting cells (SUS-1 in E – E” and CK18 in F – F”) do not co-localize with IP3R3. Note that the layer of nuclei of the supporting cells is even higher than that of the IP3R3 MV cells. The green IP3R3 MV cell in F” that seems to be labeled with the CK18 antibody is located at a different plane than the red supporting cell (z-stack of confocal images).
Fig. 6.
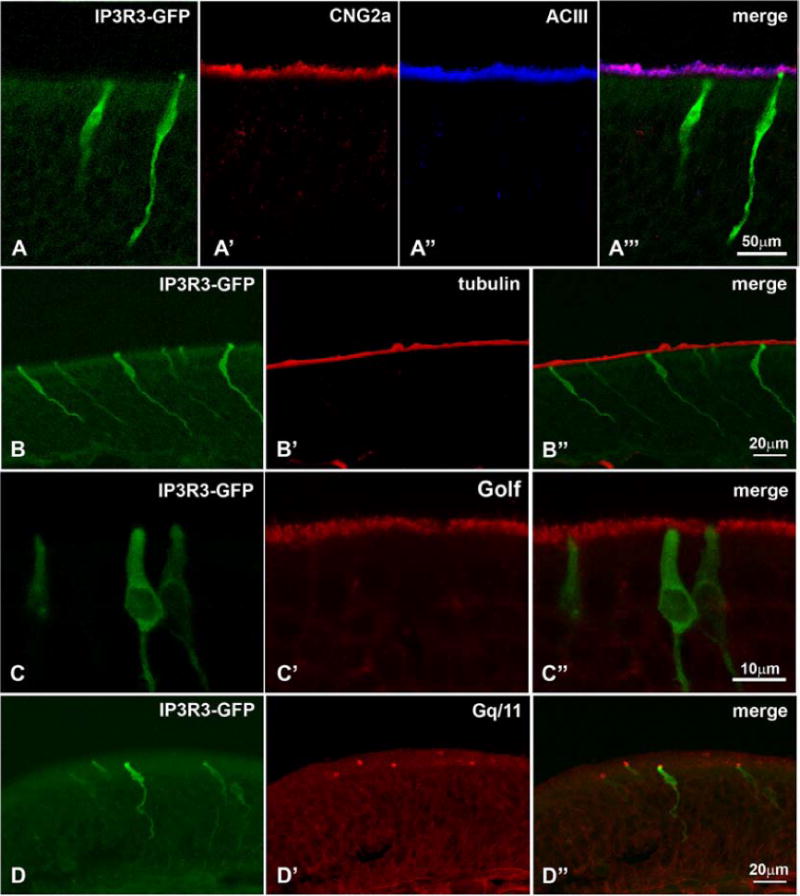
Marker for the known transduction pathways do not label the IP3R3 MV cells: (A – A″) Neither CNG2a nor ACIII, overlap with the IP3R3 MV cells. Likewise, neither acetylated tubulin (B – B″) nor Gαolf (C – C″), both of which label olfactory cilia, is expressed in the IP3R3 MV cells. (D – D″) The antibody Gαq/11 labels the apical endings of IP3R3 MV cells. Z-stacks of confocal images.
IP3R3 MV cells express receptors to bioactive compounds
Since we had noticed microvillous cells with contacts to nerve fibers in previous electron microscopic experiments, we tested antibodies against Substance P, calcitonin gene related peptide (CGRP), and tyrosine hydroxylase (TH), to test whether the IP3R3 MV cells are contacted by nerve fibers. TH-positive fibers were present beneath the basal lamina but did not enter the MOE (Fig. 7A). Occasionally, fibers immunoreactive for Substance P (Fig. 7A–B) or CGRP penetrated the MOE, apparently to contact some of the IP3R3 MV cells whereas other IP3R3 MV cells had no obvious contact with immunopositive nerve fibers (Fig. 7A–B).
Fig. 7.
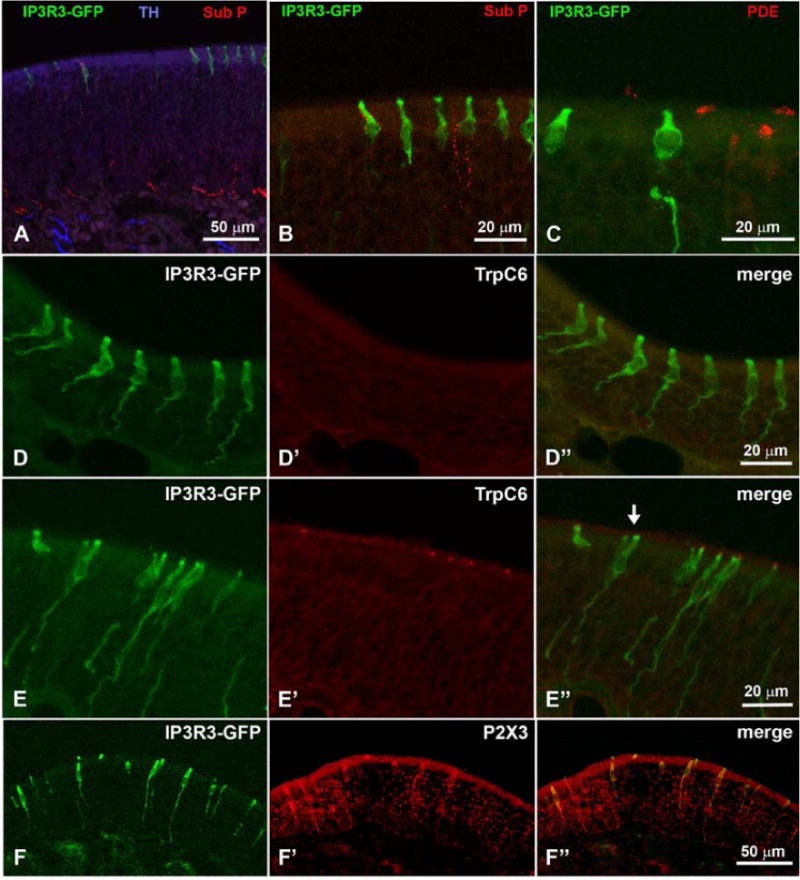
Nerve fibers expressing Substance P, a marker for trigeminal innervation, contact some of the IP3R3 MV cells but not all (A, B). C PDE2 is expressed in ciliated OSNs but not in IP3R3 MV cells. TRP C6 is expressed in some IP3R3 MV cells but not in all cells (D – E″). Also, an antibody against the purinergic receptor P2X3 labels some IP3R3 MV cells while others show no label (F – F″). Z-stacks of confocal images.
Phosphodiesterase 2 (PDE2) had been described for some cells in the MOE. We tested an antibody against this transduction component but it labeled the apices of only a subset of ciliated OSNs and never the IP3R3 MV cells (Fig. 7C). Elsaesser and coworkers reported cells in the MOE that expressed the transient receptor channel TRP C6 that have a morphology reminiscent of the IP3R3 MV cells (Elsaesser et al., 2005). We therefore tested whether the IP3R3 MV cells expressed TRP C6. The results showed that some IP3R3 MV cells did express TRP C6 while others did not (Fig. 7D,E). A distribution pattern for TRP C6-positive or –negative cells was not obvious. We concluded that there may be distinct subsets of IP3R3 MV cells. This notion was supported by utilizing antibodies against P2X3. Our experiments with these antibodies also showed that some of the IP3R3 MV cells were labeled while others showed no label (Fig. 7F). As seen in Table 1, several other antibodies (e.g. PLCβ2, ChAT, gustducin) were applied with negative results.
IP3R3 MV cells are responsive to ATP, substance P and odorants
The subset of IP3R3 MV cells expressing the P2X3 purinergic receptor may be responsive to ATP, and the subset of IP3R3 MV cells that are contacted by substance P containing fibers may be responsive to substance P. To determine if IP3R3 MV cells were responsive to bioactive compounds, we performed confocal calcium imaging on neonatal olfactory epithelial slices loaded with the long wavelength intracellular calcium indicator dye X-Rhod-1 AM. ATP (10 μM) elicited transient increases in intracellular calcium in 62.1% of IP3R3 MV cells from IP3R3+ / IP3R3− tauGFP+ mice and substance P (500 nM) induced an increase in intracellular calcium in 77.8% of IP3R3 MV cells (Table 2, Fig. 8A–B). ATP-induced increases in calcium are characterized by an initial calcium transient followed by calcium oscillations that were fused in 56.4% of the IP3R3 MV cells (53/94 cells from 16 slices; Fig. 8C) and a single monophasic transient increase in intracellular calcium in 43.6% of the IP3R3 MV cells (41/94 cells from 16 slices; Fig. 8D). The peak heights of the calcium transients were variable and ranged from 4–74% ΔF/F with a mean ± s.e.m. peak height for ATP-induced and substance P-induced transients of 25 ± 1 (n=150 cells from 21 slices) and 16 ± 1 (n=35 cells from 8 slices) %ΔF/F. There was no significant difference between time to maximum peak for ATP and substance P application (50±4 and 57±3 s for substance P and ATP, respectively; t37 = 1.36, P = 0.18, n=26 cells from 4 slices, Student’s t-test). In a subset of cells that were superfused with both ATP and substance P, 20% (15/76 cells from 9 slices) only responded to ATP, 14% (11/76 cells from 9 slices) only responded to substance P, 49% were responsive to both (37/76 cells from 9 slices) and 17% (13/76 cells from 9 slices) were unresponsive to either. Collectively, these data suggest that IP3R3 MV cells can express functional purinergic and/or neurokinin 1 receptors.
Table 2.
Response rate of IP3R3 MV cells
| ATP (10 μM) | Odorants (50 μM) | Substance P (500 nM) | ||||
|---|---|---|---|---|---|---|
| Genotype | Response/total cells | % | Response/total cells | % | Response/total cells | % |
| All cells | 172/255 | 67.5 | 99/250 | 39.6 | 48/86 | 55.8 |
| IP3R3+/IP3R3−tauGFP+ | 54/87 | 62.1 | 34/82 | 41.5 | 28/36 | 77.8 |
| IP3R3−tauGFP+/IP3R3−tauGFP+ | 118/168 | 70.2 | 65/168 | 38.7 | 20/50 | 40.0 |
Application of pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS, 25 μM), a specific but non-selective purinergic receptor antagonist, reversibly inhibited the calcium transient elicited by ATP by 65.5 ± 10.2% (Fig 8E, t8=5.0413, P = 0.0005, n=9 cells from 4 slices), suggesting that the calcium increase was mediated by a purinergic receptor. In the absence of calcium from the extracellular solution, the ATP-induced calcium transient was inhibited by 73.3 ± 3.1% (Fig. 8F,G, n = 24 cells from 3 slices, t23 = 7.764, P = 0.0001, Student’s t-test), suggesting that calcium influx via an ionotropic P2X purinergic receptor was involved in the calcium response. Collectively, these data suggest that a functional P2X receptor is expressed on a subset of IP3R3 MV cells.
We have recently identified a purinergic G protein-coupled receptor/phospholipase C mediated IP3-calcium signaling pathway in the supporting cells of the MOE (Hegg et al., 2009). To determine the role of IP3R3 in shaping the calcium dynamics in the IP3R3 MV cell we examined the responses of ATP in both IP3R3+ / IP3R3−tauGFP+ and IP3R3−tauGFP+ / IP3R3−tauGFP+ mice. We observed a calcium transient with fused calcium oscillations in a sub-population of IP3R3 MV cells, as previously observed in supporting cells (Hegg et al., 2009). In the IP3R3+ / IP3R3−tauGFP+ mice, 61% (22/36 cells from 7 slices) exhibited a calcium transient with underlying fused calcium oscillations while 39% (14/36 cells from 7 slices) exhibited a solitary calcium transient in response to ATP. In comparison, in the IP3R3− tauGFP+ / IP3R3− tauGFP+ mice, 53% (31/58 cells from 9 slices) responded with a calcium transient with underlying fused calcium oscillations and 47% (27/58 cells from 9 slices) exhibited a single calcium transient. Chi-square analysis revealed no association with the presence of the IP3R3 receptor and the occurrence of oscillations (χ21 = 0.55, P = 0.47, Chi-Squared test) or the occurrence of a single calcium transient (χ21 = 0.53, P = 0.47, Chi-Squared test). Thus, there was very little change in the ATP-induced intracellular calcium dynamics in the presence or absence of the IP3R3 receptor, suggesting there are complex calcium signaling patterns and multiple calcium responsive receptors expressed in the IP3R3 MV cells.
Occasionally, we superfused an odorant mixture at the end of the experiment in order to determine if cells in the slice preparation were viable. Interestingly, we observed that 41.5% of the IP3R3 MV cells from IP3R3+ / IP3R3−tauGFP+ mice responded to odorants (Table 2, Fig. 9). The odorant mixture responses in the IP3R3 MV cells were, in general, smaller than odorant responses from an OSN (Fig 9A–B). However, the rise and fall of intracellular calcium was similar (Fig. 9B, inset). The peak heights of the odorant-induced calcium transients were variable and ranged from 4 – 43 % ΔF/F (n=70 cells from 16 slices). The mean ± s.e.m. peak height of the odorant-mixture induced calcium transient in the IP3R3 MV cells was 16.9 ± 1.0%ΔF/F (n=70 cells from 16 slices).
Fig. 9.
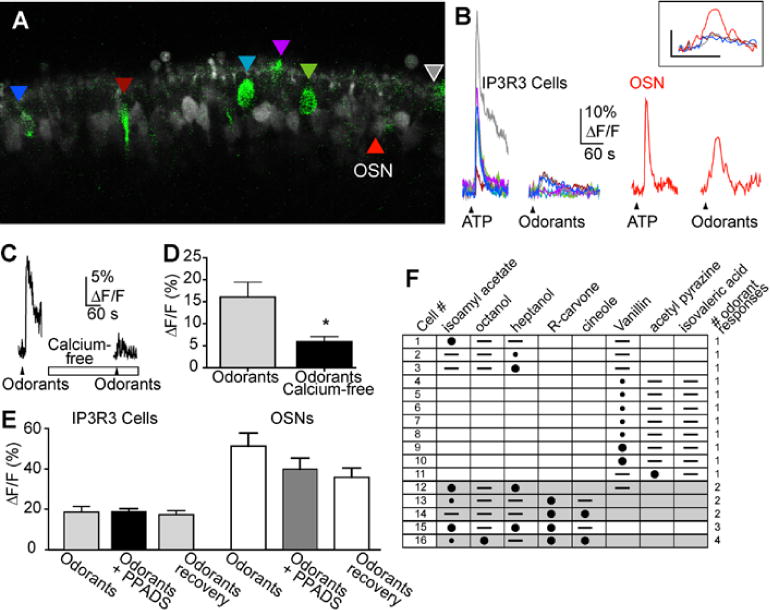
Odorant responses from IP3R3 MV cells. (A) Representative X-Rhod 1 AM loaded MOE slices containing IP3R3 MV cells (green). The GFP and X-Rhod 1 channel settings have been modified from data acquisition settings to improve visibility of the IP3R3 MV cells. Colored triangles indicate the IP3R3 MV cells analyzed and correspond to the colored traces shown in B. (B) Time course of Ca2+ transients in response to ATP (10 μM) and a mixture of odorants (10 μM each cineole, octanol, heptanol, isoamyl acetate and R-carvone) from outlined IP3R3 MV cells (left) and an OSN (right). Black triangles correspond to time of drug superfusion. Breaks in traces correspond to ~5 minutes when images were not collected. Inset: Expanded time course of the odorant-induced calcium transient from the OSN (red trace) and the 3 odorant-responsive IP3R3 MV cells. (C) Representative odorant mixture-induced calcium transient from an IP3R3 MV cell in normal and calcium-free Ringer’s solution (superfusion indicated by open rectangle). Black triangles correspond to time of drug superfusion. (D) Average peak calcium transient amplitudes are shown (mean ± s.e.m.) (n = 8 cells from 3 slices, t7=2.53, P<0.04, Student’s t-test). (E) Average peak calcium transient amplitudes induced by ATP, ATP in the presence of P2 receptor antagonist PPADS, or following washout of PPADS (recovery) from IP3R3 MV cells (left) and OSNs (right) are shown (mean ± s.e.m; t18 = 0.020, P = 0.98, n=10 cells from 3 slices and t12 = 1.33, P = 0.2, n=7 OSNs from 3 slices, Student’s t-test). (F) Matrix of 16/55 cells from 7 slices that responded to odorants singly superfused (50 μM). Large black circles indicate ΔF/F >10%, small black circle indicates 5–10% ΔF/F, – indicates no response to odorant, lack of symbol indicates odorant was not superfused.
In olfactory sensory neurons, when an odorant binds to one of hundreds of G-protein-coupled odorant receptors, the receptor activates a G-protein (Gαolf), which activates adenylate cyclase III to synthesize the internal messenger cAMP. cAMP directly activates cAMP-sensitive cyclic nucleotide-gated (CNG) channels in the plasma membrane. Subsequent Na+ and Ca2+ influx results in the initial depolarization of the cell, and at the soma, action potentials are generated and transmitted via the axon to the olfactory bulb. To elucidate the signal transduction pathway, we first determined the source of calcium in the odorant-induced calcium responses by removing calcium from the extracellular solution. The odorant-induced calcium transients were reduced by 57.1 ± 11.9% in the absence of calcium (Fig. 9C–D; t7=2.53, P<0.04, n=8 cells from 3 slices, Student’s t-test), suggesting that the majority of calcium was due to influx through a channel or receptor. Notably, the IP3R3 MV cells do not possess the canonical odorant transduction pathway components: Gαolf, adenylate cyclase III, or the CNG2A subunit of the CNG channel. One possibility is that odorants could evoke OSNs to release ATP or substance P that induces a calcium transient in the IP3R3 MV cell. To test for the possibility that the odorant-evoked ATP release from OSNs we used the purinergic receptor antagonist PPADS. Superfusion of purinergic receptor antagonist PPADS did not inhibit the odorant-induced calcium response in the IP3R3 MV cells (nor the OSNs) (Fig. 9E, t18 = 0.020, P = 0.98, n=10 cells from 3 slices and t12 = 1.33, P = 0.2, n=7 OSNs from 3 slices), suggesting that the calcium transient in IP3R3 MV cells is not due to odorant-evoked ATP release by OSNs that would then elicit a purinergic response in the IP3R3 MV cells. In support of this conclusion is the observation that an IP3R3 MV cell did not have to be in proximity to an odorant-responsive OSN in order for there to be a calcium response, e.g., Fig 9A. OSNs did not immunoreact with substance P antiserum which rules out the possibility that the odorant-evoked IP3R3 calcium transient is a response to odorant-induced release of substance P from OSNs. Further support that the IP3R3 MV cells are responding directly to odorants comes from the observation that 14% (7/49 cells) of a subset of IP3R3 MV cells that were superfused with odorants, ATP and substance P responded only to the odorants. In this subset of cells, 53% (26/49 cells from 6 slices) responded to all three, 10% (5/49 cells from 6 slices) responded to odorants and substance P, 6% (3/49 cells from 6 slices) responded to odorants and ATP, 8% (4/49cells from 6 slices) responded to both ATP and substance P, 6% (3/49 cells from 6 slices) responded to only substance P, and 2% (1/49 cells from 6 slices) were non-responsive. Finally, the lack of inhibition of the odorant-elicited response in OSNs by PPADS indicates that, in agreement with current understanding of olfactory transduction, purinergics are not directly involved in the response of OSNs to odors.
Alternatively, the Ca2+ transients may be a result of nonspecific actions of the odorant. If the odorants were activating odorant receptors, then there should be variability in responsiveness to individual odorants. Thus, we next determined the ability of multiple odorant ligands to elicit calcium transients. In this experiment, single odorants induced calcium transients in 29% of the IP3R3 MV cells (16/55 cells from 7 slices, Fig. 9F). Eleven cells responded to 1 odorant, 3 cells responded to 2 odorants and 1 cell each responded to 3 and 4 odorants. These data suggest that the odorant-induced calcium transient is not a non-specific effect. Further experiments will be required to determine the chemical sensing ability of the IP3R3 MV cells.
Discussion
In this study we describe a cell type in the MOE of mice that lacks an axon but expresses the type 3 inositol trisphosphate receptor (IP3R3) and bears microvilli at its apex. Microvillous cells in the MOE have been reported before for mammals, including humans, as early as 1969 when Andres described a microvillous cell in the MOE of cats (Andres, 1969). As to rodents, conflicting reports concerning the sensory nature of microvillous cells can be found in the literature. In the rat olfactory epithelium, Carr and coworkers described a microvillous cell that lacked an axon and survived ablation of the olfactory bulb, suggesting that this cell type is non-neural; but the cell did not react with the marker for supporting cells SUS-1 (Carr et al., 1991). However, other authors described microvillous cells with a sensory nature. Jourdan suggested that the microvillous cell type in the MOE of rats, with an axon-like basal part resembling the sensory neurons in the vomeronasal organ, is a second class of OSNs (Jourdan, 1975). If microvillous cells function as primary sensory neurons, then they should have a projection to the olfactory bulb, or have contacts with sensory nerve fibers. A study by Rowley et al. utilizing HRP tracing claimed that at least some microvillous cells project directly to the olfactory bulb (Rowley, III et al., 1989). These cells did not label with the OSN specific marker olfactory marker protein (OMP) (Johnson et al., 1993) indicating that they are distinct from the classic olfactory sensory neurons. More recently, Mombaerts and coworkers showed cells in genetically modified mice that express TrpC2-driven GFP have a microvillous cell-like morphology and also project axons to the bulb olfactory (Mombaerts, 2008). Elsaesser and coworkers reported microvillous cells in the rodent olfactory epithelium with gross morphology resembling that of the IP3R3 MV cells described in this study. Elsaesser’s microvillous cells expressed phospholipase C beta-2 (PLCβ2), ezrin, the transient receptor channel TRP C6, and IP3R3, but also did not degenerate after bulbectomy. They suggested that these cells represent a second class of olfactory chemosensory cells (Elsaesser et al., 2005). We find that a subset of the IP3R3 MV cells characterized in this publication express TRP C6 and display a morphology resembling the Elsaesser microvillous cells. However, unlike the Elsaesser microvillous cells, only some of ours did express TRP C6. This may be either due to different developmental stages of the IP3R3 MV cells or the existence of subsets of IP3R3 MV cells. Elsaesser and coworkers also reported the presence of PLCβ2. We used antibodies against PLCβ2 from the same company and tried several different lots as well as different fixation types and times. Although the antibody worked well in taste cells we could not label IP3R3 MV cells in the olfactory epithelium. We cannot rule out that the IP3R3 MV cells also express PLCβ2, however, the present lots of the antibody that Elsaesser and coworkers used does not show this protein in the IP3R3 MV cells.
In the lobster, olfactory sensory neurons express IP3 receptors on the plasma membrane (Fadool & Ache, 1992) raising the question whether the cells we found to express IP3R3 in the mouse MOE are primary sensory neurons. Taking advantage of the genetically modified IP3R3-tauGFP-mouse, we tested whether this cell type meets the criteria of primary sensory neuron (a sensory cell with an axon), secondary sensory cell or non-sensory cell. In our experiments, the IP3R3 MV cells did not disappear after bulbectomy and could not be retrogradely labeled from the olfactory bulb with DiI. In addition, even though these cells expressed tauGFP, a molecule that labels axons in other transgenic lines (e.g. Mombaerts, 2008) we did not find any GFP labeled axons extending into the glomeruli of the olfactory bulb. Tests with the typical olfactory markers OMP, PGP9.5 and Hu-C as well as markers for OSN transduction pathways and/or cilia (CNG2a, ACIII, tubulin, Gαolf) rendered negative results suggesting that these IP3R3 MV cells are not regular OSNs. Since OSNs with different molecular features exist, we used an antibody against PDE known to label a distinct subset of OSNs. In our experiments the PDE antibody only labeled obviously ciliated OSNs (Fig. 7C) (Juilfs et al., 1997) and not IP3R3 MV cells. A subpopulation of IP3R3 MV cells were contacted by Substance P- or CGRP-positive nerve fibers and responded to substance P, suggesting that some IP3R3 MV cells may be modulated by activity in the trigeminal nerve. Interestingly, the IP3R3 MV cells in this study responded to odorants. Collectively, our data indicate that the IP3R3 MV cells are chemoresponsive, and may function as a sensory cell, but they are not an olfactory sensory neuron extending a process to the olfactory bulb.
There are morphological and physiological similarities between classically described olfactory supporting cells and the IP3R3 MV cells, however, evidence suggests that IP3R3 MV cells are not a subtype of the classical supporting cells. Both cell types have microvilli, albeit different types (size, length) and both have prominent cell somata in the apical epithelium with a cytoplasmic extension. However, supporting cells display broad apical endings while IP3R3 MV cells have a narrowed apical extension. In addition, IP3R3 MV cells lack endfeet, the thick basal endings resting on the basal lamina that are typical for olfactory supporting cells. Calcium imaging data suggests that the IP3R3 MV cells have a lower resting calcium concentration than supporting cells, as evidenced by a conspicuously lower fluorescent intensity at rest (data not shown). Both cell types respond to ATP with intracellular calcium increases (Hegg et al., 2009). Notably, supporting cell responses to ATP are mediated through G-protein coupled P2Y purinergic receptors and are not dependent on extracellular calcium, whereas in the IP3R3 MV cells the ATP-mediated responses are dependent on extracellular calcium and are mediated via P2X purinergic receptors. Adjacent supporting cells are coupled to one another via gap junctions (Menco, 1988; Miragall et al., 1992; Zhang & Restrepo, 2002; Rash et al., 2005), and thus have the ability to exhibit calcium waves. Calcium waves were never observed in IP3R3 MV cells, which are not juxtaposed to each other. Consistent with these observations, the IP3R3 MV cells were not immunoreactive to supporting cell markers, providing further support that the IP3R3 MV cells are not classical supporting cells.
Our immunohistochemical results indicate that subtypes of IP3R3 MV cells may be present. The antibody against the purinergic receptor P2X3 labeled only some of the IP3R3 MV cells; similarly not all of the IP3R3 MV cells were contacted by a substance P-immunoreactive nerve fiber. Our physiological data show only a portion of the IP3R3 MV cells responding to these two bioactive compounds, corroborating the notion that subtypes of IP3R3 MV cells exist. In addition, Elsaesser at al. reported IP3R3-positive cells that also expressed TRP C6 (Elsaesser et al., 2005). In our experiments, only some of the IP3R3 cells immunoreacted with the TRP C6 antisera. This may be due to developmental stages or subtypes of IP3R3 cells may exist.
In a recent study, we described microvillous cells in mice that express the transient receptor channel TRP M5 (Hansen & Finger, 2008). Two types of microvillous cells with different morphology expressed TRP M5 but several microvillous cells were TRP M5-negative indicating the presence of multiple subtypes of microvillous cells. The function of any of the microvillous cells present in the MOE has not been clearly elucidated. Some reports suggested that they are primary OSNs; others claimed that they are a secondary chemosensory cell that functions to detect chemicals, but does not directly signal to the central nervous system (Elsaesser et al., 2005). Other evidence suggests that they may have a modulatory role in the olfactory epithelium (Elsaesser & Paysan, 2007) and that they function in the regeneration of the olfactory epithelium (Elsaesser & Paysan, 2007). Braun and Zimmermann suggested a mechanosensory function for microvillous cells in the MOE of rats (Braun & Zimmermann, 1998). The presence of different subtypes of microvillous cells may explain the different functions of microvillous cells described.
Chemosensation
Chemesthesis, the sensation of chemical irritants, occurs in the nasal cavity via the trigeminal nerve. Not only are the nerve fibers themselves directly chemosensitive but they receive input from solitary chemosensory cells (Tizzano et al., 2010). Solitary chemosensory cells that express TRP M5 respond to odorants at concentrations classified as irritants (0.5–5 mM) (Lin et al., 2008). Significantly, these solitary chemosensory cells are innervated by peptidergic nerve fibers immunoreactive to substance P, suggesting sensory information may be transmitted via the nerve fibers through synaptic transmission. In this way, the IP3R3 MV cells resemble the TRP M5-positive solitary chemosensory cells. The IP3R3 cell may form contacts with substance P and CGRP-expressing nerve fibers, suggesting innervation by trigeminal nociceptors (Bojsen-Moller, 1975; Mousley et al., 2006). We observe increases in intracellular calcium in response to both odorants and to substance P at relatively low levels, and at least one of the odorants, isoamyl acetate, is known to activate the trigeminal nerve fibers (Silver et al., 1992). However, we currently cannot determine whether odorants are directly activating the IP3R3 MV cell, or whether the odorant stimulates the release of substance P to elicit a response. In addition, we observed the expression of the P2X3 receptor, and an ATP-induced increase in intracellular calcium, presumably due to P2X3 activation. P2X3 receptors can participate in certain forms of nociceptive signaling, suggesting that IP3R3 MV cells may respond to ATP co-released from trigeminal nociceptor fibers with Substance P. Alternatively, noxious stimulation of the MOE could cause the release of ATP from injured cells, activating the IP3R3 MV cells. Collectively, the data suggest that the IP3R3 MV cells may play a role in the response to irritation or injury of the MOE.
Interestingly, the IP3R3 MV cells responded to odorants while lacking components of the canonical odorant transduction pathway such as ACIII, Gαolf, and CNGA2. One possible explanation is that the IP3R3 MV cells are responding in a non-specific manner to odorant stimulation. However, our evidence using single odorants suggests this is not the case. The microvillous cells of the vomeronasal organ (VNO) use a different transduction pathway than the canonical cAMP-ACIII cascade used by OSNs in the MOE (Zufall & Munger, 2001). This transduction pathway utilizes the following molecular components: VR-type G-protein coupled receptors, Gαo and Gαi, the PLC enzyme, the second messenger IP3, and the TRP C2 channel. IP3 can mobilize calcium from stores and activate calcium-activated channels such as the calcium-activated chloride channel or TRP channels, both of which can depolarize the cell. Thus, the IP3R3 MV cells, which express Gαq/11, could utilize a similar transduction pathway.
Our finding of IP3R3 MV cells that respond to odors, and do not express elements of the canonical cAMP pathway expressed by OSNs constitutes one more example of chemoresponsive cells that use non-canonical transduction pathways. By now, it is clear that there are subsets of OSNs that respond to odors through non-canonical olfactory transduction pathways (Munger et al., 2009). For example a subset of OSNs projecting axons to the necklace glomeruli express CNGA3 (the visual system CNG channel) instead of CNGA2 (the olfactory CNG channel). In addition, a subset of lobster OSNs respond to odors through opening of plasma membrane IP3 receptor channels instead of CNG channels (Fadool & Ache, 1992). The finding of various transduction pathways expressed in different chemosensory cells raises the question whether different transduction pathways are required to mediate specific stimulus response characteristics. Currently there is not a comprehensive understanding of the characteristics of the responses of the different chemosensory cell types and future experiments should provide information on this issue.
Role of the IP3R3 receptor
We observed two types of calcium responses following ATP application in the IP3R3 cells: a single calcium transient, and a calcium transient with fused calcium oscillations (Hegg et al., 2009). In genetically engineered cells, the IP3R1 and IP3R2 receptor subtypes have been shown to be responsible for oscillatory increases in intracellular calcium, while the IP3R3 receptor is responsible for the sustained increase in calcium (Miyakawa et al., 1999; Hattori et al., 2004). If this were true in the mouse MOE, then in the absence of the IP3R3 receptor one would expect 100% of the cells to exhibit oscillatory calcium increases in response to ATP. However, in the present study, the relative proportions of the calcium signaling patterns did not differ greatly in the presence or absence of the IP3R3 receptor, suggesting that there may be multiple IP3 receptor subtypes expressed. In rat olfactory epithelium IP3 receptors have been identified in the cilia of OSNs (Restrepo et al., 1992; Cunningham et al., 1993; DellaCorte et al., 1996), as well as in the supporting cells, lamina propria and glandular tissue (Smutzer et al., 1997). In particular, the short splice variant of the IP3R1 receptor and the IP3R3 receptor are expressed in the rat olfactory epithelium (Smutzer et al., 1997). Collectively these data indicate that multiple splice variants or IP3 receptor subtypes may be expressed in this microvillous cell population.
Conclusion
Taken together with the reports of microvillous OSNs (Moran et al., 1982; Rowley, III et al., 1989; Mombaerts, 2008), non-sensory microvillous cells (Carr et al., 1991; Asan & Drenckhahn, 2005; Hansen & Finger, 2008), sensory non-OSN cells (Braun & Zimmermann, 1998) and the present study, it is obvious that a substantial number of distinctly different types of microvillous cells exist in the olfactory epithelium. We postulate that the IP3R3 MV cells are chemoresponsive non-neuronal cells based on the following observations: IP3R3 MV cells lack an axon and are contained entirely within the epithelium, a subset of IP3R3 MV cells are contacted by nerve fibers, and IP3R3 MV cells respond to odorants with increases in calcium.
Acknowledgments
We thank Dr. Frank Margolis for the antibodies against OMP and SUS-1, and Tania Iqbal and Brad Hammond, who collected physiological data. We also thank Dr. Thomas Finger, University of Colorado and Dr. Steven D. Munger from the University of Maryland School of Medicine, for valuable comments on this manuscript.
This study was supported by NIDCD grants DC 007732 (Anne Hansen), DC 06070 (Diego Restrepo and Thomas E. Finger), and P30 DC 04657 (Diego Restrepo and Thomas E. Finger) and DC 006897 (Colleen Hegg).
List of Abbreviations
- ACIII
adenylyl cyclase III
- ATP
adenosine 5′-triphosphate
- CGRP
calcitonin gene related peptide
- CNG2A
cyclic nucleotide gated channel 2A
- GFP
green fluorescent protein
- IP3
inositol trisphosphate
- IP3R3
inositol trisphosphate receptor type 3
- MOE
main olfactory epithelium
- MV
microvillous
- OMP
olfactory marker protein
- OSN
olfactory sensory neuron
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PDE2
phosphodiesterase 2
- PFA
paraformaldehyde
- PLCβ2
phospholipase C beta-2
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid
- RE
respiratory epithelium
- TH
tyrosine hydroxylase
- TRP C2
transient receptor channel C2
- TRP C6
transient receptor channel C6
- TRPM5
transient receptor channel M5
- VNO
vomeronasal organ
Reference List
- Andres KH. Der olfaktorische Saum der Katze. Z Zellforsch. 1969;96:250–274. [PubMed] [Google Scholar]
- Asan E, Drenckhahn D. Immunocytochemical characterization of two types of microvillar cells in rodent olfactory epithelium. Histochem Cell Biol. 2005;123:157–168. doi: 10.1007/s00418-005-0759-4. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller F. Demonstration of terminalis, olfactory, trigeminal and perivascular nerves in the rat nasal septum. J Comp Neurol. 1975;159:245–256. doi: 10.1002/cne.901590206. [DOI] [PubMed] [Google Scholar]
- Braun N, Zimmermann H. Association of ecto-5′-nucleotidase with specific cell types in the adult and developing rat olfactory organ. J Comp Neurol. 1998;393:528–537. doi: 10.1002/(sici)1096-9861(19980420)393:4<528::aid-cne10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Carr VM, Farbman AI, Colletti LM, Morgan JI. Identification of a new non-neuronal cell type in rat olfactory epithelium. Neuroscience. 1991;45:433–449. doi: 10.1016/0306-4522(91)90239-k. [DOI] [PubMed] [Google Scholar]
- Chick WS, Mentzer SE, Carpenter DA, Rinchik EM, Johnson D, You Y. X-ray-induced deletion complexes in embryonic stem cells on mouse chromosome 15. Mamm Genome. 2005;16:661–671. doi: 10.1007/s00335-005-0011-5. [DOI] [PubMed] [Google Scholar]
- Cunningham AM, Ryugo DK, Sharp AH, Reed RR, Snyder SH, Ronnett GV. Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience. 1993;57:339–352. doi: 10.1016/0306-4522(93)90067-p. [DOI] [PubMed] [Google Scholar]
- DellaCorte C, Restrepo D, Menco B, PhM, Andreini I, Kalinoski DL. Gαq/Gα11: immunolocalization in the olfactory epithelium of the rat (Rattus rattus) and the channel catfish (Ictalurus punctatus) Neuroscience. 1996;74:261–273. doi: 10.1016/0306-4522(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Di VF, Steinberg TH, Silverstein SC. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Elsaesser R, Montani G, Tirindelli R, Paysan J. Phosphatidyl-inositide signaling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. Eur J Neurosci. 2005;21:2692–2700. doi: 10.1111/j.1460-9568.2005.04108.x. [DOI] [PubMed] [Google Scholar]
- Elsaesser R, Paysan J. The sense of smell, its signalling pathways, and the dichotomy of cilia and microvilli in olfactory sensory cells. BMC Neurosci. 2007;8:S1. doi: 10.1186/1471-2202-8-S3-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Ache BW. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992;9:907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Silver WL, Restrepo D. The Neurobiology of Taste and Smell. Wiley-Liss; New York: 2000. [Google Scholar]
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- Gimelbrant A, Hutchinson JN, Thompson BR, Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–1140. doi: 10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- Hansen A, Finger TE. Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci. 2008;9:115. doi: 10.1186/1471-2202-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, Inoue T, Mikoshiba K. Distinct roles of inositol 1,4,5-triphosphate receptor types 1 and 3 in Ca2+ signaling. J Biol Chem. 2004;279:11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EW, Eller PM, Jafek BW. An immuno-electron microscopic comparison of olfactory marker protein localization in the supranuclear regions of the rat olfactory epithelium and vomeronasal organ neuroepithelium. Acta Otolaryngol Stockholm. 1993;113:766–771. doi: 10.3109/00016489309135898. [DOI] [PubMed] [Google Scholar]
- Jourdan F. Ultrastructure de l’épithélium olfactif du rat: polymorphisme des récepteurs. C R Acad Sci. 1975;280:443–446. [PubMed] [Google Scholar]
- Juilfs DM, Fülle HJ, Zhao AZ, Houslay MD, Garbers DL. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Margolskee RF, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- Manzini I, Schweers TS, Schild D. Improved fluorescent (calcium indicator) dye uptake in brain slices by blocking multidrug resistance transporters. J Neurosci Meth. 2008;167:140–147. doi: 10.1016/j.jneumeth.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Harrison GS, Wood WM, Maxwell F. A DNS cassette containing a trimerized SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. BioTechniques. 1989;7:276–280. [PubMed] [Google Scholar]
- Menco BP. Tight-junctional strands first appear in regions where three cells meet in differentiating olfactory epithelium: A freeze-fracture study. J Cell Sci. 1988;89:495–505. doi: 10.1242/jcs.89.4.495. [DOI] [PubMed] [Google Scholar]
- Miragall F, Hwang TK, Traub O, Hertzberg EL, Dermietzel R. Expression of connexins in the developing olfactory system of the mouse. J Comp Neurol. 1992;325:359–378. doi: 10.1002/cne.903250304. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. TrpC2: expression outside the mouse VNO. Chem Senses. 2008;33 ISOT Abstr. S28. [Google Scholar]
- Moran DT, Rowley C, III, Jafek BW. Electron microscopy of human olfactory epithelium reveals a new cell type: The microvillar cell. Brain Res. 1982;253:39–46. doi: 10.1016/0006-8993(82)90671-0. [DOI] [PubMed] [Google Scholar]
- Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide Y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum) J Neurosci. 2006;26:7707–7717. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Nucifora FC, Jr, Sharp AH, Milgram SL, Ross CA. Inositol 1,4,5-trisphosphate recetors in endocrine cells: localization and association in hetero- and homotetramers. Mol Biol Cell. 1996;7:949–960. doi: 10.1091/mbc.7.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1.4.5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Rash JE, Davidson KGV, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson CO, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34:307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo D, Miyamoto T, Bryant BP, Teeter JH. Odor stimuli induce rapid increase in intracellular calcium in olfactory neurons. Science. 1990;249:1166–1168. doi: 10.1126/science.2168580. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Teeter JH, Honda E, Boyle AG, Marecek JF, Prestwich GD, Kalinoski DL. Evidence for an InsP3-gated channel protein in isolated rat olfactory cilia. Am J Physiol. 1992;263:C667–C673. doi: 10.1152/ajpcell.1992.263.3.C667. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- Rowley JC, III, Moran DT, Jafek BW. Peroxidase backfills suggest the mammalian olfactory epithelium contains a second morphologically distinct class of bipolar sensory neuron: the microvillar cell. Brain Res. 1989;502:387–400. doi: 10.1016/0006-8993(89)90635-5. [DOI] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- Silver Wayne L. Neural and pharmacological basis for nasal irritation. In: Tucker WG, Leaderer BP, Mohave L, Cain WS, editors. Sources of Indoor Air Contaminants. Ann NY Acad Sci. 1992. pp. 152–163. [DOI] [PubMed] [Google Scholar]
- Smutzer G, Zimmerman JE, Hahn CG, Ruscheinsky DD, Rodríguez A, Han LY, Arnold SE. Inositol 1,4,5-trisphosphate receptor expression in mammalian olfactory tissue. Molec Brain Res. 1997;44:347–354. doi: 10.1016/s0169-328x(96)00282-3. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neuroscience. 2008;9:110. doi: 10.1186/1471-2202-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill MEA, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]
- Zhang C, Restrepo D. Expression of connexin 45 in the olfactory system. Brain Res. 2002;929:37–47. doi: 10.1016/s0006-8993(01)03372-8. [DOI] [PubMed] [Google Scholar]
- Zufall F, Munger SD. From odor and pheromone transduction to the organization of the sense of smell. TINS. 2001;24:191–193. doi: 10.1016/s0166-2236(00)01765-3. [DOI] [PubMed] [Google Scholar]


