Abstract
The effects of hypoxia on gene expression have been vigorously studied, but possible effects of small changes in oxygen tension have never been addressed. SUR2A is an atypical ABC protein serving as a regulatory subunit of sarcolemmal ATP-sensitive K+ (KATP) channels. Up-regulation of SUR2A is associated with cardioprotection and improved physical endurance. Here, we have found that a 24 h-long exposure to slightly decreased ambient fractional concentration of oxygen (20% oxygen), which is an equivalent to oxygen tension at 350 m above sea level, significantly increased levels of SUR2A in the heart despite that this drop of oxygen did not affect levels of O2, CO2 and hematocrit in the blood or myocardial levels of ATP, lactate and NAD/NADH/NAD+. Hearts from mice exposed to 20% oxygen were significantly more resistant to ischaemia-reperfusion when compared to control ones. Decrease in fractional oxygen concentration of just 0.9% was associated with phosphorylation of ERK1/2, but not Akt, which was essential for up-regulation of SUR2A. These findings indicate that a small drop in oxygen tension up-regulates SUR2A in the heart by activating ERK signaling pathway. This is the first report to suggest that a minimal change in oxygen tension could have a profound signaling effect.
Keywords: Oxygen, SUR2A, Heart, ERK
Highlights
-
•
Mice were exposed for 24 h to 20% oxygen (oxygen tension at sea level is 20.9%).
-
•
Exposure to 20% oxygen did not produce measurable in vivo signs of hypoxia.
-
•
However, 20% of oxygen up-regulated cardioprotective SUR2A.
-
•
Phosphorylation of ERK1/2, but not Akt, mediated observed increase in SUR2A.
-
•
Thus, a small drop in oxygen up-regulates cardiac SUR2A by activating ERK1/2.
1. Introduction
SUR2A belongs to a group of “atypical” ABC proteins as, although possessing a structure of an ABC protein, it does not seem to mediate transport [1]. SUR2A is known to bind to inward rectifier Kir6.2 to form ATP-sensitive K+ (KATP) channels in the heart. The binding of SUR2A to Kir6.2 serves a dual purpose: 1) it allows translocation of the channel to the sarcolemma and 2) contributes to the channel regulation [2]. ATP-sensitive K + (KATP) channels were originally described in cardiomyocytes, where they exert a cardioprotective role during metabolic stress. Up-regulation of myocardial SUR2A expression increases the number of fully-assembled KATP channels and induces range of effects including an increase in myocardial resistance towards metabolic stress [3], [4], an increase in physical endurance [5], prevention of aging-induced physical decline [6] and shifting cardiomyocytes towards less differentiated state [7]. Signaling factors/pathways and conditions regulating levels of SUR2A are not yet fully understood. It has been shown that young age, female gender, nicotinamide-rich diet and exercise are associated with increased levels of SUR2A in the heart. These effects seem to be mediated by changes in estrogen and NAD/NADH levels [6], [8], [9], [10], [11], [12].
In heart embryonic H9c2 cell line exposure to 13% hypoxia up-regulates SUR2A, without affecting expression of any other gene [13]. It is well established that hypoxia induces a range of effects and regulates expression of different genes [14], but the effects of hypoxia in vivo are usually observed at fractional concentration of oxygen below 15% [15], [16]. Effects of more modest changes in oxygen tension on gene expression have been rarely investigated. In fact, there is no study so far that investigated effects of small changes in oxygen tension on gene expression.
Taking into account that in vitro experiments suggested that SUR2A is uniquely sensitive to hypoxia, we have looked into possible regulation of SUR2A in the heart by slightly decreased oxygen tension. We have exposed animals to 20% oxygen, which is equivalent to oxygen tension at ~ 350 m above sea level, and found that this activated a major signaling pathway and had a profound effect on expression of SUR2A. Such effect of seemingly negligible change in oxygen has never been previously described.
2. Materials and methods
2.1. In vivo exposure of mice to different fractional concentrations of oxygen
C57BL/6J male mice (6–8 weeks old) were exposed to either ambient oxygen (detected to be 20.9%) or fractional concentration of oxygen of 20% oxygen using integral Animal Hypoxia Chamber System; oxygen levels were controlled by ProOx Model 110 version 2.2 (Biospherix, Lacona, NY, USA). The ProOx P110 senses oxygen inside the chamber and infuses either nitrogen to lower the concentration or oxygen to raise it. Mice, in groups of 5, were placed in a chamber for 24 h in either 20.9% or 20% oxygen, which level was continuously monitored. All handling of mice was done inside the chamber. Some animals were injected i.p. with inhibitor of ERK1/2, U0126 (2 μg/mouse; volume was 200 μl and vehicle was saline; Sigma-Aldrich, Gillingham, UK). 30 min after the injection animals were introduced into the chamber. For this series of experiments, control animals were injected with only vehicle (ie. 200 μl of saline i.p. injection) and subjected to the same protocol as U0126-treated animals. The experiments have been done under authority of Project License 60/3925.
2.2. Blood gas and hematocrit analysis
Blood gas (PO2 and PCO2) and hematocrit were measured in samples (500–700 μl) taken directly from the heart using pre-heparinized (1000 IU/ml) syringes and Rapidlab 348EX blood Gas System (Siemens, Frimley, UK).
2.3. Measurement of ATP in the heart
ATP concentration in heart tissue lysates was measured using luciferase-based ATP determination kit (Invitrogen, Paisley, UK) according to the manufacturer's instructions. Luminescence was measured at 560 nm using microplate reader/multidetection reader (SPECTRAMAX M2, Molecular Devices, Wokingham, UK).
2.4. Measurement of NAD/NADH in the heart
NAD/NADH was measured in heart tissue using NAD/NADH kit (Abcam, Cambridge, UK) according to the manufacturer's instruction. Absorbance was measured at 450 nm using microplate reader/multidetection reader (SPECTRAMAX M2, Molecular Devices, Wokingham, UK). Total NAD (NADt) and NADH were estimated directly while the value of NAD+ was estimated by subtracting NADH from NADt.
2.5. Measurement of lactate in the heart
Lactate was measured in heart tissue lysates using ADVIA Chemistry Lactate Enzymatic Assay and ADVIA Chemistry System 1200 (Siemens, Frimley, UK). Lactate is oxidized by lactate oxidase to pyruvate and hydrogen peroxide and it was measured by the formation of dye from hydrogen peroxide and a chromogen in the presence of a peroxidase. Absorbance was measured at 545/694 nm.
2.6. Ischaemia-reperfusion and measurement of creatine kinase (CK) and lactate dehydrogenase (LDH)
Ischaemia-reperfusion of hearts was performed as described in ref. [10] and perfusate was recirculated during reperfusion and collected at the end of this stage. CK and LDH in perfusates from the hearts exposed ischaemia-reperfusion were measured using ADVIA Chemistry System 2400 (Siemens, Frimley, UK). CK reacts with creatine phosphate and ADP to form ATP which is coupled to the hexokinase-G6PD reaction, generating NADPH. The concentration of NADPH as a measure of CK activity was determined by changes in absorbance at 340/410 nm. LDH catalyzes the conversion of lactate to pyruvate in the presence of NAD. The enzymatic activity of lactate dehydrogenase is proportional to the rate of production of NADH. The amount of NADH produced is determined at 340/410 nm.
2.7. Western blotting
For Western blotting hearts were harvested and snap-frozen and homogenized in lysis buffer (50 mM Tris–HCl, pH 7.5; 1 mM EDTA; 1 mM EGTA; 1% (w/v) Triton X-100; 0.1% (v/v) — mercaptoethanol; 1 mM sodium orthovanadate; 50 mM sodium fluoride; 5 mM sodium pyrophosphate; 1 μM microcystin-LR; and one tablet of “complete” proteinase inhibitor per 50 ml of buffer). A 10-fold mass excess of ice-cold lysis buffer was added to the powdered.
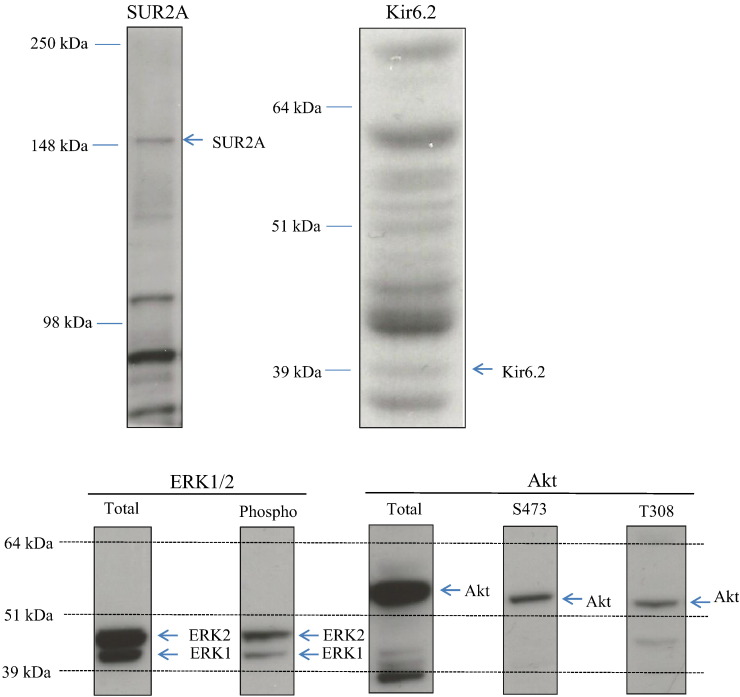
Tissue, briefly vortexed, and then centrifuged at 4 °C for 10 min at 13,000 g to remove insoluble material. The supernatant was snap-frozen in aliquots in liquid nitrogen and stored at − 80 °C. Protein concentration was determined by Bradford Assay. From each sample, 20 μg of protein was subjected to SDS/PAGE and transferred to nitrocellulose. For all blots, the nitrocellulose membranes were incubated at 4 °C for 16 h using the antibodies against SUR2A, Kir6.2 (both from Santa Cruz Biotechnology, Heidelberg, Germany), Akt total antibody, total ERK1/2 antibody, phospho-Akt antibodies (Thr308 and Ser473) and phospho-ERK1/2 (all from Millipore, Watford, UK). All antibodies were applied in 1:1000 dilution. The blots were incubated in 50 mM Tris/HCl, pH 7.5; 0.15 M NaCl; and 0.2% (by vol) Tween containing 5% (by mass) skimmed milk. Detection of total or phosphorylated protein was performed using horse radish peroxidase conjugated secondary antibodies (Pierce, Rockford, IL, USA) and enhanced chemiluminescence (ECL) reagent (Upstate, Dundee, UK). All antibodies have been characterized in details. SUR2A antibody produced a single signal in the expected region (Fig. 1). The Kir6.2 antibody produced several signals, but one of those was 39 kDa, which is the exact size of Kir6.2 (Fig. 1). Antibodies against ERK1/2, phospho-ERK1/2, Akt, S473 phospho-Akt and T308 phospho-Akt yielded solitary or double signals (where appropriate) in expected regions (Fig. 1). The band intensities were analyzed using Quantiscan software (Cambridge, UK).
Fig. 1.
Western blot signals obtained by anti-SUR2A, anti-Kir6.2, anti-ERK1/2 and anti-Akt antibodies were of appropriate molecular weight and clearly distinguishable from any other signals appearing on blots. Typical examples of original Western blots obtained in this study.
2.8. Statistical analysis
Data are presented as mean±S.E.M., with n representing the number of analyzed mice or cells. Mean values were compared by Student's t-test using SigmaStat program (Jandel Scientific, Chicago, Illinois). P < 0.05 was considered statistically significant.
3. Results
3.1. 24 h-long exposure to 20% oxygen does not induce hypoxia in mice, but it up-regulates SUR2A in the heart
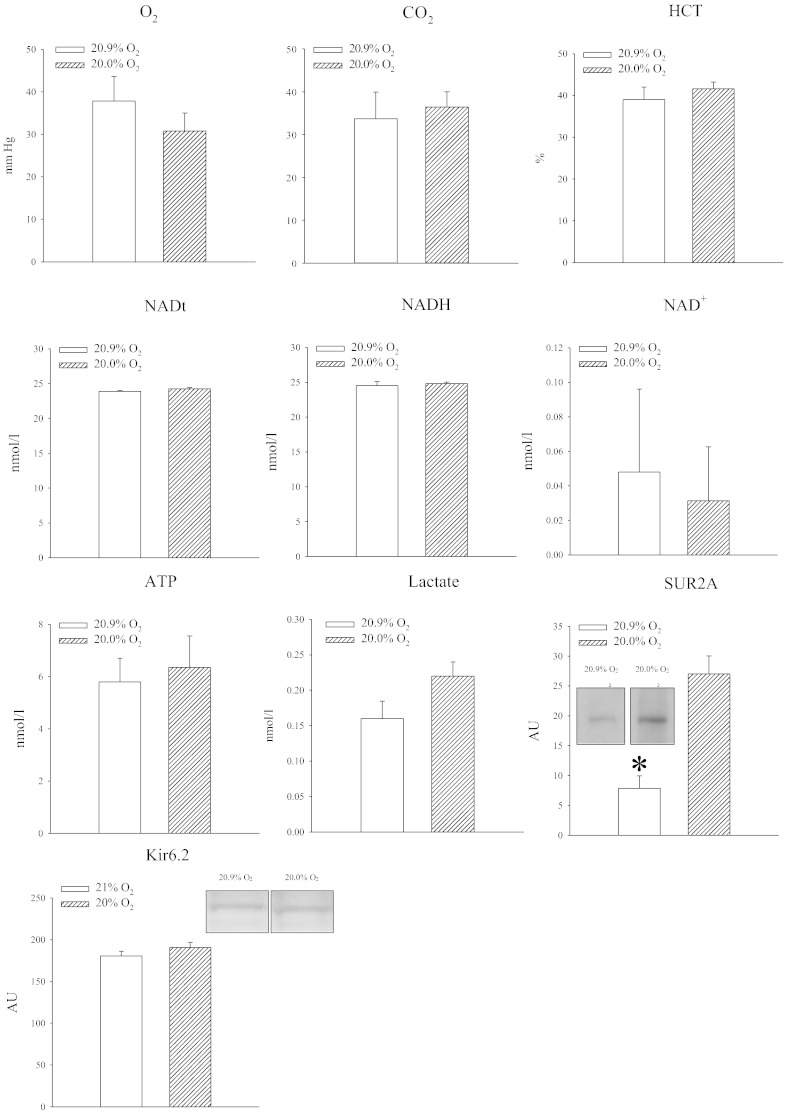
Mice were exposed to either 20.9% air oxygen or 20% oxygen. Oxygen tension in the blood was slightly decreased in mice exposed to 20% oxygen, but the difference was not statistically significant (P = 0.365; Fig. 2). No differences were found in blood partial pressure of CO2 (P = 0.753; Fig. 2). Also, hematocrit did not differ between experimental groups (P = 0.723; Fig. 2). In hearts, no differences were observed between NAD levels (NADt: P = 0.196; NADH: P = 0.772, Fig. 2). Small changes in oxygen tension also did not affect levels of ATP (P = 0.724; Fig. 2) and lactate (P = 0.09; Fig. 2). However, such seemingly negligible change in oxygen induced a substantial increase in SUR2A in the myocardium (Fig. 2; P = 0.001), while the expression of another KATP channel forming subunit, Kir6.2, was not affected (P = 0.281; Fig. 2).
Fig. 2.
Exposure to 20% oxygen up-regulates SUR2A in the heart without inducing any measurable sings of hypoxia. Bar graphs depicting PO2 (O2), PCO2 (CO2), hematocrit (HCT), total NAD (NADt), NADH (NADH), NAD + (NAD +), ATP (ATP), lactate (Lactate) SUR2A (SUR2A) and Kir6.2; insets in SUR2A and Kir6.2 bar graphs represent original Western blots for SUR2A and Kir6.2 under labeled conditions. Each bar is a mean ± SEM (n = 3–5). *P < 0.01.
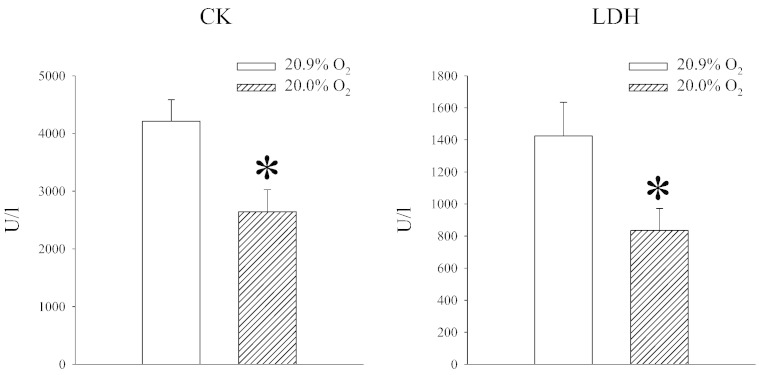
An increase in SUR2A in the heart is a significant signaling event with major implications: it increases heart resistance to ischaemia-reperfusion [10], counteracts aging-induced increase in myocardial susceptibility to stress [6] and promotes stem cells properties of cardiomyocytes [7]. We have tested whether exposure to 20% oxygen would affect myocardial resistance to ischaemia-reperfusion by measuring amounts of CK and LDH released from the muscle during this challenge, which is a good indication of necrosis of the myocardium. We have found that hearts from animals exposed to 20% oxygen released significantly less CK and LDH than animals exposed to 20.9% oxygen (CK: P = 0.019; LDH = 0.038; Fig. 3).
Fig. 3.
Exposure to 20% oxygen increases heart resistance to ischaemia-reperfusion. Bar graphs depicting CK and LDH concentration in heart perfusate following ischaemia-reperfusion challenge under labeled conditions. Each bar is a mean ± SEM (n = 5). *P < 0.05.
3.2. 24 h-long exposure to 20% oxygen activates ERK, but not Akt in the heart
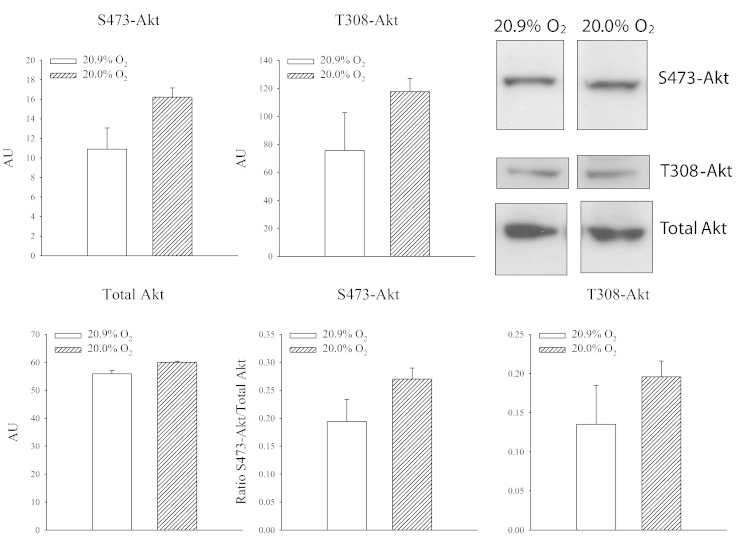
Phosphatidylinositol-4,5-bisphosphate 3-kinases (PI3K) are family of enzymes involved in crucial cell physiological functions including the cell survival. Most of PI3K functions are related to its ability to activate Akt, also known as protein kinase B (PKB) [17], [18]. Activation of Akt is a crucial signaling event in evoking cardioprotection [17], [19], [20]. Thus, we have examined a possible link between Akt and SUR2A. Antibodies targeting phosphorylation of Akt at S473 and T308 sites were used as well as an antibody targeting Akt non-selectively. We have found that exposure to 20% oxygen did not phosphorylate Akt either on S473 or on T308 (Fig. 4).
Fig. 4.
Exposure to 20% oxygen does not phosphorylate Akt in the heart. Original Western blots with phospho-S473-Akt, phospho-T308-Akt and total Akt antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 4–5).
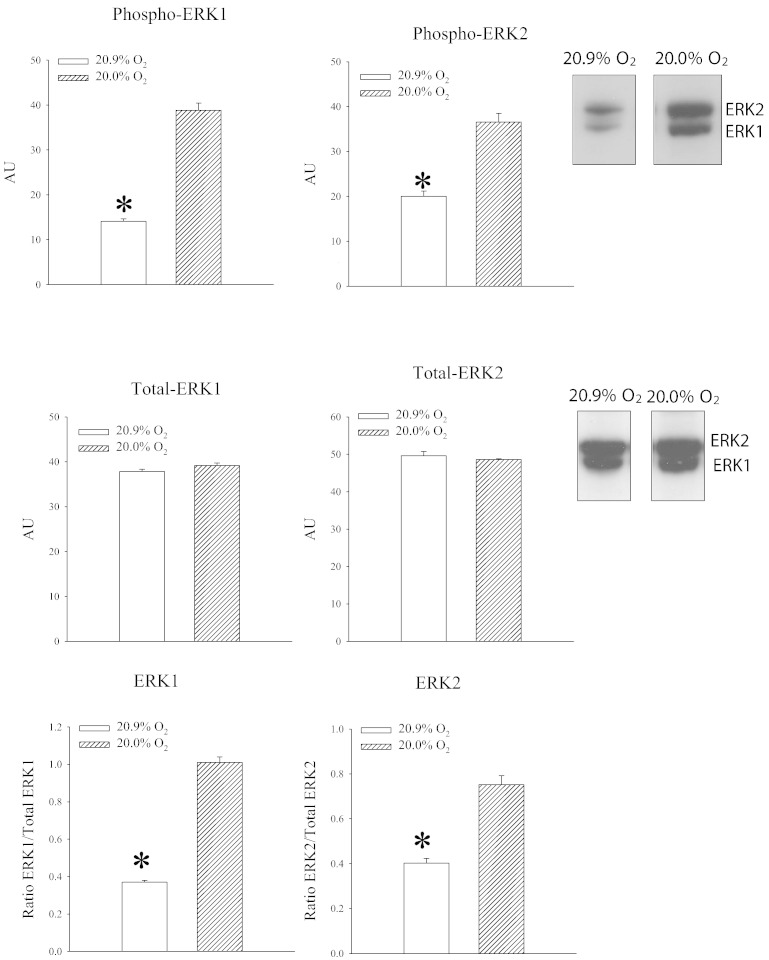
Extracellular signal regulated kinases (ERK1/2) are ubiquitously expressed and are involved in regulation of important physiological processes including cellular differentiation, proliferation, survival and others. ERK1/2 can be stimulated by a number of factors including growth factors, cytokines, G protein-regulated receptors and viral infections [21], [22]. Here, we have tested whether phosphorylation of ERK1/2 occurs during exposure to 20% oxygen. Exposure to 20% oxygen significantly increased signal for both ERK1 and ERK2 (ERK1: P < 0.01; ERK2: P < 0.01; Fig. 5). The ratio phospho vs total ERK was significantly increased for both ERK1 (P = 0.018; Fig. 4) and ERK2 (P < 0.001; Fig. 5).
Fig. 5.
Exposure to 20% oxygen phosphorylate ERK1/2 in the heart. Original Western blots with phospho-ERK1, phospho-ERK2 and total ERK1 and ERK2 antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 4–5). *P < 0.05.
3.3. Activation of ERK is responsible for up-regulation of SUR2A in the heart induced by 24 h-long exposure to 20% oxygen
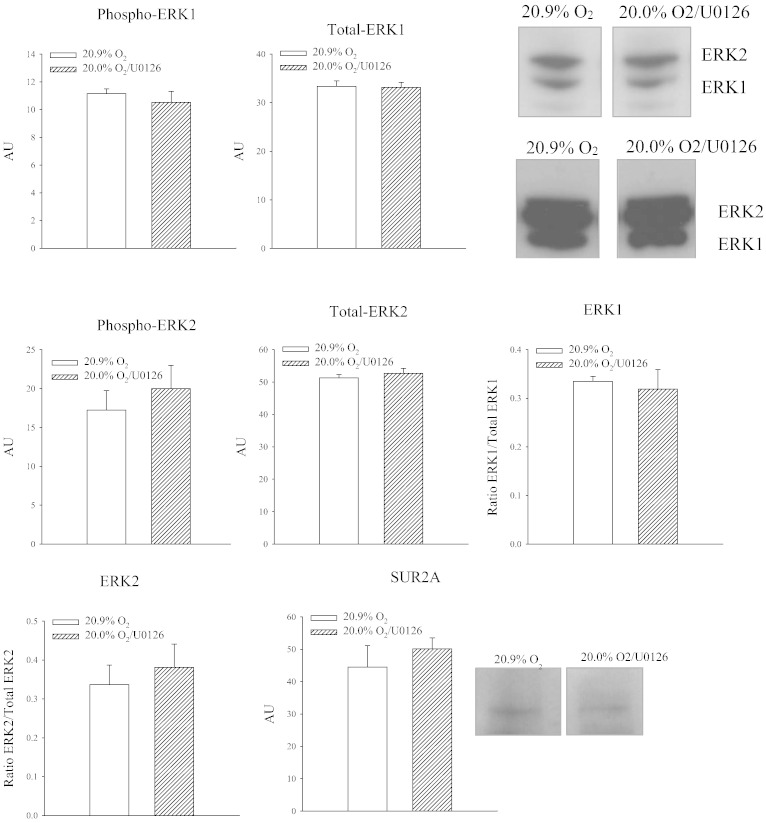
ERK1/2 activation could have been epiphenomenon that is not related to regulation of SUR2A at all. In order to find out a causal relationship between ERK activation and SUR2A, we have treated animals with U0126 (2 μg/mouse), an inhibitor of ERK1/2 phosphorylation [19]. When U0126-treated animals exposed to 20% oxygen were compared with vehicle-treated animals exposed to 20.9% oxygen, no differences in ERK1/2 phosphorylation was observed between groups (ERK1: P = 0.538; ERK2: P = 0.517; Fig. 6). The ratio phospho vs total ERK was not changed for both ERK1 (P = 0.710; Fig. 6) and ERK2 (P = 0.612; Fig. 5). At the same time, treatment with U0126 prevented increase in myocardial SUR2A in mice exposed to 20% oxygen (Fig. 6; P = 0.496).
Fig. 6.
U0126 inhibits phosphorylation of ERK1 and ERK2 and increase of SUR2A in response to 20% oxygen. Original Western blots with phospho-ERK1, phospho-ERK2, total ERK1/ERK2 and SUR2A antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 3). *P < 0.05.
4. Discussion
Cardiac response to chronic hypoxia has been studied in details due to reports suggesting that high-altitude residents have lower mortality rates for ischaemic heart disease [23], [24], [25]. In agreement with these reports were findings that exposure to moderate hypoxia confers cardioprotection in experimental animals [26], [27]. It has been suggested that this is due to myocardial metabolic reorganization and increase in utilization of carbohydrates as cardiac fuel substrate (versus fatty acids) and augmented mitochondrial respiratory capacity [30]. Metabolic gene remodeling in response to hypoxia has also been reported [28]. It has been already shown that under in vitro conditions mild hypoxia up-regulates SUR2A in rat heart embryonic H9c2 line creating cellular phenotype resistant to metabolic stress [13]. What was interesting in this study was finding that SUR2A was the only gene regulated by the level of hypoxia applied [13]. However, hypoxia applied in that study altered NAD+/NADH ratio, which was required for up-regulation of SUR2A [13]. In the present study, a slight decrease in concentration of oxygen was not associated with usual signs of hypoxia in the blood or myocardium. In hypoxia, NAD+/NADH ratio changes, levels of ATP and lactate decrease and increase respectively [29], [30]. Exposure to 20% oxygen did not affect neither cardiac NAD+/NADH levels nor levels of ATP or lactate suggesting a possibility that heart was not exposed to hypoxia irrespective to systemic exposure of mice to slightly decreased oxygen tension. This is probably not unexpected as a drop of 0.9% oxygen seems to be minimal.
However, such small decrease in oxygen tension up-regulated SUR2A. In vitro exposure to 13% oxygen altered NAD+/NADH levels which was shown to regulate expression of SUR2A [5], [10], [13]. However, in mice exposed to 20% oxygen we did not find signs that their hearts were subjected to hypoxia, and yet SUR2A was increased. An increase of SUR2A was shown to protect myocardium against ischaemia-reperfusion [3], [10] as well as adult and embryonic cardiomyocytes against different types of metabolic stresses [3], [4], [5], [6]. A range of diverse conditions such as young age, female gender, nicotinemide-rich diet and acute and chronic exercise [8], [9], [10], [11], [12] were associated with increased SUR2A levels and cardioprotection. Increase of SUR2A alone is sufficient to confer cardioprotection even if Kir6.2 levels remain unchanged [3], [4], [5], [8]. The mechanism of cardioprotection afforded by SUR2A alone is complex and seems to involve activity-dependent and activity-independent properties of KATP channels [3], [4], [5], [7], [31], [32]. CK and LDH are well established markers of myocardial injury [33], and we have used them here to determine whether exposure to 20% oxygen increases resistance to ischaemia-reperfusion. Indeed, exposure to 20% oxygen decreased the amounts of CK and LDH released from myocardium in response to ischaemia-reperfusion suggesting an increase in myocardial resistance to injury. These results fit very well with the notion that 20% oxygen increases levels of SUR2A in the heart, which in turn confers cardioprotection.
PI3K/Akt cascade is a major signaling pathway mediating cardioprotection [18], [19]. Taking into consideration that SUR2A confers cardioprotection, a link between Akt and SUR2A expression was examined in this study. We have found that exposure to 20% oxygen did not phosphorylate Akt either on S473 or on T308, excluding a possibility that activation of Akt is required for up-regulation of SUR2A induced by slight drop in oxygen tension.
It has been previously shown that mild hypoxia activates ERK to regulate expression of SUR2A in embryonic rat heart H9c2 cell line [13]. In embryonic hearts increase in SUR2A levels shifts cardiomyocytes towards less differentiated state by inhibiting ERK1/2 [7]. Here, we have found that exposure to 20% oxygen was associated with phosphorylation of ERK1/2. U0126 inhibited both phosphorylation of ERK1/2 and increase in SUR2A induced by 20% oxygen. Taking all together, it is clear that exposure to 20% oxygen activates ERK1/2 and that this activation is required for up-regulation of SUR2A and cardioprotection. It has been previously shown that ERK regulates expression of SUR2A via c-jun and regulation of SUR2 promoter activity [13]. However, it remains unclear what activates ERK1/2 in the heart of animals exposed to 20% oxygen. In H9c2 cells it has been proposed that mild hypoxia changes NAD +/NADH ratio and that this act as a trigger for signaling cascade involving ERK and SUR2A [13]. Again, a drop of only ~ 1% in oxygen concentration did not affect NAD +/NADH ratio showing that something else is responsible for ERK1/2 phosphorylation and consequent increase in SUR2A levels. How ERK in the heart is activated by a small decrease of oxygen tension in vivo remains to be established. At the moment, it is difficult to speculate about sequence of events leading to ERK activation and SUR2A up-regulation as oxygen sensor responding to such small change to oxygen tension is yet unknown.
4.1. Limitations of the study
This study has demonstrated that even very small changes in oxygen tension induce a significant signaling response. However, this study has some limitations. The major limitation is that all parameters were measured at one time point (24 h), meaning that we could have missed an important early signaling event (changes in NAD/NADH ratio as an example). Also, we did not assess contributions of various signaling factors that could have been involved. There are also other limitations that are present in almost every study such as species (mice) and gender (males alone) used.
5. Conclusion
Taken all together, we have shown in this study that a seemingly negligible decrease in oxygen tension in vivo activates ERK1/2 to up-regulate SUR2A. This is a first demonstration of a gross signaling effect induced by a small change in oxygen tension.
Authorships and disclosures
KSMA contributed to the study design, performed experiments, analyzed data obtained in those experiments and contributed to writing of the manuscript. SJ contributed to the study design, performed some experiments, analyzed data obtained in those experiments and contributed to writing of the manuscript.
AS performed some experiments and analyzed data obtained in those experiments. QD performed some experiments. AJ designed and supervised the study, analyzed data and wrote the manuscript. The authors declare that no competing interests exist.
Acknowledgements
This research was supported by the British Heart Foundation (PG/11/106/29235).
References
- 1.Burke M.A., Mutharasan R.K., Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 2008;102:164–176. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- 2.Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 3.Du Q., Jovanovic S., Clelland A., Sukhodub A., Budas G.R., Phelan K., Murray-Tait V., Malone L., Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Q., Jovanovic S., Sukhodub A., Jovanovic A. Infection with AV-SUR2A protects H9C2 cells against metabolic stress: a mechanism of SUR2A-mediated cytoprotection independent from the KATP channels activity. Biochem. Biophys. Acta Mol. Cell. Res. 2010;1803:405–415. doi: 10.1016/j.bbamcr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhodub A., Sudhir R., Du Q., Jovanovic S., Reyes S., Jovanovic A. Nicotinamide-rich diet improves physical endurance by upregulating SUR2A in the heart. J. Cell. Mol. Med. 2011;15:1703–1712. doi: 10.1111/j.1582-4934.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudhir R., Sukhodub A., Du Q., Jovanovic S., Jovanovic A. Ageing-induced decline in physical endurance in mice is associated with decrease in cardiac SUR2A and increase in cardiac susceptibility to metabolic stress: therapeutic prospects for up-regulation of SUR2A. Biogerontology. 2011;12:147–155. doi: 10.1007/s10522-010-9306-3. [DOI] [PubMed] [Google Scholar]
- 7.Land S., Walker D., Du Q., Jovanovic A. Cardioprotective SUR2A promotes stem cell properties of cardiomyocytes. Int. J. Cardiol. 2013;168:5090–5092. doi: 10.1016/j.ijcard.2013.07.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranki H.J., Budas G.R., Crawford R.M., Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- 9.Ranki H.J., Budas G.R., Crawford R.M., Davies A.M., Jovanovic A. 17β-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J. Am. Coll. Cardiol. 2002;40:367–374. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 10.Sukhodub A., Du Q., Jovanovic S., Jovanovic A. Nicotinamide-rich diet protects the heart against ischaemia-reperfusion in mice: a crucial role for cardiac SUR2A. Pharmacol. Res. 2010;61:564–570. doi: 10.1016/j.phrs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D.A., Chicco A.J., Jew K.N., Johnson M.S., Lynch J.M., Watson P.A., Moore R.L. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J. Physiol. 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown D.A., Lynch J.M., Armstrong C.J., Caruso N.M., Ehlers L.B., Johnson M.S., Moore R.L. Susceptibility of the heart to ischaemia-reperfusion injury and exercise induced cardioprotection are sex-dependent in the rat. J. Physiol. 2005;564:619–630. doi: 10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford R.M., Jovanovic S., Budas G.R., Davies A.M., Lad H., Wenger R.H., Robertson K.A., Roy D.J., Ranki H.J., Jovanovic A. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channels. J. Biol. Chem. 2003;278:31444–31455. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 15.Fradette C., Batonga J., Teng S., Piquette-Miller M., du Souich P. Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug Metab. Dispos. 2007;35:765–771. doi: 10.1124/dmd.106.013508. [DOI] [PubMed] [Google Scholar]
- 16.Fomicheva E.V., Turner I.I., Edwards T.G., Hoff J., Arden E., D'Alecy L.G., Metzger J.M. Double oxygen-sensing vector system for robust hypoxia/ischemia-regulated gene induction in cardiac muscle in vitro and in vivo. Mol. Ther. 2008;16:1594–1601. doi: 10.1038/mt.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budas G.R., Sukhodub A., Alessi D.R., Jovanovic A. 3'phosphoinositide-dependent kinase-1 (PDK1) is essential for ischaemic preconditioning of the myocardium. FASEB J. 2006;20:2556–2558. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heusch G., Boengler K., Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 21.Shaul Y.D., Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochem. Biophys. Acta Mol. Cell. Res. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto S., Nishide E. E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortimer E.A., Monson R.R., MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N. Engl. J. Med. 1977;296:581–585. doi: 10.1056/NEJM197703172961101. [DOI] [PubMed] [Google Scholar]
- 24.Ezzati M., Horwitz M.E., Thomas D.S., Friedman A.B., Roach R., Clark T., Murray C.J.L., Honigman B. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: national population-based analysis of US counties. J. Epidemiol. Community Health. 2012;66:e17. doi: 10.1136/jech.2010.112938. [DOI] [PubMed] [Google Scholar]
- 25.Hurtado A., Escudero E., Pando J., Sharma S., Johnson R.J. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol. Dial. Transplant. 2012;27(Suppl. 4):iv11–iv16. doi: 10.1093/ndt/gfs427. [DOI] [PubMed] [Google Scholar]
- 26.Tajima M., Katayose D., Bessho M., Isoyama S. Acute ischaemic preconditioning and chronic hypoxia independently increase myocardial tolerance to ischaemia. Cardiovasc. Res. 1994;28:312–319. doi: 10.1093/cvr/28.3.312. [DOI] [PubMed] [Google Scholar]
- 27.Berger M.M., Huhn R., Oei G.T., Heinen A., Winzer A., Bauer I., Preckel B., Weber N.C., Schlack W., Hollmann M.W. Hypoxia induces late preconditioning in the rat heart in vivo. Anesthesiology. 2010;113:1351–1360. doi: 10.1097/ALN.0b013e3181fce7ea. [DOI] [PubMed] [Google Scholar]
- 28.Essop M.F. Cardiac metabolic adaptations in response to hypoxia. J. Physiol. 2007;584:715–726. doi: 10.1113/jphysiol.2007.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun F., Dai C., Xie J., Hu X. Biochemical issues in estimation of cytosolic free NAD/NADH ratio. PLoS One. 2012;7:e34525. doi: 10.1371/journal.pone.0034525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClelland G.B., Brooks G.A. Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J. Appl. Physiol. 2002;92:1573–1584. doi: 10.1152/japplphysiol.01069.2001. [DOI] [PubMed] [Google Scholar]
- 31.Jovanovic S., Du Q., Sukhodub A., Jovanovic A. M-LDH physically associated with sarcolemmal KATP channels mediates cytoprotection in heart embryonic H9C2 cells. Int. J. Biochem. Cell Biol. 2009;41:2295–2301. doi: 10.1016/j.biocel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic S., Du Q., Sukhodub A., Jovanovic A. Dual mechanism of cytoprotection afforded by M-LDH in embryonic heart H9C2 cells. Biochem. Biophys. Acta Mol. Cell. Res. 2009;1793:1379–1386. doi: 10.1016/j.bbamcr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemp M., Donovan J., Higham H., Hooper J. Biochemical markers of myocardial injury. Br. J. Anaesth. 2004;93:63–73. doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]