Abstract
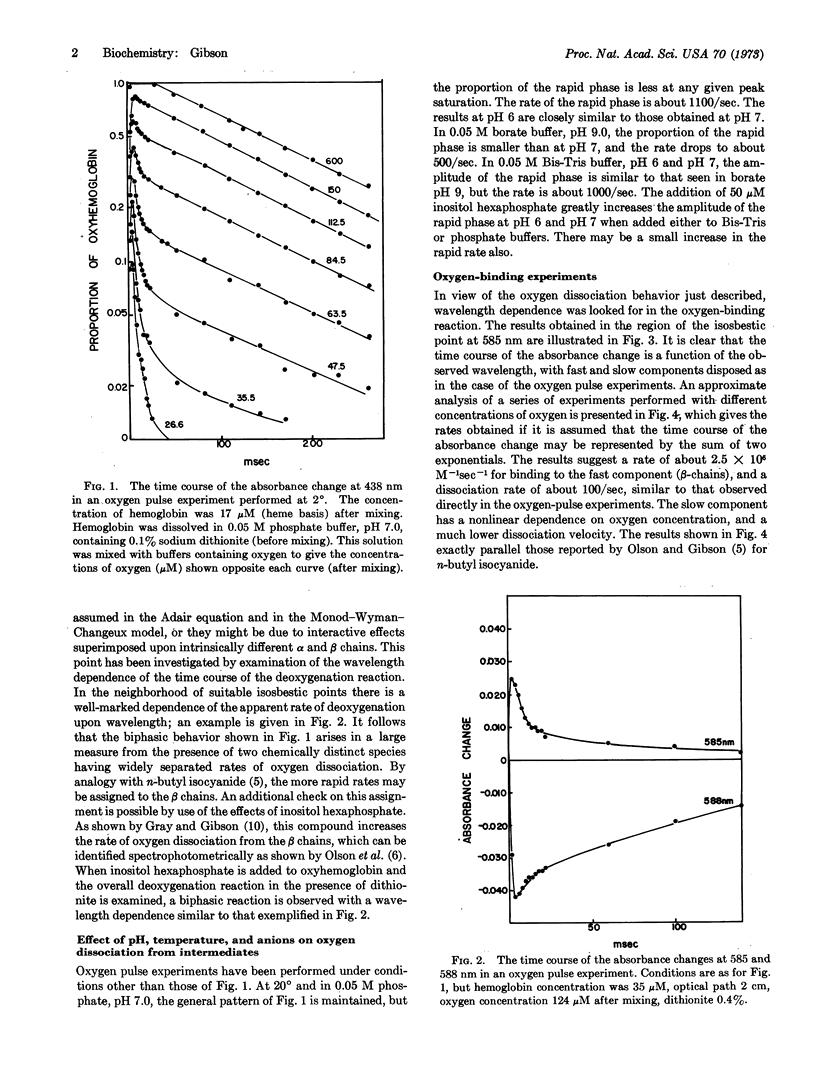
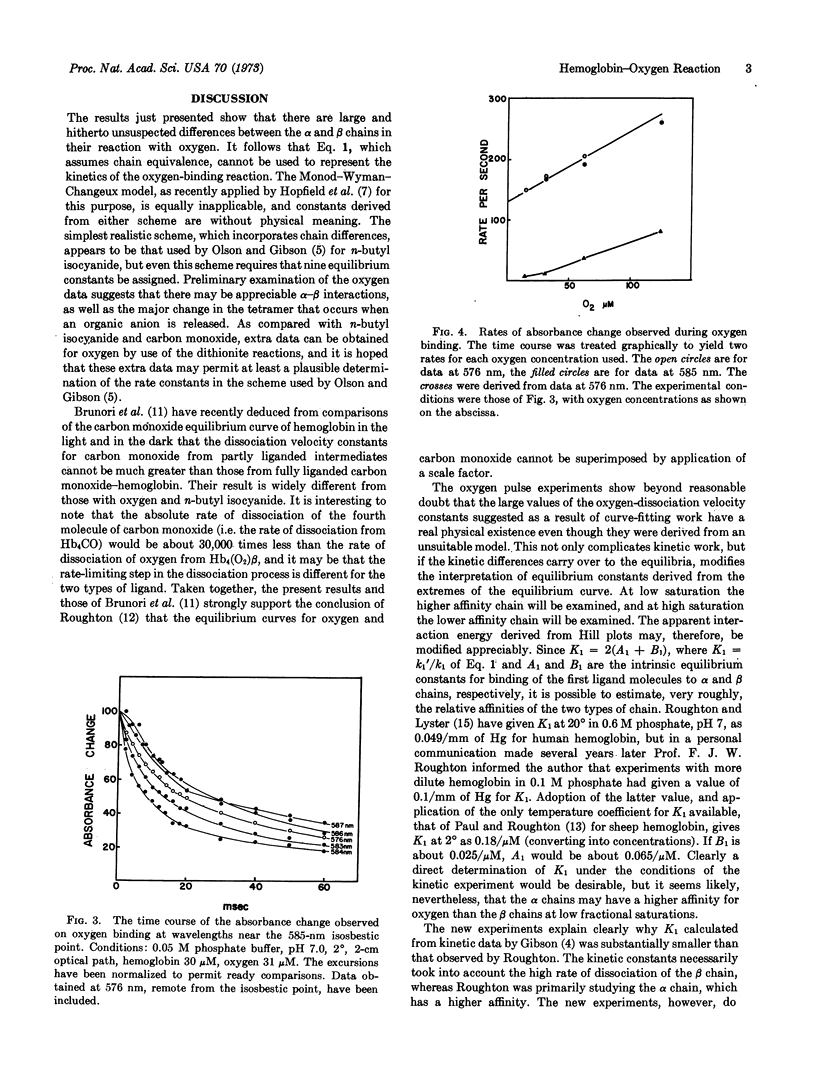
A new type of experiment in which hemoglobin is exposed briefly to oxygen has shown that the half-time of dissociation of oxygen from some partly oxygenated intermediates is about 1 msec at 20° and 10 msec at 2°. The rapid dissociation occurs selectively from one type of chain, provisionally identified as the β-chain. Chains that show the rapid rate of dissociation of oxygen also bind rapidly. It follows that the kinetic equivalent of the Adair equation and the Monod-Wyman-Changeux model are quite unsuited to represent the kinetics of the oxygen-hemoglobin reaction. The reaction of oxygen with hemoglobin closely resembles that of the alkyl isocyanides and differs radically from that of carbon monoxide.
Keywords: heme proteins, ligand binding, protein chemistry
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R. L., Antonini E., Brunori M., Wyman J., Rossi-Fanelli A. Observations on the kinetics of the reaction of hemoglobin with oxygen. J Biol Chem. 1967 Oct 25;242(20):4841–4843. [PubMed] [Google Scholar]
- Brunori M., Bonaventura J., Bonaventura C., Antonini E., Wyman J. Carbon monoxide binding by hemoglobin and myoglobin under photodissociating conditions. Proc Natl Acad Sci U S A. 1972 Apr;69(4):868–871. doi: 10.1073/pnas.69.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSa R. J., Gibson Q. H. A practical automatic data acquisition system for stopped-flow spectrophotometry. Comput Biomed Res. 1969 Oct;2(5):494–505. doi: 10.1016/0010-4809(69)90014-7. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics of dissociation of the first oxygen molecule from fully saturated oxyhaemoglobin in sheep blood solutions. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):310–334. doi: 10.1098/rspb.1955.0014. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H. The reaction of oxygen with hemoglobin and the kinetic basis of the effect of salt on binding of oxygen. J Biol Chem. 1970 Jul 10;245(13):3285–3288. [PubMed] [Google Scholar]
- Gray R. D., Gibson Q. H. The effect of inositol hexaphosphate on the kinetics of CO and O 2 binding by human hemoglobin. J Biol Chem. 1971 Dec 10;246(23):7168–7174. [PubMed] [Google Scholar]
- Hopfield J. J., Shulman R. G., Ogawa S. An allosteric model of hemoglobin. I. Kinetics. J Mol Biol. 1971 Oct 28;61(2):425–443. doi: 10.1016/0022-2836(71)90391-3. [DOI] [PubMed] [Google Scholar]
- Olson J. S., Andersen M. E., Gibson Q. H. The dissociation of the first oxygen molecule from some mammalian oxyhemoglobins. J Biol Chem. 1971 Oct 10;246(19):5919–5923. [PubMed] [Google Scholar]
- Olson J. S., Gibson Q. H. The reaction of n-butyl isocyanide with human hemoglobin. II. The ligand-binding properties of the and chains within deoxyhemoglobin. J Biol Chem. 1972 Mar 25;247(6):1713–1726. [PubMed] [Google Scholar]
- PAUL W., ROUGHTON F. J. W. The equilibrium between oxygen and sheep haemoglobin at very low percentage saturations. J Physiol. 1951 Mar;113(1):23–35. doi: 10.1113/jphysiol.1951.sp004553. [DOI] [PMC free article] [PubMed] [Google Scholar]



