Abstract
Checkpoint kinase 2 (CHK2) is a downstream effector of the DNA damage response (DDR). Dysfunctional telomeres, either owing to critical shortening or disruption of the shelterin complex, activate a DDR, which eventually results in cell cycle arrest, senescence and/or apoptosis. Successive generations of telomerase-deficient (Terc) mice show accelerated aging and shorter lifespan due to tissue atrophy and impaired organ regeneration associated to progressive telomere shortening. In contrast, mice deficient for the shelterin component TRF1 in stratified epithelia show a rapid and massive induction of DDR, leading to perinatal lethality and severe skin defects. In both mouse models, p53 deficiency can rescue survival. Here, we set to address the role of CHK2 in signaling telomere dysfunction in both mouse models. To this end, we generated mice doubly deficient for Chk2 and either Terc (Chk2−/− Terc−/−) or Trf1 (Trf1Δ/Δ K5Cre Chk2−/−). We show that Chk2 deletion improves Terc-associated phenotypes, including lifespan and age-associated pathologies. Similarly, Chk2 deficiency partially rescues perinatal mortality and attenuates degenerative pathologies of Trf1Δ/Δ K5Cre mice. In both cases, we show that the effects are mediated by a significant attenuation of p53/p21 signaling pathway. Our results represent the first demonstration of a role for CHK2 in the in vivo signaling of dysfunctional telomeres.
Keywords: aging, DNA damage, genetics, lifespan, telomerase, telomere
Introduction
Telomere dysfunction is caused by either critical telomere shortening or loss of the protein complex that protects telomeres, the so-called shelterin (de Lange, 2005a). Dysfunctional telomeres behave as double strand breaks (DSBs) and trigger a persistent DNA damage response (DDR) (d’Adda di Fagagna et al., 2003; de Lange, 2005a). DSBs are among the most deleterious lesions that challenge genomic integrity. Concomitant to the repair of DSBs, a rapid signaling cascade must be coordinated at the lesion site to prevent cell division by activation of cell cycle checkpoints. Ataxia telangiectasia mutated (ATM) and ATM and Rad-3-related (ATR) protein kinases are among the earliest signaling molecules known to initiate the transduction cascade at damage sites. This cascade ultimately results in the activation of p21 and p53, leading to senescence/apoptosis (Takai et al., 2003; von Zglinicki et al., 2005). ATM has an essential role in signaling from DSBs arising from ionizing radiation (IR) through a CHK2-dependent pathway, while ATR is typically involved in signaling from replication-linked SSBs through the CHK1 kinase. Recent findings, however, have demonstrated an active cross talk between ATM and ATR signaling pathways in response to DNA damage (Matsuoka et al., 2000; Murga et al., 2009). Both CHK1 and CHK2 can activate p53 upon DNA damage (Shieh et al., 2000) and are important for cell cycle checkpoints, however, while CHK1 is crucial for intra-S and G2/S transition, CHK2 is more important for G1/S checkpoint (Liu et al., 2000; Takai et al., 2000, 2002; Hirao et al., 2002).
Telomere shortening is one of the molecular pathways underlying organismal aging (Lopez-Otin et al., 2013). Telomerase-deficient mice show progressive telomere shortening, eventually impairing tissue regeneration and leading to tissue atrophy and premature aging phenotypes, as well as to cancer resistance (Greenberg et al., 1998; Lee et al., 1998; Rudolph et al., 1999; Gonzalez-Suarez et al., 2000). p53 deficiency can rescue both survival and tumorigenesis in Terc−/− p53−/− mice, indicating that p53 is one of the main downstream mediators of the cellular response to DDR triggered by short telomeres (Artandi et al., 2000).
TRF1 is one of the six components of shelterin, which has an essential role in protecting telomeres from fusions and from telomere fragility resulting from telomere replication (De Lange, 2005b). Abrogation of Trf1 in mice results in early embryonic lethality (Karlseder et al., 2003). Conditional deletion of Trf1 in mouse stratified epithelia leads to perinatal lethality and severe skin morphogenesis defects, which are concomitant with rapid induction of telomere-originated DNA damage and activation of the p53/p21 pathway (Martinez et al., 2009). Similar to that shown for Terc-deficient mice, deletion of p53 in Trf1Δ/Δ K5Cre mice rescues survival and leads to increased cancer incidence, indicating that p53 is also a main mediator of the cellular response to DDR induced by uncapped telomeres (Martinez et al., 2009).
Interestingly, it has been recently shown that deletion of Trf1 induces phosphorylation of both the ATR and ATM downstream kinases CHK1 and CHK2, respectively, suggesting that both checkpoint kinases may be important to mediate telomere dysfunction owing to telomere uncapping (Martinez et al., 2009; Sfeir et al., 2009). In line with this, inhibition of either ATM or ATR kinases in Trf1-deficient MEFs can significantly rescue DNA damage in vitro (Martinez et al., 2009). However, whether attenuation of these pathways rescues Trf1 deficiency-associated phenotypes in vivo is not known.
Several studies have previously addressed the role of CHK2 in the response to dysfunctional telomeres in vitro cultured human cells (Thanasoula et al., 2012; Cesare et al., 2013). Thus, Cesare et al. (2013) showed that telomere deprotection resuting from TRF2 downregulation to levels that do not induce telomere–telomere fusions, results in p53-mediated G1 cell cycle arrest independently of CHK2 phosphorylation. Only when TRF2 was fully abrogated leading to chromosome fusions, the authors observed CHK2 phosphorylation (Cesare et al., 2013). The authors concluded that very few TRF2 molecules at telomeres are sufficient to prevent the nonhomologous end joining pathway (NHEJ) and to inhibit CHK2 activation, thereby allowing the cells to continue through mitosis avoiding the G2/M arrest (Cesare et al., 2013). In contrast, Thanasoula et al. (2012) showed that TRF2 depletion leads to CHK2 phosphorylation and to G2/M arrest, preventing fusions and genome instability.
In the case of the mouse, we have previously shown that dysfunctional telomeres induced by Trf1 deletion lead to Chk2 phosphorylation and G2/M arrest (Martinez et al., 2009). Furthermore, abolishment of end-to-end fusions owing to Trf1 deficiency by simultaneously deleting 53 bp1, and essential component of the NHEJ, does not inhibit Chk2 phosphorylation or ‘in vivo’ G2/M arrest (Martinez et al., 2009). Similarly, mouse cells depleted for Trf2 and lacking ligase 4, another essential component of the NHEJ, do not show telomere–telomere fusions but show Chk2 phosphorylation (Celli & de Lange, 2005). Thus, in contrast to human cells, mouse telomere dysfunction induced by either depleting TRF1 or TRF2 leads to Chk2 phosphorylation independently of chromosome fusions.
Here, we set to address the role of CHK2 activation in response to dysfunctional telomeres in vivo. In particular, we set to address whether Chk2 deficiency could rescue phenotypes associated to telomere dysfunction in Terc and Trf1 deficient mouse models previously generated by us (Blasco et al., 1997; Martinez et al., 2009).
Chk2-deficient mice are viable and do not show increased cancer incidence (Donehower et al., 1992; Barlow et al., 1996; Hirao et al., 2002), constituting an interesting model to test the importance of this checkpoint kinase in mediating telomere dysfunction in vivo. Thus, here, we generated Trf1Δ/Δ K5Cre Chk2−/− and Chk2−/− Terc−/− compound mice. The findings described here indicate that Chk2 abrogation can partially rescue both survival and some of the phenotypes associated to both Terc and Trf1 deficiencies, revealing a potential for Chk2 inhibition in ameliorating phenotypes associated to both critically short telomeres and severe telomere dysfunction.
Results
Chk2 deficiency rescues mouse survival and degenerative pathologies in Trf1-deficient mice
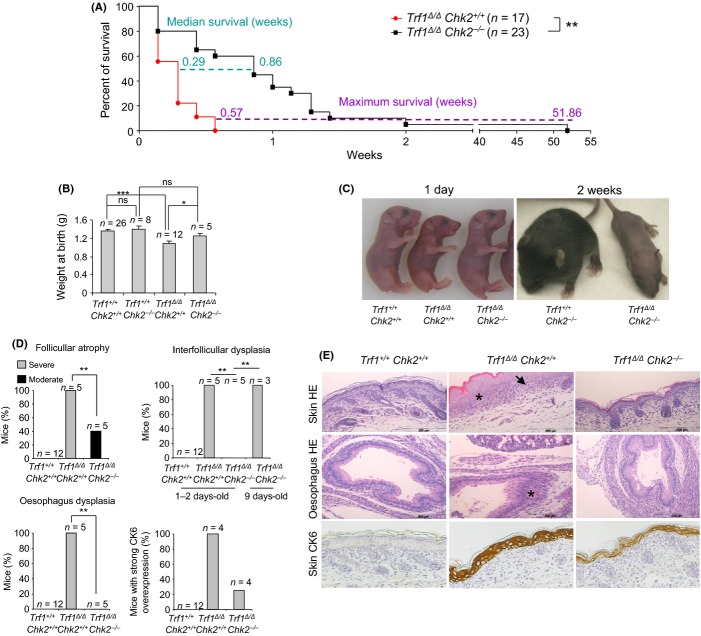
To address the in vivo role of CHK2 in signaling telomere dysfunction owing to Trf1 deficiency, we crossed Trf1Δ/Δ K5Cre mice (Martinez et al., 2009) with mice with whole-body Chk2 deletion, Chk2−/− (Hirao et al., 2002). Interestingly, Chk2 deficiency significantly rescued the survival of Trf1Δ/Δ K5Cre mice, with one doubly deficient mice surviving for as long as 1 year of age compared to a maximum survival of 4 days for single Trf1-deficient littermates (Fig. 1A). On average, the median survival of the Trf1Δ/Δ K5Cre Chk2−/− mice was threefold higher than the Trf1Δ/Δ K5Cre mice, 0.9 and 0.3 weeks, respectively (Fig. 1A). In agreement with the increased survival, we also observed a significant rescue of body weight in Trf1Δ/Δ K5Cre Chk2−/− newborns compared to the Trf1Δ/Δ K5Cre newborns (Fig. 1B–C).
Figure 1.
Chk2 deficiency partially rescues Trf1Δ/Δ K5Cre associated phenotypes and lethality (A) Survival curve of Trf1Δ/Δ K5Cre Chk2−/− and Trf1Δ/Δ K5Cre Chk2+/+ mice. Statistical analysis was done by the Log-rank (Mantel–Cox) test. (B) Weight at birth of the indicated genotypes. (C) Representative images of newborns and 2-week-old mice of the indicated genotypes. (D) Percentage of newborns that show severe or moderate skin follicular atrophy, skin interfollicular dysplasia, esophagus dysplasia, and strong CK6 expression. Chi-square test was performed for statistical analysis. Severe follicular atrophy is characterized by a reduced number of hair follicle primordial that are completely undeveloped. Moderate follicular atrophy is characterized by a decreased differentiation degree as compared to wild-type. Interfollicular dysplasia is characterized by undifferentiated keratinocytes, cellular depolarization and hyperkeratosis. (E) Representative images of hematoxylin–eosin and cytokeratin 6 staining in newborn skin and esophagus of the indicated genotypes. Dysplasic areas are marked with an asterisk and undeveloped hair follicles with an arrow. *P < 0.05; **P < 0.01; ***P < 0.001.
In an analogous manner, the frequency and severity of epithelial pathologies associated to Trf1 deficiency such as hair follicle atrophy, skin interfollicular dysplasia and esophagus dysplasia were also significantly rescued in Trf1Δ/Δ K5Cre Chk2−/− newborns (1–2 day old mice) compared to the Trf1Δ/Δ K5Cre Chk2+/+ age-matched controls (Fig 1D,E). Aberrant overexpression of cytokeratin 6 associated to Trf1 deficiency was also rescued in the Chk2-deficient background (Fig. 1D,E). Interestingly, at 9 days of age, at which point all single mutant Trf1Δ/Δ K5Cre mice were dead, surviving Trf1Δ/Δ K5Cre Chk2−/− mice showed extensive dysplasia in the skin, suggesting an increased preneoplastic growth associated to massive Trf1-induced DNA damage in the absence of Chk2 (Fig. 1D; see also Fig. 2).
Figure 2.
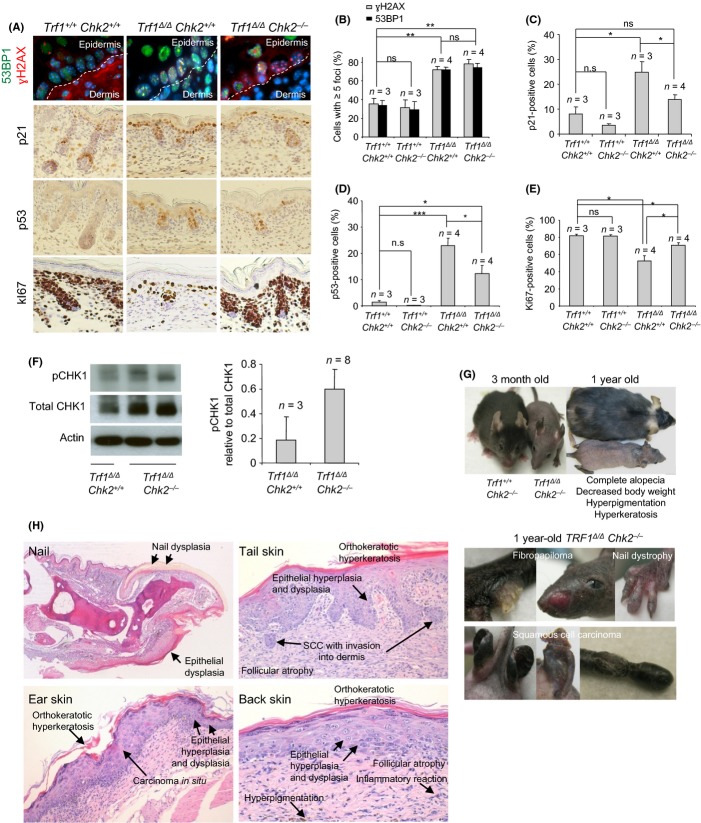
Chk2 deficiency increases epithelial tumor incidence in Trf1Δ/Δ K5Cre mice (A) Representative images of γH2AX and 53BP1 double immunofluorescence, and of p21, p53 and Ki67 immunohistochemistries performed on newborn skin. (B–E) Percentage of cells showing 5 or more γH2AX and 53BP1 damage foci (B), p21 positive cells (C), p53 positive (D) and Ki67 positive cells (E) in the newborn basal skin layer of the indicated genotypes. Of 1500–2000 cells were analyzed per genotype. T test was performed for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars represent standard error. n, number of mice analyzed per genotype. (F) Representative image and quantification by western blot of phospho-CHK1 normalized to total CHK1 in newborn keratinocytes. n represents the number of mice analyzed per genotype. (G–H) Representative images of the longer-lived TRF1Δ/Δ K5Cre Chk2−/− mouse compared to a wild-type littermate at different ages. Macroscopic lesions, fibropapiloma, nail dystrophy, and squamous cell carcinoma in ear and tail skin are shown.
Chk2 deficiency reduces p53 induction and increases proliferation of the skin of K5Cre Trf1Δ/Δ Chk2−/− newborns
To further investigate how Chk2 deficiency could rescue both the severity of the skin pathologies and survival of Trf1Δ/Δ K5Cre Chk2−/− mice compared to Trf1Δ/Δ K5Cre littermates, we first set to quantify DNA damage in the skin of these mice by determining the number of cells showing DNA damage foci as indicated by staining with γH2Ax and 53BP1. Both Trf1Δ/Δ K5Cre Chk2+/+ and Trf1Δ/Δ K5Cre Chk2−/− newborn skin showed approximately 80% of the basal cells positive for γH2Ax and 53BP1 staining (>5 foci per cell), indicating similarly high amounts of DNA damage in both cases, in agreement with similarly high levels of telomere uncapping owing to TRF1 deficiency (Fig. 2A–B). Conditional Trf1deletion in MEFs by adenoviral infection with the Cre recombinase induces Chk2 phosphorylation (Fig. S1A,B) (Martinez et al., 2009). We also found similar amounts of DNA damage, as well as similar frequencies of chromosomal aberrations associated to Trf1 deficiency in in vitro grown MEFs deficient for both Trf1 and Chk2 (Fig. S1), thus confirming the in vivo findings.
Interestingly, although the amount of DNA damage was similar independently of the Chk2 status, we found a decreased induction of p21 and p53 in the epidermis of Trf1Δ/Δ K5Cre Chk2−/− doubly deficient mice compared to that of single Trf1 knockouts (Fig. 2A,C–D). This was also paralleled by a higher proliferation in Trf1Δ/Δ K5Cre Chk2−/− compared to Trf1Δ/Δ K5Cre Chk2+/+ newborn epidermis as determined by Ki67-positive cells (Fig. 2A,E). Of note, despite this rescue, p53 levels were still significantly higher in Trf1Δ/Δ K5Cre Chk2−/− epidermis compared to wild-type, which may be indicative of p53 activation through the alternative ATR/Chk1 pathway (d’Adda di Fagagna et al., 2003; Zou & Elledge, 2003; Jazayeri et al., 2006; Martinez et al., 2009; Sfeir et al., 2009). In this regard, we observed a 3-fold increase in p-CHK1 levels in Trf1Δ/Δ K5Cre Chk2−/− compared to Trf1Δ/Δ K5Cre keratinocytes (Fig. 2F).
Chk2 deficiency in Trf1Δ/Δ K5Cre Chk2−/− mice leads to epithelial abnormalities characteristic of telomere syndromes, including increased skin cancer
Interestingly, cell division in the presence of persistent DNA damage in long-lived Trf1Δ/Δ K5Cre Chk2−/− mice resulted in development of epithelial pathologies, which recapitulate some of the skin abnormalities characteristic of the human telomere syndromes, such as occurrence of nail dystrophy and fibropapilomas (Fig. 2G,H). In addition, the back skin displayed preneoplasic lesions such as epithelial hyperplasia and dysplasia, orthokeratotic hyperkeratosis, and dermal infiltrates. In addition, long-lived Trf1Δ/Δ K5Cre Chk2−/− mice also showed spontaneous development of invasive squamous cell carcinomas (SCC) both in the tail and ear skin (Fig. 2G,H).
Altogether, these results demonstrate that Chk2 deletion attenuates induction of p53/p21 as well as rescues proliferative defects in the skin of Trf1Δ/Δ K5Cre mice, which in turn leads to a partial rescue of survival but also to increased tumorigenesis.
Chk2 deficiency increased median survival of G1 and G2 Terc-deficient mice
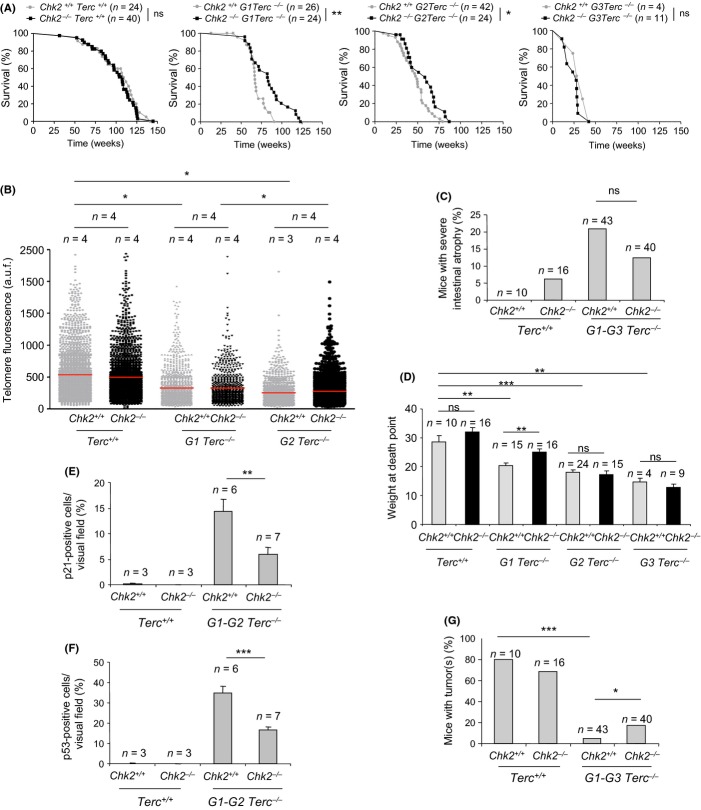
To address the effect of Chk2 deletion in a model of telomeric dysfunction originated by telomere shortening, we crossed Chk2−/− with telomerase-deficient Terc−/− knockout mice (Blasco et al., 1997; Hirao et al., 2002; Takai et al., 2002). The Chk2+/− Terc+/− mice were further crossed to generate successive generations (G1–G3) of Chk2−/− Terc−/− mice, which progressively shorten telomeres. Similar to the results obtained in Trf1Δ/Δ MEFS, critically short telomeres induce Chk2 phosphorylation and the Chk2−/− G1-G3 Terc−/− presented similar incidence of chromosomal aberrations and senescent cells as the Chk2+/+ G1-G3 Terc−/− MEFs (Fig. S2). Again, in vivo analysis of successive generations of these mice revealed that Chk2 deletion could significantly rescue the survival of the first two generations (G1 and G2) of telomerase–deficient mice (Fig. 3A). This rescue was not due to an effect of Chk2 deletion on telomere length, as we detected similar telomere shortening in successive of Chk2−/− G1-G3 Terc−/− and Chk2+/+ G1-G3 Terc−/− mice (Fig. 3B).
Figure 3.
Chk2 deficiency improves mouse survival, intestinal degenerative pathologies, and body weight of G1-G2 Terc-deficient mice (A) Survival curves of G1-G3 Terc/Chk2 mouse cohorts. Statistical analysis was done by the Log-rank (Mantel–Cox) test. ns, no significant; *P < 0.05; **P < 0.01. (B) Telomere length analysis by q-FISH in the intestine of G1-G3 Terc/Chk2 mouse cohorts. (C) Quantification of severe intestinal degenerative lesions in Terc+/+ and G1-G3 Terc−/− in Chk2-proficient and deficient background at death point. (D) Body weight of G1-G3 Terc/Chk2 mouse cohorts at death point. (E–F) Percentage of p21-positive (E) and p53-positive cells (F) in the intestinal crypts of the indicated G1-G2 Terc/Chk2 mouse cohorts. (G) Percentage of mice that presented malignant tumors among G1-G3 Terc/Chk2 cohorts at death point. T-Test was performed for statistical analysis in B, D, and E. Chi-square was performed for statistical analysis in F. Error bars represent standard error. *P < 0.05; **P < 0.01; ***P < 0.001. n, number of mice of each genotype.
In line with the increased survival, Chk2−/− Terc−/− mice showed a reduced incidence of severe intestinal atrophy, a frequent cause of death in the Terc-deficient mice (Fig. 3C). Furthermore, decreased body weight presented by Terc−/− mice at their time of death was rescued by Chk2 deficiency in the first generation G1 Terc−/− (Fig. 3D). At the molecular level, we observed a significantly decreased expression of p21 and p53 in intestinal crypts of G1-G2 Chk2−/− Terc−/− mice compared to the Chk2+/+ G1-G2 Terc−/− controls (Fig. 3E,F), in line with the findings with Chk2−/− Trf1−/− mice (Fig. 2C,D). Of note, p53/p21 levels were still significantly higher in G1-G2 Chk2−/− Terc−/− intestines compared to wild-type, which indicates alternative mechanisms for p53/p21 activation (d’Adda di Fagagna et al., 2003; Zou & Elledge, 2003; Jazayeri et al., 2006; Martinez et al., 2009; Sfeir et al., 2009).
Terc deficiency has been previously shown to have a potent tumor suppressor effect with increasing mouse generations (Greenberg et al., 1998; Lee et al., 1998; Rudolph et al., 1999; Gonzalez-Suarez et al., 2000). In this regard, we found that, Chk2 deletion lead to a slightly increased tumor incidence compared to the single Terc-deficient cohorts (Fig. 3G), suggesting that Chk2 could partially abolish the tumor suppressor effect of Terc deficiency.
Discussion
Here, we studied the role of the checkpoint kinase Chk2 in mediating the in vivo phenotypes induced by telomere dysfunction owing to either critical telomere shortening (Terc deficiency) or severe telomere uncapping (Trf1 deficiency). We found that Chk2 deficiency partially rescues both Terc deficiency in early generations and Trf1 deficiency-associated phenotypes, thus demonstrating a role for Chk2 in signaling telomere dysfunction in vivo. Furthermore, we demonstrate that this rescue is not mediated by a significant rescue of the amount of DNA damage but instead reflects an attenuation of the proliferative defects associated to p53/p21 induction. A previous work reported that Chk2 deletion in mice did not rescue survival or pathologies associated to Terc deficiency (Nalapareddy et al., 2010). Although our findings may seem to stand in contrast to those reported by Nalapareddy (Nalapareddy et al., 2010), it is relevant to note that these authors only focused their study in late generation iG4 telomerase-deficient mice which correspond to our G3 generation bearing very short telomeres and where we also do not see Chk2-mediated rescue. In contrast to our current study, Nalapareddy et al. did not address the effect of Chk2 deficiency in signaling critically short telomeres in G1 or G2 telomerase-deficient mice, which is where we observe a significant partial rescue in median survival by Chk2 deficiency. In addition, the different outcomes might also be due to the different ways by which both groups have generated the Terc-deficient mouse cohorts. Thus, Nalapareddy et al. crossed G3 Terc−/− Chk2+/− with Terc+/− Chk2+/− to generate their experimental cohorts; namely, Terc+/− Chk2+/+ (iF1 Chk2+/+), Terc+/− Chk2−/− (iF1 Chk2−/−), Terc−/− Chk2+/+ (iG4 Chk2+/+), and Terc−/− Chk2−/− (iG4 Chk2+/+). The zygotes in all these cohorts harbor half of its chromosomes with long telomeres (those coming from the Terc+/− parental) and half with short telomeres (those coming from the G3 Terc−/− parental). In our work, we carried the Terc− allele in homozygosis from the first generation of G1 Terc−/− Chk2+/− throughout the G2 and G3 generations, thus in a setting where all chromosomes present progressively shorten telomeres. It is conceivable that the Chk2-mediated response in G3 Terc-deficient mice is masked by a compensatory induction of a Chk2-independent mechanism that leads to p53/p21 activation. In fact, Nalapareddy et al. detected significantly higher levels of phosphorylated Chk1 in iG4 Chk2−/− as compared to iG4 Chk2+/+ (Nalapareddy et al., 2010). In agreement with Nalapareddy et al., we also detected a slight increase in Chk1 phosphorylation in the absence of Chk2 in Trf1-deficient keratinocytes, which explains the fact that p53 levels were still elevated in Chk2 cohorts compared to the wild-types. This fact also explains that Chk2 deficiency only partially rescued survival and phenotypes associated to Trf1 deficiency, while p53 deficiency was previously described by us to fully rescue Trf1Δ/Δ K5-Cre perinatal lethality (Martinez et al., 2009). Thus, in the absence of Chk2, other components of the DDR, including Chk1 may be important to signal telomere dysfunction.
Finally, although single Chk2 deficiency has not been associated to increased cancer (Donehower et al., 1992; Barlow et al., 1996; Hirao et al., 2002), we found a slightly increased cancer incidence in the absence of Chk2 in the context of both Trf1 and Terc deficiencies. These findings are in line, with increased cancer incidence associated to telomere dysfunction in the absence of p53. Interestingly, abrogation of other components of the DDR, such as p21, Pms2 and Exo-1, can rescue survival of Terc-deficient mice without increasing cancer (Choudhury et al., 2007; Schaetzlein et al., 2007; Siegl-Cachedenier et al., 2007).
In summary, our findings represent the first demonstration in vivo for a role of Chk2 in signaling telomere dysfunction owing to both short telomeres and severe telomere uncapping. Furthermore, we show a role for Chk2 in blocking tumorigenesis associated to dysfunctional telomeres.
Acknowledgments
We are indebted to R. Serrano for animal care.
Author contributions
M.A.B., P.M. and M.G.B. designed experiments and wrote the manuscript. M.G.B. performed most of the experiments. J.M.F. performed the histopathologic analysis.
Funding
Research in the Blasco Lab was funded by European Research Council (ERC) Project TEL STEM CELL (GA#232854), European Union FP7 Projects 2007-A-20088 (MARK-AGE) and 2010-259749 (EuroBATS), Spanish Ministry of Economy and Competitiveness Projects SAF2008-05384 and CSD2007-00017, Regional of Government of Madrid Project S2010/BMD-2303 (ReCaRe), AXA Research Fund (Life Risks Project), Lilly 2010 Preclinical Biomedicine Research Award (Fundación Lilly, Spain), Fundación Botín (Spain).
Conflict of interest
The authors declare that there is no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Fig. S1 Chk2 deficiency does not rescue the Trf1-associated phenotypes in MEFs.
Fig. S2 Chk2 deficiency does not rescue Terc-associated phenotypes in MEFs.
Data S1 Experimental procedures.
References
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Hayashi MT, Crabbe L, Karlseder J. The telomere deprotection response is functionally distinct from the genomic DNA damage response. Mol. Cell. 2013;51:141–155. doi: 10.1016/j.molcel.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, Lee HW, von Zglinicki T, Ganser A, Schirmacher P, Nakauchi H, Rudolph KL. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb. Symp. Quant. Biol. 2005b;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Samper E, Flores JM, Blasco MA. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat. Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Kachatrian L, Takai H, Mercer K, Hingorani S, Jacks T, de Lange T. Targeted deletion reveals an essential function for the telomere length regulator Trf1. Mol. Cell. Biol. 2003;23:6533–6541. doi: 10.1128/MCB.23.18.6533-6541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005a;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl Acad. Sci. USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga M, Bunting S, Montana MF, Soria R, Mulero F, Canamero M, Lee Y, McKinnon PJ, Nussenzweig A, Fernandez-Capetillo O. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat. Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K, Choudhury AR, Gompf A, Ju Z, Ravipati S, Leucht T, Lechel A, Rudolph KL. CHK2-independent induction of telomere dysfunction checkpoints in stem and progenitor cells. EMBO Rep. 2010;11:619–625. doi: 10.1038/embor.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Schaetzlein S, Kodandaramireddy NR, Ju Z, Lechel A, Stepczynska A, Lilli DR, Clark AB, Rudolph C, Kuhnel F, Wei K, Schlegelberger B, Schirmacher P, Kunkel TA, Greenberg RA, Edelmann W, Rudolph KL. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130:863–877. doi: 10.1016/j.cell.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- Siegl-Cachedenier I, Munoz P, Flores JM, Klatt P, Blasco MA. Deficient mismatch repair improves organismal fitness and survival of mice with dysfunctional telomeres. Genes Dev. 2007;21:2234–2247. doi: 10.1101/gad.430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, Nagashima K, Sawa H, Ikeda K, Motoyama N. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Thanasoula M, Escandell JM, Suwaki N, Tarsounas M. ATM/ATR checkpoint activation downregulates CDC25C to prevent mitotic entry with uncapped telomeres. EMBO J. 2012;31:3398–3410. doi: 10.1038/emboj.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Chk2 deficiency does not rescue the Trf1-associated phenotypes in MEFs.
Fig. S2 Chk2 deficiency does not rescue Terc-associated phenotypes in MEFs.
Data S1 Experimental procedures.