Abstract
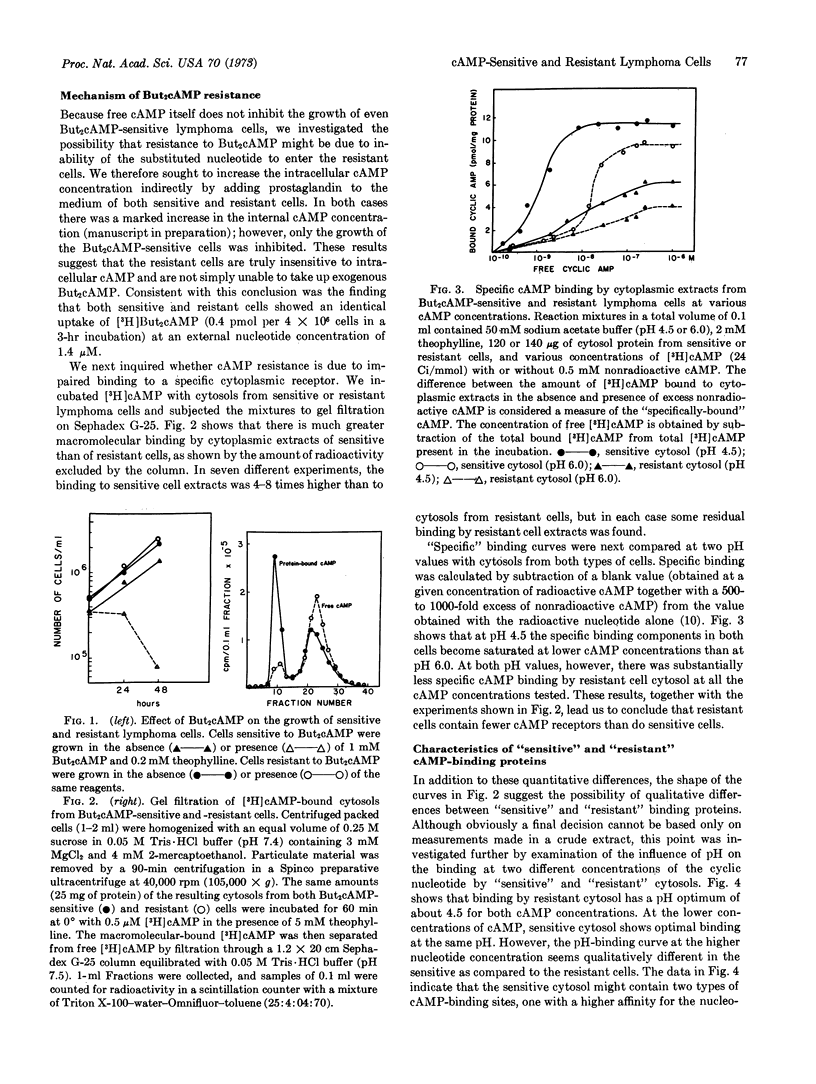
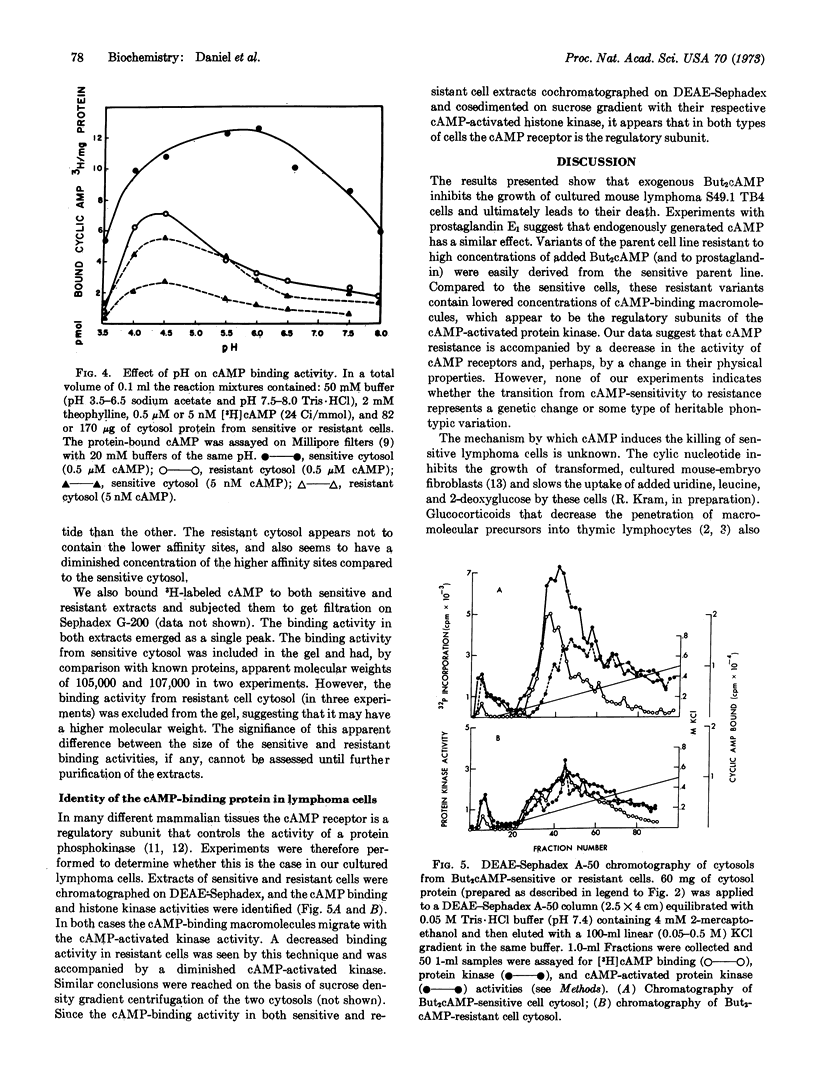
Cultured mouse lymphosarcoma cells are killed on exposure to 0.1 mM N6,O2′-dibutyryl-adenosine 3′:5′-cyclic monophosphate. A population of cells resistant to the killing effect of dibutyryl cyclic AMP at concentrations as high as 1 mM was selected. The growth characteristics of the resistant cells were similar to those of the sensitive parental line. However, the resistant cells contain less cytoplasmic cyclic AMP-binding proteins and decreased cyclic AMP-stimulated protein kinase activity. It is proposed that transition from sensitivity to resistance to dibutyryl cyclic AMP in lymphoma cells is connected with a modification of the cyclic AMP-binding protein, which appears to be the regulatory subunit of the cyclic AMP-activated protein kinase.
Keywords: cytoplasmic receptor molecules, protein kinase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. A cell-free system with ribosomal protein kinase activity. Biochemistry. 1971 Jan 19;10(2):197–203. doi: 10.1021/bi00778a001. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Evidence for induction by cortisol in vitro of a protein inhibitor of transport and phosphorylation processes in rat thymocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1269–1273. doi: 10.1073/pnas.68.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]



