Abstract
Objectives:
Many efforts have shown multi-oncologic roles of galectin-3 for cell proliferation, angiogenesis, and apoptosis. However, the mechanisms by which galectin-3 is involved in cell proliferation are not yet fully understood, especially in human colon cancer cells.
Methods:
To cluster genes showing positively or negatively correlated expression with galectin-3, we employed human colon cancer cell lines, SNU-61, SNU-81, SNU-769B, SNU-C4 and SNU-C5 in high-throughput gene expression profiling. Gene and protein expression levels were determined by using real-time quantitative polymerase chain reaction (PCR) and western blot analysis, respectively. The proliferation rate of human colon cancer cells was measured by using a 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay.
Results:
Expression of γ-aminobutyric acid B receptor 1 (GABABR1) showed a positive correlation with galectin-3 at both the transcriptional and the translational levels. Downregulation of galectin-3 decreased not only GABABR1 expression but also the proliferation rate of human colon cancer cells. However, Korean herbal extract, HangAmDan-B (HAD-B), decreased expression of GABABR1 without any expressional change of galectin-3, and offset γ-aminobutyric acid (GABA)-enhanced human colon cancer cell proliferation.
Conclusions:
Our present study confirmed that GABABR1 expression was regulated by galectin-3. HAD-B induced galectin-3-independent down-regulation of GABABR1, which resulted in a decreased proliferation of human colon cancer cells. The therapeutic effect of HAD-B for the treatment of human colon cancer needs to be further validated.
Keywords: HAD-B, GABABR1, galectin-3, human colon cancer, proliferation, 5-fluorouracil
1. Introduction
Galectin-3 is a member of the family of β-galactoside-binding proteins that bind to the carbohydrate portion of cellsurface glycoproteins and glycolipids [1]. Galectin-3 has a chimera-type structure consisting of three different structural domains: a short NH2-terminal domain of 12 amino acids that contains a serine phosphorylation site; a repeated collagen-like sequence that rich in glycine, tyrosine, and proline amino acid residues, which serves as a substrate for matrix metalloproteinases (MMPs); and a COOHterminal carbohydrate recognition domain [1 - 3]. Galectin-3 is a multifunctional oncogene [1], which regulates cell growth [4], adhesion [5], proliferation [6], angiogenesis [7], and apoptosis [8].
Many studies have shown that galectin-3 regulates cancer cell proliferation. Galectin-3-stimulated cell proliferation of IMR-90 human lung fibroblasts [6]; a decrease of galectin-3 expression in activated T lymphocytes paralleled a downregulation or even a blocking of proliferation [9]; and the introduction of galectin-3 cDNA caused human lymphoma Jurkat T cells to grow faster [10]. A recent report provided evidence that downregulation of galectin-3 led to diminished human colon cancer cell proliferation via modulation of the hete-rogeneous nuclear ribonucleoprotein Q (hnRNP Q) level [11].
Overexpression of galectin-3 has been reported in gastric cancer [12]. Positive galectin-3 expression was observed in 84% of gastric cancer cases. In enhanced cells of a cancerous lesion, 48% showed stronger nuclear immunoreactivity than a cytoplasmic one whereas adjacent epithelial cells showed little or weak nuclear immunoreactivity [12]. In addition, decreased galectin-3 expression was found in breast [13], ovary [14], prostate [15], epithelial skin cancer [16], and head-and-neck squamous cell carcinomas [17] than in corresponding normal tissue.
HangAmDan (HAD)-B consists of eight species of Korean medicinal plants and animals (Table1), and is an upgraded version of HangAmDan (HAD) used traditionally for solid masses, which also shows anti-angiogenic activity [18]. A mixture of these plants has been shown to exert strong anticancer activity against solid tumors, including pancreatic, lung, colorectal, and stomach cancers. Additionally, anti-angiogenesis effects and inhibition of cancer cell proliferation and metastasis have been reported [19]. In particular, case reports observed with HAD have been selected as part of the National Cancer Institute’s Best Case Series Program [20]. HAD-B has shown efficacy in inhibiting migration and proliferation of human umbilical vein endothelial cells and in limiting the formation of capillary tube structures [21]. Furthermore, a safety evaluation of HAD-B has revealed no side-effects in both healthy subjects and cancer patients [22].
Table. 1. Ingredients of HAD-B.
| Scientific name | Relative amount (mg) |
|---|---|
| Panax notoginseng Radix | 84.0 |
| Cordyceps Militaris | 64.0 |
| Santsigu Tuber | 64.0 |
| Ginseng Radix | 64.0 |
| Bovis Calculus | 64.0 |
| Margarita | 64.0 |
| Bostaurus var.domesticus Gmelin | 48.0 |
| Commiphora myrrha | 48.0 |
| Total amount (1 capsule) | 500.0 |
Even though a number of studies have reported the functions of galectin-3 in many types of cancer, the mechanisms by which galectin-3 is involved in cell proliferation are not yet fully understood, especially in human colon cancer cells. In the present study we report that γ-aminobutyric acid B receptor 1 (GABABR1) expression is linked to galectin-3 in human colon cancer cell line, and we discuss the effect of galectin-3- independent down-regulation of GABABR1 by treatment with Korean herbal extract HAD-B in human colon cancer cells.
2. Materials and methods
2.1. Human colon cancer cell lines
Human colon cancer cell lines, SNU-61, SNU-81, SNU-769B, SNU-C4 and SNU-C5, were obtained from the Korean Cell Line Bank (Seoul, Korea).
2.2. Preparation of water extract of HAD-B
HAD-B was provided from the East-West Cancer Center of Dunsan Oriental Medical Hospital, Daejeon University, Daejeon, Korea (Table 1). The water extract of HAD-B was prepared by extracting HAD-B powder with 10-times (v/w) the amount of distilled water at room temperature for 24 hrs. The extract was centrifuged at 1000×g for 30 mins and was then filtered and lyophilized. The extract powder was dissolved directly in distilled water.
2.3. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
A colorimetric assay using tetrazolium salt, MTT, was used to assess cell proliferation after galectin-3 suppression. MTT assays were performed as described in a previous report [11]. Briefly, equal numbers of cells were incubated in each well in 0.18 ml of culture medium to which 0.02 ml of 10 × 5-FU (Choongwae Pharma Corporation), HAD-B, GABA or PBS (for untreated 100% survival control) had been added. After 4 days of culture, 0.1 mg of MTT was added to each well and incubated at 37°C for a further 4 hrs. Plates were centrifuged at 450 × g for 5 mins at room temperature, and the medium was removed. Dimethyl sulfoxide (0.15 ml) was added to each well to solubilize the crystals, and plates were immediately read at 540 nm by using a scanning multiwell spectrometer (Bio-Tek Instruments Inc., Winooski, VT). All experiments were performed three times, and the IC50 (μg/ml) values are presented as means ± standard deviations.
2.4. Western blot analysis
Western blot analyses were performed as described in a previous report [11]. Primary antibodies against galectin-3 (Abcam, Cambridge, UK), γ-aminobutyric acid B receptor 1 (GABABR1) (Abcam) and actin (Abcam) (1:1,000) were used.
2.5. Immunoprecipitation
All procedures were performed at 4°C unless otherwise specified. Approximately 107 cells in 1 ml of cold 1 × radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (Roche Diagnostics) were incubated on ice for 30 mins with occasional mixing. Cell lysates were centrifuged at 12,000 × g for 10 mins, and the supernatant was collected carefully without disturbing the pellet. The supernatant was mixed with primary antibody against either galectin-3 (Abcam) or GABABR1 (Abcam) and was incubated for 2 hrs on a rocking platform. Prepared protein G sepharose beads (GE Health Care Life Sciences) were added and further incubated on ice for 1 hrs on a rocking platform. The mixture was centrifuged at 10,000 g for 30 s, and the supernatant was removed completely. Protein G sepharose beads were washed 5 times with 1 ml of cold 1 × RIPA to minimize the background. Next, 100 μl of 2 × sodium dodecyl sulfate (SDS) sample buffer was added to the bead pellets and heated to 100°C for 10 mins. After boiling, immunoprecipitates were centrifuged at 10000 × g for 5 mins, and the supernatant was collected for the Western blot analysis.
2.6. Intracellular cAMP measurement
The intracellular cAMP for human colon cancer cells was determined by using a cAMP Direct Immunoassay Kit (Abcam), as recommended by the manufacturer.
2.7. RNA preparation and Affymetrix GeneChip hybridization
Total RNA was extracted using Trizol reagent (Life Technologies, Inc., Carlsbad, CA), according to the manufacturer’s instructions. Genes expressed in the chemosensitive and chemoresistant groups were analyzed on a high-density oligonucleotide microarray (HG-U133A; Affymetrix, Santa Clara, CA) containing 22,283 transcripts. Target preparation and microarray processing procedures were performed, following the Affymetrix GeneChip Expression Analysis Manual (Affymetrix). Briefly, total RNA extracted was purified with an RNeasy kit (Qiagen). Double-stranded cDNA was synthesized from total RNA (20 μg) with SuperScript II reverse transcriptase (Life Technologies, Inc. Rockville, MD) and a T7-(dT)24 primer (Metabion, Germany). Biotinylated cRNA was synthesized from double-stranded cDNA by using a RNA Transcript Labeling kit (Enzo Life Sciences, Farmingdale, NY), purified, and fragmented. Fragmented cRNA was hybridized to the oligonucleotide microarray, which was washed and stained with streptavidinphycoerythrin. Scanning was performed with an Agilent Microarray Scanner (Agilent Technologies, Santa Clara, CA).
2.8. Affymetrix GeneChip data analysis
A GeneChip analysis was performed based on the Affymetrix GeneChip Manual (Affymetrix) with Data Mining Tool (DMT) 2.0 and Microarray Database software. All genes represented on the GeneChip were globally normalized and scaled to a signal intensity of 500. Fold changes were calculated by comparing transcripts between the cell lines tested. The DMT 2.0 software employed changed calls (increased or decreased) to analyze the expression of a particular transcript statistically and to determine whether it had been relatively increased, decreased or remained unchanged. After filtration through a "present" call (p〈0.05), a transcript was considered differentially expressed at a fold change of greater than 2.0.
2.9. Real-time quantitative reverse transcription polymerase chain reaction
Four genes (ELF3, AXIN2, ENO2 and SACS) were selected for real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) for validation of the microarray data. Using the SuperScript Pre-amplification System for first strand cDNA synthesis, 5 mg of total RNA was used for creation of singlestranded cDNA (Life Technologies). The cDNA was diluted and quantitatively equalized for PCR amplification. For real-time qRT-PCR, the ABI Prism 7900 sequence detection system (Applied Biosystems) was used. AccuPower GreenStar PCR Master Mix (Bioneer Corporation, Daejeon, Korea) was used for each PCR reaction, and the GAPDH gene was simultaneously run as a control and was used for normalization. Non-template-control wells without cDNA were included as negative controls. Each test sample was run in triplicate. The primer sets for PCR amplification were designed as follows: ELF3-F: 5’-TGAGCTGCTGGAGAAGGATG- 3’, ELF3-R: 5’-CCCTTCTTGCAGTCACGAAA- 3’, AXIN2-F: 5’-AATCATTCGGCCACTGTTCA-3’, AXIN2-R: 5’- CACAGGCAAACTCATCGCTT-3’, ENO2-F: 5’-CTGATGCTGGAGTTGGATGG- 3’, ENO2-R: 5’-CCATTGATCACGTTGAAGGC-3’, SACS-F: 5’-CCATTTGTTGGCATTTTTGG-3’, and SACS-R: 5’- CGCTCATGTTTCAGTGCCTT-3’. Following the standard curve method, the expressed quantities of the examined genes were determined using the standard curves and the CT values and were normalized using the GAPDH expression quantities.
3. Results
3.1. Galectin-3 expression related to 5-FU susceptibility in human colon cancer cells
To confirm the correlation between galectin-3 expression and 5-FU susceptibility in human colon cancer cells, we performed Western blot and MTT analyses on three human colon cancer cell lines, SNU-769B, SNU-C4 and SNU-C5. 5-FU susceptibility showed a decreasing tendency that depended on both the transcriptional (Fig 1 A) and the translational (Fig 1 Ba) levels of galectin-3. To cluster the genes showing positively or negatively correlated expression with galectin-3, we employed SNU-61, which had almost the same 5-FU susceptibility as SNU-769B, in a high-throughput gene expression profiling experiment (Fig. 1 B & Tables 2, 3). Figure 1 Ba shows an example of 19 genes clustered in a galectin-3 expression pattern, which was confirmed by real-time PCR (Fig 1 Bb). The top 50 down- and up-regulated genes in SNU-C5, compared to SNU-769B, are listed in Tables 2, 3 respectively.
Fig. 1. Galectin-3 expression correlated with 5-FU susceptibility in human colon cancer cell lines and gene expression profiling linked to galectin-3.
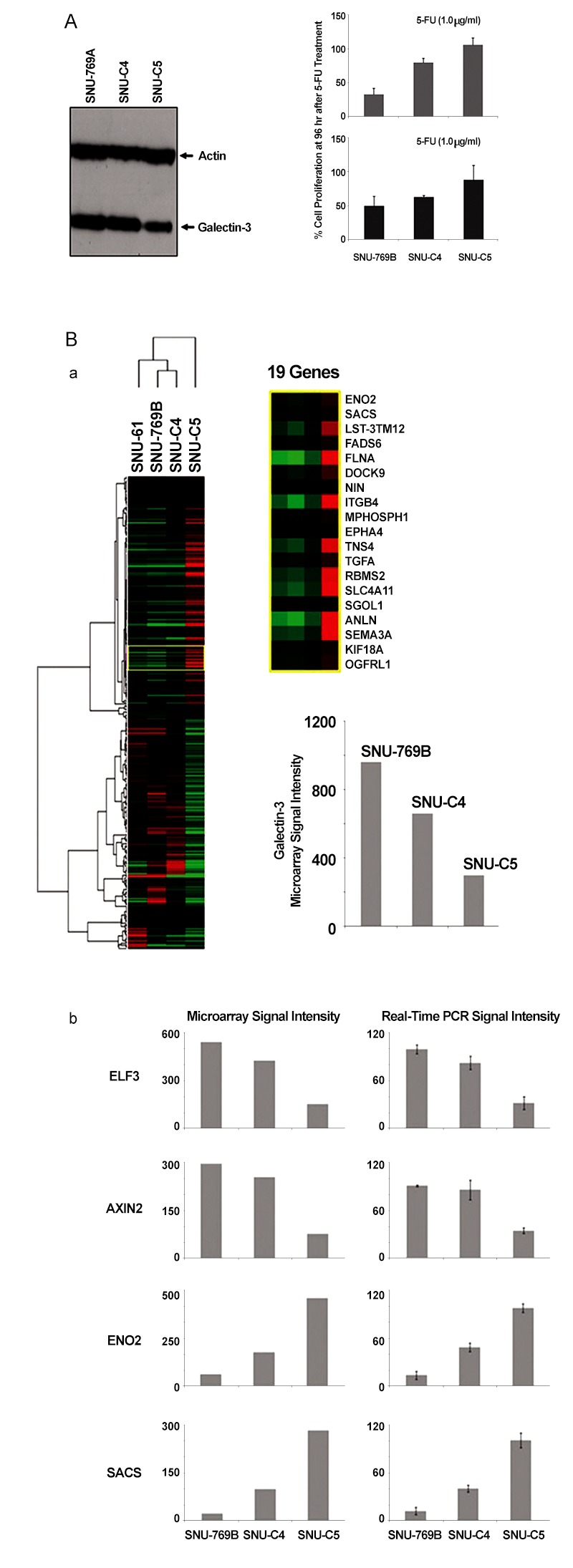
(A) Galectin-3 protein expression correlated with 5-FU susceptibility in three human colon cancer cell lines, SNU-769B, SNU-C4 and SNU-C5. Whole proteomes obtained from the human colon cancer cell lines employed were subjected to SDS-PAGE and were electro-transferred to PVDF membranes for western blot analysis. When galectin-3 expression was higher, human colon cancer cell lines showed more 5-FU susceptibility. (B) Gene expression profiling liked to galectin-3. To satisfy minimum clustering sample size, we added SNU-61, which has almost the same 5-FU susceptibility as SNU-769B, and as shown in the enlarged yellow box, genes linked to galectin-3 expression were selected (a). The expressional profiling was further confirmed by using real-time PCR as shown in panel (b). All genes showing positive and negative expressional correlations with galectin-3 are listed in Tables2, 3respectively.
Table. 2. Top 50 down-regulated genes in SNU-C5 compared to SNU-769B.
| Probe Set ID | Gene Symbol | SNU- 769A | SNU- C4 | SNU- C5 | FC | GO Biological Process Term | GO Cellular Component Term | GO Molecular Function Term |
|---|---|---|---|---|---|---|---|---|
| 8101893 | ADH1C | 2263 | 1125 | 14 | -7.3 | alcohol metabolic process | cytoplasm | alcohol dehydrogenase activity, zinc-dependent |
| 7989501 | CA12 | 2428 | 789 | 26 | -6.5 | one-carbon compound metabolic process | membrane | carbonate dehydratase activity |
| 8022692 | DSC3 | 904 | 35 | 10 | -6.5 | cell adhesion | membrane fraction | calcium ion binding |
| 7979658 | GPX2 | 2129 | 1408 | 24 | -6.5 | response to oxidative stress | cytoplasm | glutathione peroxidase activity |
| 7919055 | HMGCS2 | 2091 | 1229 | 28 | -6.2 | acetyl-CoA metabolic process | mitochondrion | hydroxymethylglutaryl-CoA synthase activity |
| 8036591 | LGALS4 | 5079 | 4762 | 69 | -6.2 | cell adhesion | cytosol | sugar binding |
| 7928770 | PCDH21 | 1522 | 291 | 21 | -6.2 | homophilic cell adhesion | membrane | calcium ion binding |
| 7953200 | CCND2 | 2210 | 60 | 37 | -5.9 | regulation of progression through cell cycle | nucleus | protein binding |
| 7928766 | C10orf99 | 1970 | 493 | 33 | -5.9 | |||
| 8138392 | AGR3 | 292 | 174 | 6 | -5.7 | |||
| 7919984 | SELENBP1 | 2409 | 1499 | 54 | -5.5 | selenium binding | ||
| 8174654 | KLHL13 | 650 | 164 | 16 | -5.3 | protein binding | ||
| 7967107 | C12orf27 | 367 | 229 | 9 | -5.3 | |||
| 8161884 | PRUNE2 | 396 | 230 | 12 | -5.1 | |||
| 8106354 | IQGAP2 | 704 | 95 | 22 | -5.0 | signal transduction | intracellular | actin binding |
| 8134339 | PEG10 | 658 | 28 | 21 | -5.0 | negative regulation of transforming growth factor beta receptor signaling pathway | cytoplasm | nucleic acid binding |
| 8135378 | PRKAR2B | 543 | 85 | 18 | -4.9 | protein amino acid phosphorylation | cAMP-dependent protein kinase complex | nucleotide binding |
| 8091283 | PLOD2 | 358 | 98 | 13 | -4.8 | protein modification process | endoplasmic reticulum | iron ion binding |
| 8128123 | RRAGD | 311 | 58 | 12 | -4.7 | nucleus | nucleotide binding | |
| 7983606 | EID1 | 759 | 568 | 30 | -4.7 | negative regulation of transcription from RNA polymerase II promoter | cellular component | protein binding |
| 8100734 | UGT2B17 | 125 | 6 | 5 | -4.6 | metabolic process | membrane fraction | glucuronosyltransferase activity |
| 8080964 | PPP4R2 | 293 | 130 | 12 | -4.6 | protein modification process | centrosome | protein binding |
| 8151592 | CA1 | 256 | 23 | 11 | -4.6 | one-carbon compound metabolic process | cytoplasm | carbonate dehydratase activity |
| 8101757 | GPRIN3 | 711 | 256 | 31 | -4.5 | |||
| 7926545 | PLXDC2 | 499 | 87 | 22 | -4.5 | multicellular organismal development | membrane | receptor activity |
| 7916185 | ZCCHC11 | 266 | 262 | 12 | -4.5 | intracellular | nucleic acid binding | |
| 8008172 | B4GALNT2 | 693 | 38 | 30 | -4.5 | UDP-N-acetylgalactosamine metabolic process | membrane | acetylgalactosaminyltransferase activity |
| 8040374 | FAM84A | 998 | 492 | 44 | -4.5 | |||
| 8168589 | ZNF711 | 378 | 106 | 19 | -4.4 | regulation of transcription, DNA-dependent | intracellular | DNA binding |
| 8043981 | IL1R2 | 663 | 54 | 33 | -4.3 | immune response | membrane | receptor activity |
| 7923578 | FMOD | 359 | 74 | 18 | -4.3 | transforming growth factor beta receptor complex assembly | proteinaceous extracellular matrix | protein binding |
| 8138553 | FAM126A | 210 | 69 | 11 | -4.3 | biological process | cellular component | signal transducer activity |
| 8077323 | CNTN4 | 168 | 11 | 10 | -4.1 | cell adhesion | plasma membrane | protein binding |
| 7999553 | FLJ11151 | 337 | 207 | 20 | -4.1 | hydrolase activity | ||
| 7940565 | FADS2 | 502 | 380 | 31 | -4.0 | lipid metabolic process | membrane fraction | iron ion binding |
| 7951554 | RDX | 259 | 67 | 16 | -4.0 | cytoskeletal anchoring | cytoplasm | actin binding |
| 8044212 | SULT1C2 | 215 | 39 | 13 | -4.0 | amine metabolic process | cytoplasm | sulfotransferase activity |
| 7903742 | GSTM2 | 946 | 183 | 59 | -4.0 | metabolic process | glutathione transferase activity | |
| 7937335 | IFITM1 | 402 | 343 | 25 | -4.0 | regulation of progression through cell cycle | plasma membrane | receptor signaling protein activity |
| 8041383 | LTBP1 | 470 | 213 | 30 | -4.0 | biological process | proteinaceous extracellular matrix | transforming growth factor beta receptor activity |
| 8142171 | SLC26A3 | 185 | 18 | 12 | -4.0 | transport | membrane fraction | transcription factor activity |
| 7951789 | FAM55D | 318 | 209 | 21 | -3.9 | |||
| 8078544 | MLH1 | 155 | 121 | 10 | -3.9 | mismatch repair | nucleus | single-stranded DNA binding |
| 8111772 | DAB2 | 344 | 72 | 23 | -3.9 | cellular morphogenesis during differentiation | coated pit | protein C-terminus binding |
| 8094988 | FLJ21511 | 270 | 75 | 18 | -3.9 | |||
| 7918223 | C1orf59 | 122 | 101 | 8 | -3.9 | |||
| 8095110 | KIT | 160 | 29 | 11 | -3.9 | protein amino acid dephosphorylation | external side of plasma membrane | nucleotide binding |
| 8125149 | SLC44A4 | 1306 | 1163 | 88 | -3.9 | membrane | ||
| 8178653 | NEU1 | 1306 | 1163 | 88 | -3.9 | metabolic process | lysosome | exo-alpha-sialidase activity |
| 8179861 | NEU1 | 1306 | 1163 | 88 | -3.9 | metabolic process | lysosome | exo-alpha-sialidase activity |
FC: Fold-change was calculated from the signal Log ratio value.
Table. 3. Top 50 up-regulated genes in SNU-C5, compared to SNU-769B.
| Probe Set ID | Gene Symbol | SNU- 769A | SNU- C4 | SNU- C5 | FC | GO Biological Process Term | GO Cellular Component Term | GO Molecular Function Term |
|---|---|---|---|---|---|---|---|---|
| 7954330 | SLCO1B3 | 6 | 551 | 921 | 7.3 | ion transport | integral to plasma membrane | transporter activity |
| 7959856 | PIWIL1 | 11 | 17 | 834 | 6.3 | multicellular organismal development | cytoplasm | single-stranded RNA binding |
| 7954090 | EMP1 | 23 | 51 | 1597 | 6.1 | multicellular organismal development | membrane fraction | |
| 7954344 | LST-3TM12 | 14 | 236 | 875 | 6.0 | transport | membrane | transporter activity |
| 8026490 | LOC729642 | 29 | 34 | 1815 | 5.9 | |||
| 8108217 | TGFBI | 36 | 105 | 2089 | 5.9 | cell adhesion | proteinaceous extracellular matrix | integrin binding |
| 8176026 | FLNA | 67 | 699 | 2852 | 5.4 | cell motility | nucleus | actin binding |
| 7954356 | SLCO1B1 | 10 | 55 | 327 | 5.1 | ion transport | membrane fraction | transporter activity |
| 8124437 | HIST1H3F | 45 | 49 | 1482 | 5.0 | nucleosome assembly | nucleosome | DNA binding |
| 7997139 | CALB2 | 33 | 35 | 1020 | 5.0 | calcium ion binding | ||
| 8102950 | INPP4B | 25 | 131 | 786 | 5.0 | signal transduction | phosphatidylinositol-3, 4-bisphosphate 4-phosphatase activity | |
| 8095728 | EREG | 62 | 230 | 1896 | 4.9 | regulation of progression through cell cycle | extracellular space | epidermal growth factor receptor binding |
| 8155849 | ANXA1 | 65 | 980 | 1942 | 4.9 | lipid metabolic process | cornified envelope | phospholipase inhibitor activity |
| 8089082 | DCBLD2 | 131 | 308 | 3841 | 4.9 | cell adhesion | integral to plasma membrane | protein binding |
| 8140668 | SEMA3A | 36 | 190 | 1067 | 4.9 | multicellular organismal development | extracellular region | chemorepellant activity |
| 7920128 | S100A11 | 41 | 806 | 1209 | 4.9 | signal transduction | ruffle | calcium ion binding |
| 8098470 | WWC2 | 13 | 20 | 358 | 4.8 | |||
| 8067233 | TMEPAI | 50 | 115 | 1311 | 4.7 | androgen receptor signaling pathway | membrane | molecular function |
| 7909789 | TGFB2 | 18 | 22 | 440 | 4.6 | cell morphogenesis | extracellular region | beta-amyloid binding |
| 8015016 | TNS4 | 42 | 240 | 1027 | 4.6 | apoptosis | cytoskeleton | actin binding |
| 8095744 | AREG | 44 | 240 | 1080 | 4.6 | cell-cell signaling | extracellular space | cytokine activity |
| 8021442 | ZNF532 | 28 | 73 | 673 | 4.6 | intracellular | nucleic acid binding | |
| 7933312 | LOC653110 | 27 | 29 | 648 | 4.6 | |||
| 7981514 | AHNAK2 | 22 | 42 | 532 | 4.6 | protein binding | ||
| 8027778 | FXYD5 | 65 | 433 | 1459 | 4.5 | ion transport | membrane | actin binding |
| 7908072 | LAMC2 | 73 | 317 | 1591 | 4.4 | cell adhesion | basement membrane | protein binding |
| 8075310 | LIF | 54 | 56 | 1139 | 4.4 | immune response | extracellular region | cytokine activity |
| 8138466 | 7A5 | 61 | 316 | 1226 | 4.3 | |||
| 7986446 | ALDH1A3 | 88 | 114 | 1741 | 4.3 | alcohol metabolic process | 3-chloroallyl aldehyde dehydrogenase activity | |
| 8041179 | CLIP4 | 18 | 20 | 356 | 4.3 | |||
| 8124413 | HIST1H4D | 63 | 338 | 1234 | 4.3 | |||
| 7924029 | LAMB3 | 77 | 169 | 1473 | 4.3 | electron transport | proteinaceous extracellular matrix | structural molecule activity |
| 8129379 | ECHDC1 | 38 | 685 | 717 | 4.3 | metabolic process | catalytic activity | |
| 7945321 | LOC89944 | 33 | 510 | 625 | 4.2 | carbohydrate metabolic process | beta-galactosidase complex | catalytic activity |
| 8179731 | HLA-C | 153 | 616 | 2862 | 4.2 | ciliary or flagellar motility | axonemal dynein complex | microtubule motor activity |
| 8064613 | SLC4A11 | 72 | 239 | 1300 | 4.2 | anion transport | membrane | inorganic anion exchanger activity |
| 8167185 | TIMP1 | 215 | 857 | 3816 | 4.1 | multicellular organismal development | extracellular region | enzyme inhibitor activity |
| 8120602 | OGFRL1 | 28 | 141 | 438 | 4.0 | membrane | receptor activity | |
| 8178489 | HLA-C | 190 | 730 | 2990 | 4.0 | ciliary or flagellar motility | axonemal dynein complex | microtubule motor activity |
| 8060758 | PRNP | 108 | 486 | 1632 | 3.9 | copper ion homeostasis | cytoplasm | copper ion binding |
| 8178498 | HLA-B | 164 | 517 | 2463 | 3.9 | antigen processing and presentation of peptide antigen via MHC class I | cellular component | molecular function |
| 8124911 | HLA-B | 131 | 408 | 1955 | 3.9 | antigen processing and presentation of peptide antigen via MHC class I | cellular component | molecular function |
| 7973985 | MIPOL1 | 9 | 92 | 134 | 3.9 | |||
| 7944722 | STS-1 | 32 | 58 | 473 | 3.9 | nucleus | ||
| 8124901 | HLA-C | 205 | 724 | 2974 | 3.9 | ciliary or flagellar motility | axonemal dynein complex | microtubule motor activity |
| 8095736 | LOC727738 | 43 | 170 | 615 | 3.8 | |||
| 8091411 | TM4SF1 | 38 | 220 | 538 | 3.8 | biological process | integral to plasma membrane | molecular function |
| 7917875 | F3 | 78 | 87 | 1078 | 3.8 | immune response | plasma membrane | transmembrane receptor activity |
| 8092726 | CLDN1 | 57 | 195 | 772 | 3.8 | cell adhesion | integral to plasma membrane | structural molecule activity |
| 8126820 | GPR110 | 16 | 20 | 217 | 3.8 | signal transduction | membrane | receptor activity |
FC: Fold-change was calculated from the signal Log ratio value.
3.2. Galectin-3-dependent γ-aminobutyric acid B receptor 1 (GABABR1) expression in human colon cancer cells
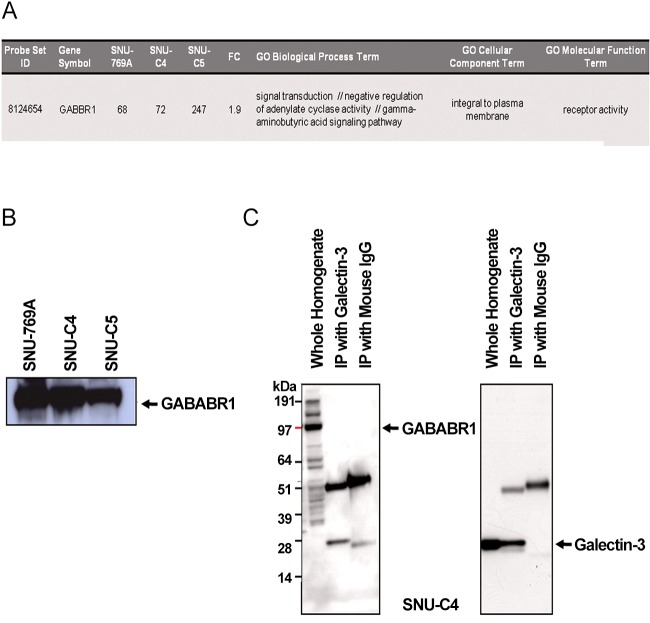
Although the genes listed in Tables 2, 3 contain γ-aminobutyric acid B receptor 1 (GABABR1), its expression was positi-vely correlated with galectin-3 as previously reported (Fig 2 A) [11]. GABABR1 expression at the translational level was highest in SNU-769B among the three human colon cancer cell lines tested (Fig 2 B). To validate the interaction between galectin-3 and GABABR1, we performed reverse immunoprecipitation: however, galectin-3 did not form a complex with GABABR1 (Fig 2 C).
Fig. 2. Gene and protein expressions of GABABR1 positively linked to galectin-3 expression.
(A) GABABR1 in the list of the genes showing positive expressional correlation with galectin-3. (B) Protein expression of GABABR1 in the three human colon cancer cell lines tested. GABABR1 protein expression also showed positive correlation with galectin-3 expression. (C) Reverse immunoprecipitation using anti-galectin and GABABR1 antibody. Results demonstrated that two proteins did not interact to form a complex in SNU-C4 with modest expression of galectin-3 and GABABR1.
3.3. Galectin-3-independent down-regulation of GABABR1 protein by HAD-B in human colon cancer cells
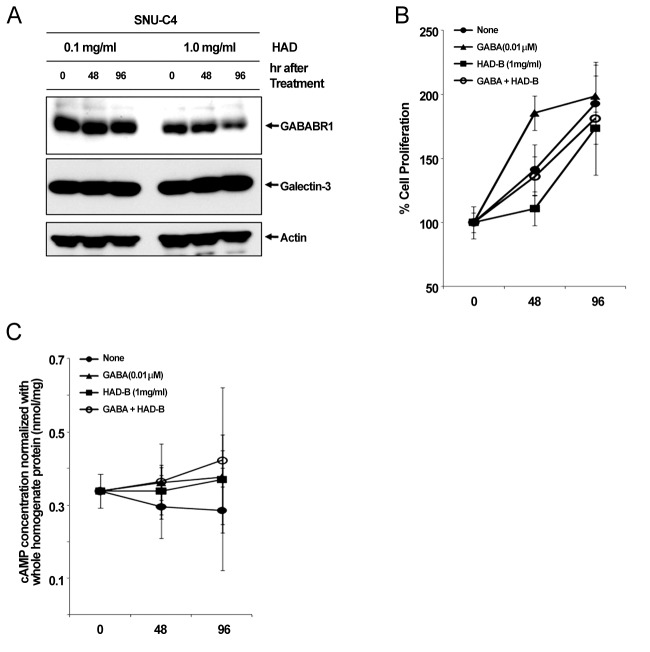
To check the effect of HAD-B treatment on the expression level of galectin-3 and GABABR1, we cultured SNU-C4 with modest expression of galectin-3 and GABABR1 in the presence of HADB, and we performed a Western blot analysis. At 96 hrs after treatment with 1 mg/ml HAD-B, expression of GABABR1 was reduced, but galectin-3 did not show any expressional change (Fig 3 A).
Fig. 3. Reduced GABABR1 expression and suppressed cell proliferation of SNU-C4 by treatment with HAD-B.
(A) Decreased GABABR1 expression by treatment with HAD-B. At 96 hrs after treatment of with 1 mg/ml HAD-B, protein expression of GABABR1 was decreased in SNU-C4. (B) Suppressed cell proliferation by treatment with HAD-B. GABA treatment recovered the rate of proliferation of SNU-C4 that had been suppressed by HAD-B treatment. (C) Increase in the intracellular cAMP by either GABA or HAD-B treatment. Treatment with GABA or HAD-B increased the basal level of intracellular cAMP.
3.4. GABABR1-mediated proliferation of human colon cancer cells suppressed by HAD-B treatment
Treatment with γ-aminobutyric acid (GABA) in the culture medium promoted proliferation of the human colon cancer cell line SNU-C4 (Fig 3 B). At 48 hrs after treatment with GABA, cell proliferation was increased up to ~50% compared to nonetreated controls, but rate of increase of proliferation was not maintained (Fig 3 B). HAD-B significantly decreased cell proliferation at 48 hrs after treatment compared to the control, but the suppressed proliferation had recovered at 96 hrs (Fig 3 B). Cells co-treated with GABA and HAD-B showed almost the same pattern of proliferation as that of the control (Fig 3 B). Either GABA or HAD-B treatment slightly increased the intracellular cAMP in SNU-C4 compared to that in the nontreated control (Fig 3 C).
4. Discussion
Colon cancer causes almost a half million deaths every year [23]. In the past 3 decades, 5-fluorouracil (5-FU) chemotherapy and 5-FU-based chemotherapy have been the mainstream in adjuvant treatment of colon cancer [24]; however, partial or complete responses of colon cancer to 5-FU are generally followed by eventual tumor re-growth [25]. Numerous studies have focused on identifying the mechanisms and key molecules involved in natural or acquired 5-FU resistance. Nevertheless, conclusive and consistent results have not been obtained so far. A recent proteome approach identified galectin-3 as a protein affecting 5-FU resistance and the proliferation rate of human colon cancer cells [11]. Our present study confirmed the correlation between galectin-3 expression and 5-FU susceptibility in three human colon cancer cell lines. 5-FU susceptibility of human colon cancer cells was different depending on both the transcriptional and the translational levels of galectin-3 (Fig 1A,B). Because the identification of genes showing positively or negatively correlated expression with galectin-3 can provide further information on how galectin-3 regulates proliferation of human colon cancer cells, a high-density oligonucleotide microarray was performed. From this transcriptional analysis, we were able to list the genes down- and up-regulated based on the level of galectin-3 expression (Fig. 1B, Tables2, 3). Though γ-aminobutyric acid B receptor 1 (GABABR1) was not in the top 50 genes linked to galectin-3 (Table2and3), interestingly we found that both the transcriptional and the translational levels of GABABR1 were positively correlated with galectin-3 (Fig 2A,B). Even though the biological functions of each individual protein have been well studied, we could not find a report describing the relation between galectin-3 and GABABR1.
GABABRs have been found to play a key role in regulating membrane excitability and synaptic transmission in the brain [26]. GABABRs are G-protein coupled receptors that associate with a subset of G-proteins that trigger cAMP cascades [26]. GABABR subtypes exist; two GABAB-receptor splice variants, GABABR1a and GABABR1b, have been cloned [27], and a new GABABR subtype, GABABR2, does not bind with available GABAB antagonists with measurable potency [28]. GABABR1a, GABABR1b and GABABR2 alone do not activate Kir3-type potassium channels efficiently, but co-expression of these receptors yields a robust coupling to activation of Kir3 channels. GABABR2 and GABABR1a/b proteins immunoprecipitate and localize together at dendritic spines [28]. The heteromeric receptor complexes exhibit a significant increase in agonist- and partial agonist-binding potencies as compared with individual receptors and probably represent the predominant native GABAB receptor [28]. As a previous report also showed that the transcriptional level of GABABR1 was decreased by transfection of galectin-3 small interfering RNA (siRNA) [11], expression of GABABR1 could be regulated by galectin-3. However, reverse immunoprecipitation to validate the interaction between two proteins revealed that galectin-3 did not affect the protein stability of GABABR1 because it formed a complex with GABABR1 (Fig 2C).
Gamma-aminobutyric acid (GABA) has been reported to affect cancer development. For example, GABA can be a potential tumor suppressor for small airway-derived lung adenocarcinomas [29]. The GABA agonist nembutal has been reported to be a potent inhibitor of primary colon cancer and metastasis [30]. The GABABR agonist baclofen induced G(0)/G(1) phase arrest of human hepatocellular carcinomas (HCCs), which suggested the possibility of developing baclofen as a therapeutic drug for the treatment of HCCs [31]. Furthermore, stimulation of GABABR signaling has been suggested as a novel target for the treatment and the prevention of pancreatic cancer [32]. However, in our present study, treatment with GABA promoted proliferation of the human colon cancer cell line SNU-C4 (Fig 3B). The Korean herbal extract HAD-B not only decreased GABABR1 expression but also reduced proliferation of human colon cancer cells without any expressional change of galectin-3 (Fig 3A,B). GABABR activation can lead to down-regulation of the intracellular cAMP level in human cancer cells [30, 32]. Downregulation of GABABR1 by HAD-B treatment increased the basal level of intracellular cAMP in SNU-C4 (Fig 3C). However, such an increased cAMP was also observed after GABA treatment (Fig 3C). The overall findings in the present study were inconsistent with those in previous reports describing the activation of GABABR1 to prevent the progression of a human carcinoma. Nevertheless, our present results showed a link between galectin-3 and GABABR1 in human colon cancer cell proliferation. Galectin-3 regulated GABABR1 expression [11]. Decreased galectin-3 expression reduced not only GABABR1 expression but also the proliferation rate of human colon cancer cells [11]. Even GABA promoted human colon cancer cell proliferation by activating GABABR1 signaling, and the increased proliferation was offset by HAD-B treatment because HAD-B led to galectin-3-independent down-regulation of GABABR1 (Fig. 3).
5. Conclusion
Our present study confirmed that GABABR1 expression was regulated by galectin-3. Korean herbal extract HAD-B induced galectin-3-independent down-regulation of GABABR1, which resulted in a decreased proliferation of human colon cancer cells. The therapeutic effect of HAD-B for the treatment of human colon cancer needs to be further validated.
Acknowledgments
This work was supported by a research grant (NCC-1010050) from the National Cancer Center, Korea.
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–20810. [PubMed] [Google Scholar]
- 2.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59(24):6239–6245. [PubMed] [Google Scholar]
- 3.Herrmann J, Turck CW, Atchison RE, Huflejt ME, Poulter L, Gitt MA et al. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J Biol Chem. 1993;268(35):26704–26711. [PubMed] [Google Scholar]
- 4.Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cell Mol Life Sci. 2003;60(2):267–276. doi: 10.1007/s000180300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inohara H, Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 1995;55(15):3267–3271. [PubMed] [Google Scholar]
- 6.Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res. 1998;245(2):294–302. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- 7.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66(6):3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- 9.Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, Von Bernstorff, W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69(4):555–564. [PubMed] [Google Scholar]
- 10.Yoshii T, Inohara H, Takenaka Y, Honjo Y, Akahani S, Nomura T et al. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol. 2001;18(4):787–792. doi: 10.3892/ijo.18.4.787. [DOI] [PubMed] [Google Scholar]
- 11.Yoo BC, Hong SH, Ku JL, Kim YH, Shin YK, Jang SG et al. Galectin-3 stabilizes heterogeneous nuclear ribonucleoprotein Q to maintain proliferation of human colon cancer cells. Cell Mol Life Sci. 2009;66(2):350–364. doi: 10.1007/s00018-009-8562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki J, Hokari R, Kato S, Tsuzuki Y, Kawaguchi A, Nagao S et al. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9(6):1307–1312. doi: 10.3892/or.9.6.1307. [DOI] [PubMed] [Google Scholar]
- 13.Castronovo V, Van Den Brule FA, Jackers P, Clausse N, Liu FT, Gillet C et al. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996;179(1):43–48. doi: 10.1002/(SICI)1096-9896(199605)179:1%3C43::AID-PATH541%3E3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Van den Brule FA, Berchuck A, Bast RC, Liu FT, Gillet C, Sobel ME et al. Differential expression of the 67-kD lamininreceptor and 31-kD human laminin-binding protein in human ovarian carcinomas. Eur J Cancer. 1994;30(8):1096–1099. doi: 10.1016/0959-8049(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 15.Pacis RA, Pilat MJP, Pienta KJ, Wojno KJ, Raz A, Hogan V et al. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44(2):118–123. doi: 10.1002/1097-0045(20000701)44:2%3C118::AID-PROS4%3E3.3.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Mollenhauer J, Deichmann M, Helmke B, Müller H, Kollender G, Holmskov U et al. Frequent down-regulation of DMBT1 and galectin-3 in epithelial skin cancer. Int J Cancer. 2003;105(2):149–157. doi: 10.1002/ijc.11072. [DOI] [PubMed] [Google Scholar]
- 17.Choufani G, Nagy N, Saussez S, Marchant H, Bisschop P, Burchert M et al. The levels of expression of galectin-1, galectin-3, and the Thomsen-Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer. 1999;86(11):2353–2363. doi: 10.1002/(SICI)1097-0142(19991201)86:11%3C2353::AID-CNCR25%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Choi YJ, Shin DY, Lee YW, Cho CK, Kim GY, Kim WJ et al. Inhibition of cell motility and invasion by HangAmDan-B in NCI-H460 human non-small cell lung cancer cells. Oncol Rep. 2011;26(6):1601–1608. doi: 10.3892/or.2011.1440. [DOI] [PubMed] [Google Scholar]
- 19.Bang JY, Kim KS, Kim EY, Yoo HS, Lee YW, Cho CK et al. Anti-angiogenic effects of the water extract of HangAmDan (WEHAD), a Korean traditional medicine. Sci China Life Sci. 2011;54(3):248–254. doi: 10.1007/s11427-011-4144-3. [DOI] [PubMed] [Google Scholar]
- 20.Park HM, Kim SY, Jung IC, Lee YW, Cho CK, Yoo HS. Integrative tumor board: a case report and discussion from East- West Cancer Center. Integr Cancer Ther. 2010;9(2):236–245. doi: 10.1177/1534735410371479. [DOI] [PubMed] [Google Scholar]
- 21.Bang JY, Kim EY, Shim TK, Yoo HS, Lee YW, Kim YS et al. Analysis of anti-angiogenic mechanism of HangAmDan-B (HAD-B), a Korean traditional medicine, using antibody microarray chip. BioChip J. 2010;4(4):350–355. doi: 10.1007/s13206-010-4412-5. [DOI] [Google Scholar]
- 22.Yoo HS, Lee HJ, Kim JS, Yoon J, Lee GH, Lee YW et al. A toxicological study of HangAmDan-B in mice. J Acupunct Meridian Stud. 2011;4(1):54–60. doi: 10.1016/S2005-2901(11)60007-1. [DOI] [PubMed] [Google Scholar]
- 23.Xu R, Zhou B, Fung PCW, Li X. Recent advances in the treatment of colon cancer. Histol Histopathol. 2006;21(8):867–872. doi: 10.14670/HH-21.867. [DOI] [PubMed] [Google Scholar]
- 24.Nordman IC, Iyer S, Joshua AM, Clarke SJ. Advances in the adjuvant treatment of colorectal cancer. ANZ J Surg. 2006;76(5):373–380. doi: 10.1111/j.1445-2197.2006.03726.x. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FUinduced apoptosis in colon cancer cells. Ann Surg Oncol. 2009;16(3):761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 26.Padgett CL, Slesinger PA. GABAB receptor coupling to Gproteins and ion channels. Adv Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- 27.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386(6622):239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 28.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396(6712):683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 29.Schuller HM, Al-Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29(10):1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaker PH, Yokoi K, Jennings NB, Li Y, Rebhun RB, Rousseau DL, Jr et al. Inhibition of experimental colon cancer metastasis by the GABA-receptor agonist nembutal. Cancer Biol Ther. 2005;4(7):753–758. doi: 10.4161/cbt.4.7.1827. [DOI] [PubMed] [Google Scholar]
- 31.Wan T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 2008;82(9-10):536–541. doi: 10.1016/j.lfs.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112(4):767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]