Abstract
Objectives:
Toad venom, called Chan-Su, is a traditional Oriental medicine secreted from the auricular and the skin glands of the Bufo bufo gargarizanz Cantor or B. melanosticus Schneider and has been widely used in China, Korea and other parts of Asia for the treatment of pain, heart conditions, and cancer. We examined the concentrations of the main chemical constituents within a commerciallyavailable toad venom product and compared the levels for different extraction methods.
Methods:
Toad venom was extracted using either cold or hot water, ethanol (EtOH), methanol (MeOH), or ethyl acetate (EtOAc), was fractionated using precipitation or reflux, and was then analyzed using thin layer chromatography (TLC), high-performance liquid chromatography (HTLC), and liquid chroma-tography - mass spectrometry (LC-MS). Individual components were identified by comparisons of the retention times, the ultraviolet spectra, and mass spectras and differences in chemical constituents for different solvents and extraction methods are presented.
Results:
Components with authentic standards, including serotonin and bufodienolides (cinobufagen, bufalin, cinobufalin, and resibufogenin), were detected. The water extract of toad venom contained the greatest amount of serotonin (75.7 ± 0.1 mg/g), but very small amounts of bufodienolides (3.8 ± 0.0 mg/g). In contrast, the use of MeOH or EtOH extraction solutions resulted in 5-26 times higher concentrations of bufodienolides, with only trace amounts of serotonin. The relative and the absolute concentrations of the component also varied based on the extraction method; i.e., EtOH extracts yielded the greatest total amounts of bufodienolides, and EtOAc precipitation had the lowest amounts of bufodienolides.
Conclusions:
Toad venom consists of serotonin and several bufodienolides, and the choice of solvent to extract chemical the constituents is important as a way to enrich the purported active components for treating different conditions.
Keywords: Bufonis venenum, bufadienolides, bufotoxin, HPLC, LC/MS, TLC
1. Introduction
Venenum Bufonis (VB), called Chan-Su (Sum-So), is a traditional Oriental medicine prepared from the dried white secretions of the auricular and the skin glands of the toads Bufo bufo gargarizans or Bufo melanosticus Schneider [1]. Although it is toxic, Chan-Su has been used in China, Korea and other parts of Asia for the treatment of pain, heart conditions, and cancers. Recent evidence has shown effects on myocardial contraction, anti-inflammation, pain relief, and tumor suppression [2, 3]. Chan-Su has been shown to induce apoptosis in T24 by down-regulation of antiapoptoic bcl-2 and bcl-X (S/L) expressions, up-regulation of pro-apoptotic bax expression, and proteolytic activation of caspase-3 and caspase-9 [4]. These findings are consistent with previous findings that support the anti-tumor effects of Chan-Su [5 - 11].
Chemical investigations on toad venom have led to the characterization of its many chemical constituents, including biogenic amines, alkaloids, peptides, proteins and many bufadienolides, such as bufalin, cinobufagin and resibufogenin [12, 13]. Exploration of these new substances has been tempered by their possible toxicities due to the potent cardiotonic activity and narrow therapeutic index of bufadienolides [14].
As with other forms of traditional medicine, variations exist in the types and the amounts of the active compounds present among toad venom samples, depending on the species, geographic region, storage, and preparation [13]. Chan-Su is commonly administered intravenously as a sterile hot-water extract derived from the toad’s skin and can be taken orally in combinations with other herbs such as Liu-Shen-Wan (Yuk-Sin-Huan) and Kyushin (Gu-shim) [2, 3]. Some reports analyzing the components of Chan-Su already exist, but given the diversity of modes of preparation and administration of this substance in traditional medical practice, comparative analyses of the concentrations of active components in Venenum Bufonis prepared by using various extraction methods and solvents are insufficient. Based on this identified dearth of needed information, we decided to investigate changes in the concentrations of several control markers of Chan-Su prepared by using various extraction solvents and extraction methods. In the present study, we used various extraction methods to extract the components of Chan-Su, and we evaluated these components by using thin-layer chromatography (TLC), high-performance liquid chromatography (HTLC), and liquid chromatography - mass spectrometry (LC-MS).
2. Materials and methods
The Chan-Su used in preparatory experiments was purchased in two groups. The first one was dried secretions collected and processed from the parotid glands of toads, and the second one was from toad skin. The two groups were purchased from a herbal market in Shandong Province in China. Preparatory experiments showed that the quantities of the target extracts were higher in the dried secretion than in the toad skin, so we decided to use the dried secretion of the parotid glands of toads in the present study (Fig. 1).
Fig. 1. Pictures of toad venom purchased in Shandong Province, China.
Toad venom is a secretion collected and processed from the parotid glands (upper) and skin lines (lower). About 50 ㎎ of venom is obtained from each toad.
Kiesel gel 60 (particle size: 70-230 mesh, ASTM, Merck) silica gel was used for column chromatography decomposition and a Kiesel gel 60 F254 (ART.5715, Merck) TLC plate was used for component identification. Acetonitrile (solvent for HPLC, Burdick & Jackson) was used for the HPLC analysis. Extraction, graduation, sample preparation and component identification were conducted with guaranteed reagent-grade solvents. Reference materials of serotonin and authentic bufodienolides (cinobufagen, bufalin, cinobufotalin, resibufogenin) were purchased from Santa Cruz Biotechnology, Inc (Canada), for the HPLC component identification. The TLC analyses were performed using an Agilent 1200 series system (USA). The samples were separated on a C18 column (4.6 ㎜×250 ㎜, 5 ㎛ 120 A, INNOPIA, Korea). LC-MS analyses were conducted with an Agilent 6530 Accurate-Mass Q-TOF LC/MS System (USA).
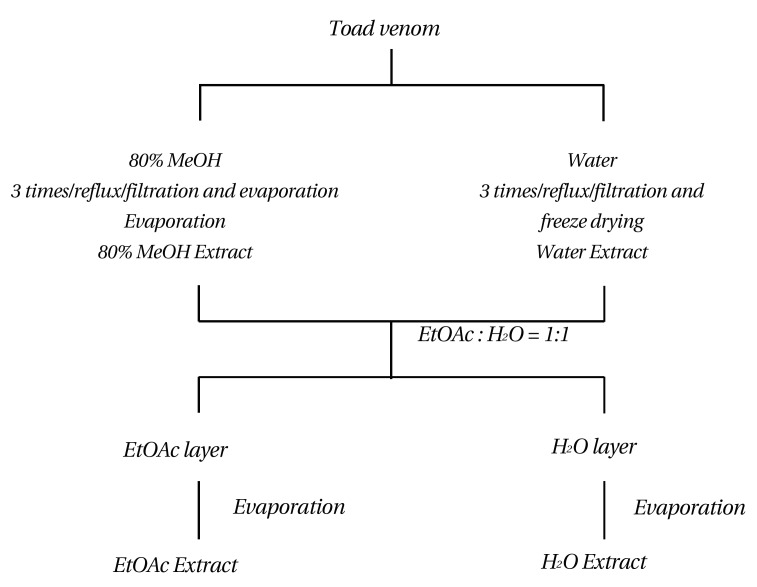
Each extraction and fractionation of toad venom was made as described in Fig. 2. Toad venom (50 g) was added to 500 mL of 80% methanol (ratio 1:10) and refluxed for 5 h 3 times. Then, the resulting 80% MeOH extraction (33.4 g) was obtained after filtration and evaporation. For the hotwater extract, 100 g of toad venom was added to 100 mL of hot water (ratio 1:1) and was refluxed for 5 hours 3 times. A 45.3 g was obtained after filtration and freeze drying.
Fig. 2. Extraction and fractionation of toad venom.
An Ethyl acetate fraction, MeOH extraction, hot-water extraction was obtained after evaporation and condensation.
10 g of the 80 % MeOH extract was added to ethyl acetate with water (ratio of EtOAc to water 1:1). Then, the ethylacetate fraction (3.05 g) of the MeOH extraction was obtained after evaporation and condensation. 20 g of the hot-water extraction was added to ethyl acetate with water (ratio of EtOAc to water 1:1). Then, the ethyl-acetate fraction (2.2 g) of the hot-water extraction was obtained after evaporation and condensation (Fig. 2).
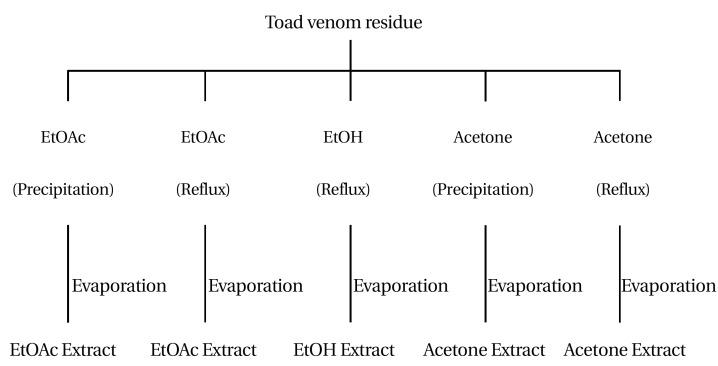
Each 5 g of toad venom residue after dehydration extraction was extracted again with organic solvent (EtOH, EtOAc, acetone), as shown in Fig. 3Each toad venom residue was then divided into two groups. The first group consisted of the residue after hot-water extraction. The second group was composed of the residue after coolwater extraction. Then, the residue in first group was extracted with organic solvents (EtOH, EtOAc, acetone). The residue in the second group was extracted with organic solvents (EtOAc, acetone). Finally five divisions of residue extractions were obtained after dehydration extraction (Fig. 3).
Fig. 3. Extraction and fractionation of toad venom residue.
Toad venom residue after dehydration extraction was extracted again with an organic solvent (EtOH, EtOAc, acetone.)
TLC was performed to distinguish between and to detect bufadienolide compounds from the toad venom extracts. Two grams of EtOH extract were used. Analytical conditions were as follows: Plate, TLC silica gel 60 F254 ; developing solvent, CHCl3 : acetone = 10 : 3 ; detection, 10% H2 SO4 10%. After chromatography had been performed, the chromatogram was divided into three fractions. Standards of cinobufagin, resibufogenin, bufalin, cinobufotalin and separate toad venom extracts were detected in the TLC plate. Then, comparative analyses were performed among standards and toad venom samples.
Each toad venom extract was accurately weighed (1.0 mg), placed in 1 ml of MeOH, and spun for 15 seconds. Then, this solution was passed through a 0.45 ㎛ membrane filter and used as a test liquid. A stock solution of 1.0 mg/1 ml was prepared. A serial dilution was made on each stock solution to prepare standard solutions at concentrations of 25, 50, 100, 200, and 400 ㎍/㎖, from each of which 10 ㎛ was used for plotting the standard curves. The conditions of the chromatographic analysis were based on the conditions of Huming Gao [13]. Analyses were performed using a C18 column (4.6 ㎜ × 250 ㎜, 5 ㎛, 120 A, Innopia). The column was equilibrated with KH2 PO4 buffer and acetonitrile at a flow rate of 0.8 ml/min. The column temperature was kept constant at 35 ℃. The UV wavelength was monitored at 296 nm (Table1).
Table. 1. Operating conditions of HPLC for determination of serotonin, cinobufotalin, bufalin, resibufogenin and cinobufagin.
| Mobile phase | ||||
|---|---|---|---|---|
| Time (min) |
Flow (ml/min) |
A (Buffer/ACN) 95 / 5 Buffer: 10 mM KH2 PO4 (Adjusted to pH 2.5 with phosphoric acid) |
B (ACN) | |
| Gradient profile | 0 | 0.8 | 100 | 0 |
| 30 | 0.8 | 70 | 30 | |
| 40 | 0.8 | 70 | 30 | |
| 60 | 0.8 | 10 | 90 | |
| 70 | 0.8 | 100 | 0 | |
| 90 | 0.8 | 100 | 0 | |
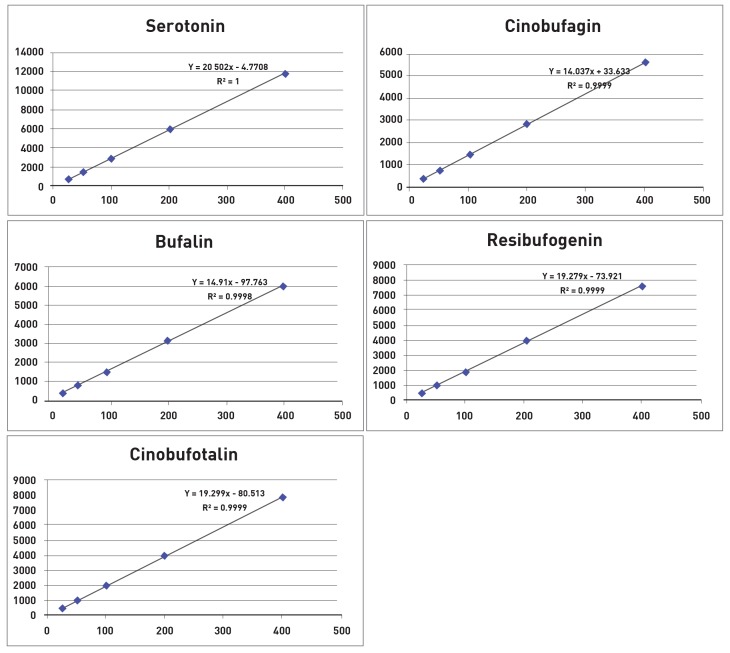
The calibration curves were prepared with five standards at concentrations, of 25, 50, 100, 200, and 400 ㎍/㎖ under optimized HPLC conditions. The five standards, serotonin, cinobufotalin, bufalin, resibufogenin, and cinobufagin, are known to be major control markers of toad venom. The regression equations were calculated in the form of y = ax + b, where y and x correspond to the peak area and the concentration, respectively. The calibration curves were linear in the tested concentration range. The correlation coefficients (R2) were all greater than 0.99 (0.9999, 0.9998, 0.9999, 0.9999), indicating high correlation and good linearity (Fig. 4).
Fig. 4. Calibration curves of serotonin, cinobufotalin, bufalin, resibufogenin and cinobufagin.
For the quantative HPLC analysis on toad venom for different extraction methods, calibration curves were prepared with five standards (serotonin, cinobufotalin, bufalin, resibufogenin and cinobufagin) at concentration of 25, 50, 100, 200, and 400 ㎍/㎖ under optimized HPLC conditions.
The test liquid was analyzed three times under optimized HPLC conditions. The contents of toad venom in the each test liquid were determined by using a calibration curve of concentration versus peak area.
For the LC-MS analyses, the mobile phase was 0.05 % and 0.1 % acetone solution at a flow rate of 1 ml/min. The column temperature was kept constant at 30℃. Gradients were 100~97 %, 10 min; 97~50%, 10 min; 50~0 %, 15 min.
3. Results
3.1. Result of TLC analysis
After the toad venom standard had been located on the TLC plate, each location was confirmed. The results of separating the EtOH extract by using open column chromatography are shown in Fig. 5. Components that have polarities lower than that of bufalin were detected in fraction 1, bufalin was detected in the fraction 2, and components with polarities higher than that of bufalin were detected in the fraction 3. EtOH extractions were compared with the bufalin standard to check their components in fraction 2 (Fig 5).
Fig. 5. Comparative TLC chromatography results for Venenum Bufonis(plate: TLC silica gel 60 F254, developing solvent: CHCl3 : acetone = 10 : 3, and detection: 10% H2 SO4 10%).
The HPLC results of analyses on the standard preparations according to analysis conditions of toad venom are shown in Fig. 6. The retention times were 8.185 min for serotonin, 48.602 min for cinobufotalin, 51.305 min for bufalin, 53.706 min for cinobufagin, and 54.200 min for resibufogenin. The HPLC calibration curves of the standard preparations were linear for all five concentrations (25, 50, 100, 200 and 400 ㎍/㎖). A comparative analysis of the contents of toad venom based on the extraction method was performed using the calibration curves (Fig 7). As showed in Table 2, the 80% MeOH extract contained serotonin (35.4 ± 1.2 ㎎/g), cinobufota-lin (8.4 ± 0.5 ㎎/g), bufalin (11.7 ± 0.4 ㎎/g), resibufogenin (20.9 ± 0.4 ㎎/g), and cinobufagin (27.0 ± 2.1 ㎎/g). The hot-water extract contained serotonin (75.7 ± 0.1 ㎎/g), cinobufotalin (1.5 ± 0.0 ㎎/g), bufalin (negligible amount), resibufogenin (0.8 ± 0.0 ㎎/g), and cinobufagin (1.5 ± 0.0 ㎎/g). The ethyl acetate extract that was fractionated with 80% MeOH extract with ethyl acetate contained serotonin (0.3 ± 0.0 ㎎/g), cinobufotalin (16.9 ± 0.9 ㎎/g), bufalin (26.3 ± 0.4 ㎎/ g), resibufogenin (35.8 ± 0.3 ㎎/g) and cinobufagin (43.8 ± 0.1 ㎎/g). The ethyl acetate extract fractionated with hot-water extract and ethyl acetate contained cinobufotalin (2.8 ± 0.1 ㎎/g), bufalin (1.8 ± 0.0 ㎎/g), resibufogenin (1.8 ± 0.0 ㎎/g), and cinobufagin (2.8 ± 0.1 ㎎/g); serotonin was not detected.
Fig. 6. HPLC chromatogram of serotonin, cinobufotalin, bufalin, resibufogenin and cinobufagin.
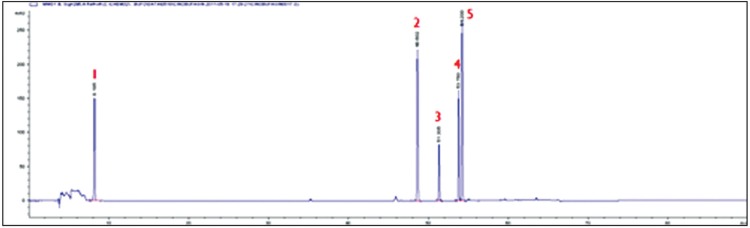
1: serotonin, 2: cinobufotalin, 3: bufalin, 4: cinobufagin, 5: resibufogenin.
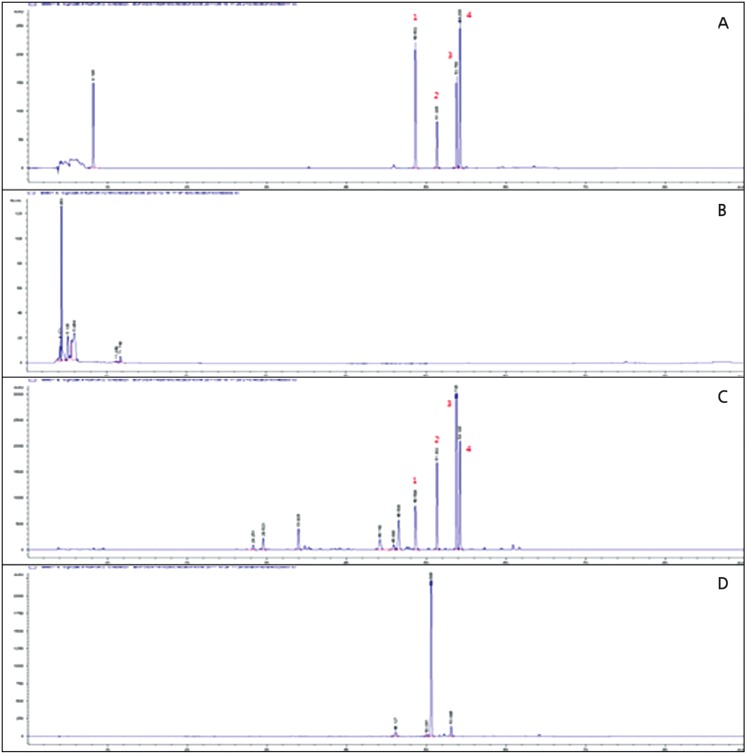
Fig. 7. HPLC chromatograms of Venenum Bufonis.
(A: standard mix, B: water extract, C: EtOH extract, D: isolation of bufalin).
Table. 2. Content analyses of the Bufonis Venomum by using HPLC for different extraction methods.
| Extraction method | Sample | Constituent | Area | Content (㎎/g) |
|---|---|---|---|---|
| Evaporation after 80%
MeOH extraction |
80% MeOH ext. | Serotonin | 776.3 ± 12.0 | 35.4 ± 1.2 |
| Cinobufotalin | 202.0 ± 8.4 | 8.4 ± 0.5 | ||
| Bufalin | 228.2 ± 10.2 | 11.7 ± 0.4 | ||
| Resinobufogenin | 253.6 ± 9.9 | 20.9 ± 0.4 | ||
| Cinobufagin | 463.9 ± 32.7 | 27 ± 2.1 | ||
| EtOAc Ext | Serotonin | 19.4 ± 1.2 | 0.3 ± 0.0 | |
| Cinobufotalin | 879.7 ± 61.6 | 16.9 ± 0.9 | ||
| Bufalin | 1059.7 ± 67.3 | 26.3 ± 0.4 | ||
| Resinobufogenin | 1268.5 ± 50.2 | 35.8 ± 0.3 | ||
| Cinobufagin | 2145.2 ± 22.7 | 43.8 ± 0.1 | ||
| Freeze dry after water extraction | Water Ext | Serotonin | 1228.1± 20.9 | 75.7 ± 0.1 |
| Cinobufotalin | 112.6 ± 2.7 | 1.5 ± 0.0 | ||
| Bufalin | 57.8 ± 0.6 | - | ||
| Resinobufogenin | 46.2 ± 2.1 | 0.8 ± 0.0 | ||
| Cinobufagin | 105.9 ± 8.4 | 1.5 ± 0.0 | ||
| EtOAc Ext | Serotonin | - | - | |
| Cinobufotalin | 632.1 ± 21.8 | 2.8 ± 0. | ||
| Bufalin | 363.7 ± 7.1 | 1.8 ± 0.0 | ||
| Resinobufogenin | 293.1 ± 4.3 | 1.8 ± 0.0 | ||
| Cinobufagin | 624.3 ± 8.9 | 2.8 ± 0.0 |
Table. 3. Content analyses of the Bufonis Venomum by using HPLC after extraction dehydration.
| Constituent | Area | Content (㎎/g) | |
|---|---|---|---|
| EtOH Ext | Serotonin | 303.4 ± 11.0 | 1.0 ± 0.0 |
| Cinobufotalin | 9319.4 ± 21.3 | 43.9 ± 1.7 | |
| Bufalin | 13256.9 ± 33.6 | 80.8 ± 1.3 | |
| Resinobufogenin | 24321.8 ± 44.2 | 158.5 ± 6.5 | |
| Cinobufagin | 16075.3 ± 55.8 | 76.0 ± 0.3 | |
| EtOH Ext (hot-water reflux) | Serotonin | - | - |
| Cinobufotalin | 6565.6 ± 20.9 | 25.3 ± 0.2 | |
| Bufalin | 9706.6 ± 12.7 | 48.5 ± 0.3 | |
| Resinobufogenin | 20068.7 ± 33.7 | 107.3 ± 1.1 | |
| Cinobufagin | 11818.6 ± 93.5 | 45.8 ± 0.7 | |
| EtOH Ext (precipitation) | Serotonin | - | - |
| Cinobufotalin | 6200.3 ± 66.1 | 5.6 ± 0.1 | |
| Bufalin | 9081.9 ± 21.6 | 10.6 ± 0.1 | |
| Resinobufogenin | 19757.9 ± 93.5 | 24.7 ± 2.7 | |
| Cinobufagin | 11545.4 ± 95.8 | 10.5 ± 0.2 | |
| EtOH Ext (hot-water reflux) | Serotonin | 303.0 ± 10.2 | 0.8 ± 0.0 |
| Cinobufotalin | 7939.3 ± 82.4 | 31.8 ± 0.1. | |
| Bufalin | 11826.3 ± 33.6 | 61.4 ± 0.1 | |
| Resinobufogenin | 23067.6 ± 212.1 | 128.0 ± 0.2 | |
| Cinobufagin | 14320.4 ± 77.3 | 57.6 ± 0.3 | |
| EtOH Ext (precipitation) | Serotonin | 45.8 ± 1.0 | 0.1 ± 0.0 |
| Cinobufotalin | 9086.5 ± 43.1 | 31.5 ± 0.1 | |
| Bufalin | 13695.0 ± 77.9 | 61.6 ± 0.2 | |
| Resinobufogenin | 25570.1 ± 101.5 | 123.0 ± 0.2 | |
| Cinobufagin | 16807.7 ± 21.9 | 58.7 ± 0.1 |
In the MeOH extract, cinobufotalin was found to be 5.6 times higher, resibufogenin 26 times higher, and cinobufagin 18 times higher than in the hot-water extract. However, serotonin was found to be present at much higher concentrations than in the hot-water extract. Bufalin was not detected in the hot-water extract. In the ethylacetate extract that was fractionated with MeOH extract and ethyl acetate, cinobufotalin was found to be 6 times higher, bufalin 14.6 times higher, resibufogenin 19 times higher, and cinobufagin 15.6 times higher than in the ethylacetate extract that was fractionated with hotwater extract with ethyl acetate.
The contents of the toad venom residue after dehydration were analyzed with the organic solvents EtOH, EtOAc, and acetone. The EtOH extraction contained serotonin (1.0 ± 0.0 ㎎/g), cinobufotalin (43.9 ± 1.7 ㎎/g), bufalin (80.8 ± 1.3 ㎎/g), resibufogenin (158.5 ± 6.5 ㎎/g), and cinobufagin (76.0 ± 0.3 ㎎/g). The EtOAc extraction (hotwater reflux) contained cinobufotalin (25.3 ± 0.2 ㎎/g), bufalin (48.5 ± 0.3 ㎎/g), resibufogenin (107.3 ± 1.1 ㎎/g), and cinobufagin (45.8 ± 0.7 ㎎/g). Serotonin was not detected in this preparation. The EtOAc extraction (precipitation) contained cinobufotalin (5.6 ± 0.1 ㎎/g), bufalin (10.6 ± 0.1 ㎎/g), resibufogenin (24.7 ± 2.7 ㎎/g), and cinobufagin (10.5 ± 0.2 ㎎/g). Serotonin was not detected in this preparation. Either the acetone extract (hot-water reflux) contained serotonin (0.8 ± 0.0 ㎎/g), cinobufotalin (31.8 ± 0.1 ㎎/g), bufalin (61.4 ± 0.1 ㎎/g), resibufogenin (128.0 ± 0.2 ㎎/g), and cinobufagin (57.6 ± 0.3 ㎎/g). The acetone extraction (precipitation) contained serotonin (0.1 ± 0.0 ㎎/g), cinobufotalin (31.5 ± 0.1 ㎎/g), bufalin (61.6 ± 0.2 ㎎/g), resibufogenin (123.0 ± 0.2 ㎎/g), and cinobufagin (58.7 ± 0.1 ㎎/g).
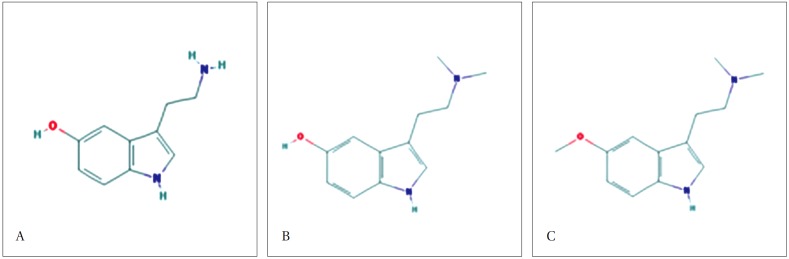
A LC/MS analysis was performed to identify the constituents of the hot-water extract of toad venom. The results of HPLC analysis showed that most of the hot-water extract was composed of serotonin. The LC/MS analysis showed three broad peaks after 6-8 min. When the molecular weight of each peak was measured, the molecular weights of the first and the second peaks were 160.0, and the molecular weight of the third peak was 219.0. When the LC/MS analysis results were compared to a serotonin standard, the molecular weight of serotonin was 177. Thus, the substances composing the first and the second peak structures were nitrogen, carbon, and hydrogen, eliminating the possibility of identifying the substance as serotonin. Similarly, the substance of the third peak was concluded to be bufotenin or methoxybufotenin (Figs. 8 and 9).
Fig. 8. LC-MS chromatograms of water extract in Venenum Bufonis.
(A: the molecular weight of the first peak was 160.0, B: the molecular weight of the second peak was 160.0, C: the molecular weight of the third peak was 219.1, and D: the molecular weight of the standard serotonin was 177.0).
Fig. 9. Molecular structures.
(A: serotonin, B: bufotenin, and C: methoxybufotenin).
4. Discussion
The dried white secretion from the auricular and the skin glands of toads, called Chan-Su, is widely used for treating heart failure, arrhythmia, cancer and tonsillitis in Korea and China. It contains bufotoxins such as bufotenine, cinobufotalin, bufalin, resibufogenin, and cinobufagin. These bufotoxins can pose serious toxicity to humans; thus, Chan-Su has been used carefully in clinical settings. Bufotoxin poisoning manifests primarily as digitalis toxicity-like cardiac effects, including bradycardia, atrioventricular conduction blocks, ventricular tachycardia, ventricular fibrillation, and sudden death [15]. The cardiotonic effect of Chan-Su is due to its major bufadienolides such as bufalin, cinobufagin and resibufogenin [16]. Bufalin is known to block vasodilation and to increase vasoconstriction, vascular resistance and blood pressure by inhibiting Na and K-ATPase [17]. Bufotenine belongs to a class of indole alkaloids with documented effects of aphrodisia, hallucination, and paralysis [18, 19]. The serotonin component may be useful for improving sleep quality and cancer-related fatigue. Cinobufagin has effects of myocardial contraction, and resibufogenin has been used to strengthen respiration but in overdose induces heart palpitation [20]. Therefore, careful dosage and use is very important for the best use of Chan-Su. Also, better understanding of the properties of the chemicals in toad venom prepared by using solvent extraction may provide the potential for targeted therapy with one or several bufodienolides.
This study was performed as part of a precedent study to explore the use of toad venom as an anti-cancer drug. To investigate the constituent differences of toad venom prepared by using various extraction method, we used water, EtOH, MeOH, and ethyl acetate as extraction solvents, and we analyzed the extracts by using TLC, HPLC and Q-TOF(LC-MS) based on the comparisons of the retention times, the UV spectra, or the mass spectra. TLC is a qualitative analysis method to detect the existence of a specific substance. An advantage of TLC is that results can be obtained in a relatively short time. In the separation results for the EtOH extract using an open column, components where polarities were lower than that of bufalin were detected in fraction 1, bufalin was detected in fraction 2, and other components that had polarities higher than that of bufalin were detected in fraction 3. HPLC is also a quantitative analysis method. The standards (serotonin, cinobufotalin, bufalin, resibufogenin and cinobufagin) for the main components of toad venom were analyzed by using HPLC under optimized conditions and retention times (RT). The RT of serotonin was 8.185 min, that of cinobufotalin was 48.602 min, that of bufalin was 51.305 min, that of cinobufagin was 53.760 min, and that of resibufogenin was 54.200 min.
The calibration curves for HPLC quantitative analysis on toad venom according to different extract methods were prepared with five standards (serotonin, cinobufotalin, bufalin, resibufogenin, and cinobufagin) at concentrations of 25, 50, 100, 200, 400 ㎍/㎖ under optimized HPLC conditions. In the HPLC analysis on toad venom for various extraction methods, the extract with 80% MeOH showed various compounds including serotonin, cinobufotalin, bufalin, resibufogenin, and cinobufagin. The ethyl acetate extract after 80% MeOH extraction showed more bufadienolides (cinobufotalin, bufalin, resibufogenin and cinobufagin) compared to the MeOH extract, but not serotonin. In the water extract, a great part of the solution was comprised of serotonin, and only a small amount consisted of bufadienolides. The ethylacetate extract after water extraction showed small amounts of bufadienolides, but not serotonin. Therefore, different solvents are necessary for the extraction of target substances and the choice of solvent and extraction method allows for flexible manipulation of substance extraction.
A mass analysis was used to measure the molecular weight by measuring the ratio of mass to charge after the material had been ionized. The mass analysis was also applied to establish the molecular structure through an analysis on the fragmentation of ions. A mass spectrometer has several types, such as the time-of-flight mass spectrometer, magnetic sector mass spectrometer, quadrupole spectrometer, etc. Among them, the mostfrequently- used type for LC/MS analysis is the quadrupole spectrometer. A quadrupole time-of-flight mass spectrometer was used in this present study [21].
The HPLC quantitative analysis confirmed that bufadienolides such as cinobufotalin, bufalin, resibufogenin and cinobufagin were extracted well in MeOH or EtOH and that serotonin was extracted well in water. To identify the components of the water extract more accurately, we performed a LC/MS analysis. In the LC/MS analysis, 3 peaks were observed between 6 and 8 min. The molecular weights of the peaks were 160, 160, and 219, respectively. The first and the second peaks with molecular weights of 160 were assumed to be variations of the basic structure of serotonin. The third peak with a molecular weight of 219 was assumed to be bufotenin (C12 H16 N2O ) or methoxybufotenin (C13 H18 N2O ).
Gao et al. [13] reported that the chemical profiles of toad venoms from different Bufo species, and even identical Bufo species form different geographic locations, differed significantly, not only in the absolute and relative contents but also in the number and the types of the constituents. Our study indicated that the relative and absolute concentrations of the components varied based on the extraction method; i.e., EtOH extracts yielded the greatest total amounts of bufodienolides and EtOAc precipitation had the lowest amounts of bufodienolides. These findings can be used to standardize the bioactive compounds in toad venom and to improve delivery of target compounds in the form of oral supplements or pharmacopuncture.
Toad venom consists of serotonin and several bufodienolides, and the choice of solvent to extract the chemical constituents is important as a way to enrich the purported active components for treating different conditions. For example, the bufodienolides are implicated as the components responsible for the analgesic, antimicrobial, and anticancer properties of toad venom. The serotonin component, on the other hand, may be useful for improving sleep quality and cancer-related fatigue. Further studies on this subject should be conducted to yield more concrete evidence.
5. Conclusion
Toad venom was shown to consist of serotonin and several bufadienoldies. The choice of solvent is very important for the extraction of targeted components, and a combination of solvent and extraction methods allows for the selection of active components that can be used to treat specific illnesses.
References
- 1.Chen KK, Kovarikova A. Pharmacology and toxicology of toad venom. J Pharm Sci. 1967;56(12):1535–1541. doi: 10.1002/jps.2600561202. [DOI] [PubMed] [Google Scholar]
- 2.Hong Z, Chan K, Yeung HW. Simultaneous determination of bufadienolides in the traditional Chinese medicine preparation, liu-shen-wan by liquid chromatography. J Pharm Pharmacol. 1992;44(12):1023–1026. doi: 10.1111/j.2042-7158.1992.tb07086.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan WY, Ng TB, Yeung HW. Examination for toxicity of a Chinese drug, the total glandular secretion product Chan Su in pregnant mice and embryos. Biol Neonate. 1995;67(5):376–380. doi: 10.1159/000244188. [DOI] [PubMed] [Google Scholar]
- 4.Ko WS, Park TY, Park C, Kim YH, Yoon HJ, Lee SY et al. Induction of apoptosis by Chan Su, a traditional Chinese medicine, in human bladder carcinoma T24 cells. Oncol Rep. 2005;14(2):475–480. doi: 10.3892/or.14.2.475. [DOI] [PubMed] [Google Scholar]
- 5.Gowda RM, Cohen RA, Khan IA. Toad venom and Liushen tablet by β-cyclodextrin modified micellar electrokinetic chromatography. J Pharm Biomed Anal. 2006;41(1):124–128. doi: 10.1016/j.jpba.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 6.WY W, Chai XS, Li GY, Zhang HB, Long SQ, Xue XG et al. Preliminary results of combination therapy with Chinese herbs and vinorelbine in elderly advanced non-small cell lung cancer. Journal of US-China Medical Science. 2006;3(2):72–77. [Google Scholar]
- 7.Kim JS, Jeong TY, Cho CK, Lee YW, Yoo HS. Antitumor effect of skin of venenum bufonis in a NCI-H460 tumor regression model. J Acupunct Meridian Stud. 2010;3(3):181–187. doi: 10.1016/S2005-2901(10)60034-9. [DOI] [PubMed] [Google Scholar]
- 8.Yun HR, Yoo HS, Shin DY, Hong SH, Kim JH, Cho CK et al. Apoptosis induction of human lung carcinoma cells by Chan Su (Venenum Bufonis) through activation of caspases. J Acupunct Meridian Stud. 2009;2(3):210–217. doi: 10.1016/S2005-2901(09)60057-1. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Dasgupta SC, Gomes A. Immunomodulatory and antineoplastic activity of common Indian toad (Bufo melanostictus, Schneider) skin extract. Indian J Pharmacol. 1998;30(5):311–317. [Google Scholar]
- 10.Giri B, Gomes A, Debnath A, Saha A, Biswas AK, Dasgupta SC et al. Antiproliferative, cytotoxic and apoptogenic activity of Indian toad (Bufo melanostictus, Schneider) skin extract on U937 and K562 cells. Toxicon. 2006;48(4):388–400. doi: 10.1016/j.toxicon.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Nogawa T, Kamano Y, Yamashita A, Pettit GR. Isolation and structure of five new cancer cell growth inhibitory bufadienolides from the Chinese traditional drug Ch’an Su. J Nat Prod. 2001;64(9):1148–1152. doi: 10.1021/np0101088. [DOI] [PubMed] [Google Scholar]
- 12.Krenn L, Kopp B. Bufadienolides from animal and plant sources. Phytochemistry. 1998;48(1):1–29. doi: 10.1016/S0031-9422(97)00426-3. [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Zehl M, Leitner A, Wu X, Wang Z, Kopp B. Comparison of toad venoms from different Bufo species by HPLC and LC-DAD-MS/MS. J Ethnopharmacol. 2010;131(2):368–376. doi: 10.1016/j.jep.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta A. Review of abnormal laboratory test results and toxic effects due to use of herbal medicines. Am J Clin Pathol. 2003;120(1):127–137. doi: 10.1309/P024-K7VR-DDPJ-CTVN. [DOI] [PubMed] [Google Scholar]
- 15.Gowda RM, Cohen RA, Khan IA. Toad venom poisoning: resemblance to digoxin toxicity and therapeutic implications. Heart. 2003;89(4):e14. doi: 10.1136/heart.89.4.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morishita S, Shoji M, Oguni Y, Ito C, Higuchi M, Sakanashi M. Pharmacological actions of ‘Kyushin’, a drug containing toad venom: cardiotonic and arrhythmogenic effects, and excitatory effect on respiration. Am J Chin Med. 1992;20(3-4):245–256. doi: 10.1142/S0192415X92000254. [DOI] [PubMed] [Google Scholar]
- 17.Pamnani MB, Chen S, Bryant HJ, Schooley JF, Jr, Eliades DC, Yuan CM et al. Effects of three sodium-potassium adenosine triphosphatase inhibitors. Hypertension. 1991;18(3):316–324. doi: 10.1161/01.HYP.18.3.316. [DOI] [PubMed] [Google Scholar]
- 18.Barry TL, Petzinger G, Zito SW. GC/MS Comparison of the west Indian Aphrodisiac “Love Stone” to the Chinese Medication “Chan Su”: Bufotenine and related Bufodienolides. J Forensic Sci. 1996;41(6):1068–1073. [PubMed] [Google Scholar]
- 19.Fuller RW, Snoddy HD, Perry KW. Tissue distribution, metabolism and effects of bufotenine administered to rats. Neuropharmacology. 1995;34(7):799–804. doi: 10.1016/0028-3908(95)00049-C. [DOI] [PubMed] [Google Scholar]
- 20.Akizawa T, Mukai T, Matsukawa M, Yoshioka M, Morris JF, Butler VP., Jr Structures of novel bufadienolides in the eggs of a toad, Bufo marinus. Chem Pharm Bull. 1994;42(3):754–756. doi: 10.1248/cpb.42.754. [DOI] [PubMed] [Google Scholar]
- 21.Ye M, Guo H, Han J, Guo D. Simultaneous determination of cytotoxic bufadienolides in the Chinese medicine ChanSu by high-performance liquid chromatography coupled with photodiode array and mass spectrometry detections. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838(2):86–95. doi: 10.1016/j.jchromb.2006.04.042. [DOI] [PubMed] [Google Scholar]