Abstract
Objectives:
Buxus Microphylla var. Koreana Nakai Extract (BMKNE) is used as a folk remedy for malaria and veneral disease. In the present study, we investigated the effects of BMKNE in the growth and the survival of AGS cells, the most common human gastric adenocarcinoma cell lines.
Methods:
The AGS cells were treated with varying concentrations of BMKNE. Analyses of the sub G1 peak, the caspase-3 and -9 activities, and the mitochondrial depolarization were conducted to determine whether AGS cell death occured by apoptosis. Also, to identify the role of transient receptor potential melastatin (TRPM) 7 channels in AGS cell growth and survival, we used human embryonic kidney (HEK) 293 cells overexpressed with TRPM7 channels.
Results:
Experimental results showed that the sub G1 peak, the caspase-3 and -9 activities, and the mitochondrial depolarization were increased. Therefore, BMKNE was found to induce the apoptosis of these cells, and this apoptosis was inhibited by SB203580 (a p38 mitogen-activated protein kinase (MAPK) inhibitor), and by a c-jun NH2-terminal kinase (JNK) II inhibitor. Furthermore, BMKNE inhibited TRPM7 currents and TRPM7 channel over-expressions in HEK 293 cells, exacerbating BMKNE-induced cell death.
Conclusions:
These findings indicate that BMKNE inhibits the growth and the survival of gastric cancer cells due to a blockade of the TRPM7 channel’s activity and MAPK signaling. Therefore, BMKNE is a potential drug for treatment of gastric cancer, and both the TRPM7 channel and MAPK signaling may play an important role in survival in gastric cancer cells.
Keywords: AGS cells, apoptosis, Buxus Microphylla var. Koreana Nakai, gastric cancer, mitogen-activated protein kinase (MAPK) inhibitor, transient receptor potential melastatin 7 (TRPM7) channel
1. Introduction
Among cancer, gastric cancer causes nearly one million deaths worldwide per year. Gastric cancer is one of the leading causes of cancer-related death worldwide due to its frequency, poor prognosis and limited treatment options. Although the incidence of gastric cancer has been declining for several decades in most countries, it remains a crucial public health problem in some countries [1].
In previous studies, we suggested that human gastric adenocarcinoma cells expressed the transient receptor potential melastatin 7 (TRPM7) channel, which is essential for cell survival and is a potential target for pharmacological gastric cancer treatment [2]. TRPM7 is endogenously expressed in a wide variety of tissues including brain and hematopoietic tissues [3], as well as kidney and heart tissues [4 - 6]. The TRPM7 cation channel supports multiple cellular and physiological functions, including cellular Mg2+ homeostasis [7, 8], cell viability and growth [8 11], anoxic neuronal cell death [12], synaptic transmission [13], cell adhesion [14, 15], and intestinal pacemaking [16]. Wykes et al. [17] suggested that TRPM7 channels were critical for human mast cell survival, and Jiang et al. [18] suggested that activation of TRPM7 channels was critical for the growth and proliferation of human head and neck carcinoma cells. Also, Abed et al. [19] proposed the importance of TRPM7 in human osteoblast-like cell proliferation. However, the role of the TRPM7 channel in the survival of gastric cancer cells after incubation with Buxus Microphylla var. Koreana Nakai extract (BMKNE) is unknown.
BMKN is distributed widely in Korea as an ornamental plant [20]. In this study, we examined the effects of BMKN and the role of TRPM7 channels in BMKN-inhibited apoptosis of AGS cells, the most common human gastric adenocarcinoma cell lines.
2. Materials and Methods
2.1. Preparation of BMKNE
The BMKNE used in this study were purchased from the Korea Research Institute of Bioscience and Biotechnology (KRIBB).
2.2. Cell
AGS lines were used. The AGS cell lines were established at the Cancer Research Center, College of Medicine, Seoul National University, Korea. The cell lines were propagated in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco-BRL) supplemented with 10% heat-inactivated fetal bovine serum and 20-μg/mL penicillin and streptomycin in an atmosphere of 5% CO2 at 37℃.
2.3. Flow cytometric analysis
In order to investigate whether the cell cycle of AGS cells was redistributed, we used a flow cytometric analysis with propidium iodine (PI) stain [21, 22]. We placed 1 × 106 cells in an e-tube. An ice-cold fixation buffer (ethyl alcohol), 700 μL was slowly added with vortexing. The tubes were sealed with parafilm and incubated at 4℃ overnight. Samples were spun for 3 min at 106 g at 4℃, and the supernatant was aspirated and discarded. The cell pellet was resuspended in 200 μL of PI staining solution (PI [5 mg/ mL] 2 μL and RNase 2 μL in PBS 196 μL) at 20817 g for 5 s. After 30 min in the dark at room temperature, the samples were analyzed in a fluorescence activated cell sorter (FACScan; Becton-Dickinson, Moutain View, CA, USA) at λ= 488 nm by using Cell-Quest software (Becton-Dickinson). The DNA content distribution of normal growing cells was characterized by using two peaks, the G1/G0 and the G2/M phases. The G1/G0 phase comprises the normal functioning and resting state of the cell cycle with the most diploid DNA content, while the DNA content in the G2/M phase is more than diploid. Cells in the sub-G1 phase have the least DNA content in the cell cycle distribution; this is termed hypodiploid. Hypoploid DNA contents represent the DNA fragmentation [23].
2.4. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay
Cell viability was assessed by using a MTT (3-[4, 5-dimethylthiazol- 2-yl]-2, 5-diphenyltetrazolium bromide) assay. The AGS cells were seeded into each well of 12-well culture plates and then cultured in Roswell Park Memorial Institute medium (RPMI)-1640 supplemented with other reagents for 72 h. After incubation, 100 μL of MTT solution (5 mg/mL in phosphate-buffered saline (PBS)) was added to each well, and the plates were incubated at 37℃ for 4 h. After the supernatant had been removed and shaken with 200 μL of dimethyl sulfoxide (Jersey Lab Supply, Livingston, NJ, USA) for 30 min, the absorbance was measured at 570 nm. All experiments were repeated at least 3 times.
2.5. Caspase assay
Caspase-3 and -9 assay kits (Cellular Activity Assay Kit Plus) were purchased from BioMol (Plymouth, PA, USA). After experimental treatment, cells were centrifuged (10000 g, 4℃, 10 min) and washed with PBS. Cells were re-suspended in ice-cold cell lysis buffer and incubated on ice for 10 min. Sample were centrifuged at 10000 g (4℃, 10 min), and the supernatant was removed. Supernatant samples (10 μL) were incubated with 50 μL of substrate (400-μM Ac-DEVD-pNA) in 40 μL of assay buffer at 37℃. The absorbance at 405 nm was read at several time points. The pNA concentrations in the samples were extrapolated from a standard created using the absorbances of sequential pNA concentrations.
2.6. Assessment of mitochondrial membrane depolarization
Mitochondrial membrane depolarization was evaluated using a JC-1 fluorescence probe according to the manufacturer’s instructions (Santa Cruz). The AGS cells were labeled with 2 μM JC-1 for 30 min at 37℃ and then analyzed by using flow cytometry with 488-nm excitation and 530/30- or 585/42-nm bypass emission filters. The cells without red fluorescence were regarded as the cells manifesting mitochondrial membrane depolarization.
2.7. Patch-clamp experiments
A whole-cell configuration of the patch-clamp technique experiment was performed at room temperature (22–25℃). The AGS cells were transferred to a small chamber on an inverted microscope stage (IX70, Olympus, Japan) and were constantly perfused with a solution containing 2.8-mmol/L KCl, 145-mmol/L NaCl, 2-mmol/L CaCl2, 10-mmol/L glucose, 1.2-mmol/L MgCl2, and 10-mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), adjusted to a pH of 7.4 with NaOH. The pipette solution contained 145-mmol/L Cs-glutamate, 8-mmol/L NaCl, 10-mmol/L Cs-2-bis (2-aminophenoxy)- ethane-N,N,N′,N′-tetraacetic acid, and 10-mmol/L HEPES-CsOH, adjusted to a pH of 7.2 with CsOH. Axopatch I-D (Axon Instruments, Foster City, CA, USA) was used to amplify the membrane currents and potentials. For data acquisition and the application of command pulses, pCLAMP software v.9.2 and a Digidata 1322A units (Axon Instruments) were used. Results were analyzed using pClamp and Origin software (Microcal Origin version 6.0).
2.8. TRPM7 expression in human embryonic kidney- 293 cells
Human embryonic kidney (HEK)-293 cells were transfected with the Flag-murine long transient receptor potential channel 7 (LTRPC7)/pCDNA4-TO construct and grown on glass coverslips in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, blasticidin (5 μg/mL), and zeocin (0.4 mg/mL). TRPM7 (LTRPC7) expression was induced by adding 1 μg/mL of tetracycline to the culture medium. Whole-cell patch-clamp experiments were performed at 21-25℃ with cells that had been grown on the glass coverslips.
2.9. Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Differences between the data were evaluated by using the student’s t-test. A P-value of 0.05 was taken to indicate a statistically significant difference.
3. Results
3.1. BMKNE-induced cell death in AGS cells
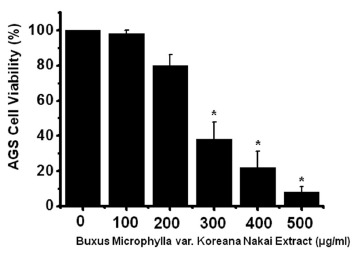
To ascertain whether BMKNE kills AGS cells, we performed MTT assays. The viable cell population was gradually reduced with increasing concentrations of BMKNE for 24 h in AGS cells (Fig 1). Thus, our results demonstrate that BMKNE induces cell death in AGS cells.
Fig. 1. Buxus Microphylla var. Koreana Nakai Extract (BMKNE) induces cell death in AGS cells. MTT (3-[4, 5-dimethylthiazol- 2-yl]-2, 5-diphenyltetrazolium bromide)–based viability assay. The AGS cells were treated with increasing concentrations of BMKNE for 24 h. Distilled water was used as a vehicle. Cell viability is expressed as a value relative to that of the untreated cells which is set to 100%. The figures show means ± SEMs. * P < 0.01.
3.2. BMKNE-triggered apoptosis in AGS cells
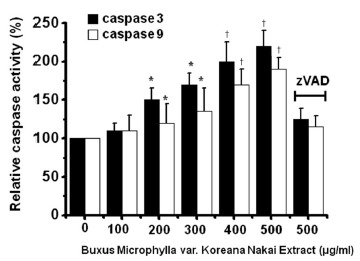
To determine whether AGS cell death occurs by apoptosis, we conducted sub-G1 analysis [24, 25]. In this protocol, cells were incubated with BMKNE and stained with a fluorescent DNA stain (PI). The action of endogenous endonucleases in apoptotic cells cleaves DNA into endonucleosomal fragments of typical size, which are extracted from the cells. The loss of DNA is detected by using an FACS analysis, as the reduced nuclear staining in apoptotic cells, which results in a novel (sub-G1) fluorescence peak to the left of the regular fluorescence peak. Flow cytometric analysis showed that the percentage of sub-G1 phase cells markedly increased in the AGS cells treated with BMKNE in a dose-dependent manner (Fig 2). In addition, BMKNE elevated mitochondrial membrane depolarization, an early event of an intrinsic apoptosis signaling (Fig 3). Thus, our findings suggest that BMKNE induces apoptosis via intrinsic apoptotic mechanism(s). Caspase-3 and -9 activations are hallmarks of apoptotic cell death. We also measured the enzyme activity in AGS cells after BMKNE incubation. Using a synthetic substrate, we detected the caspase-3 and -9 activities in AGS cells. BMKNE increased the activities of caspase-3 and -9 (Fig 4).
Fig. 2. BMKNE increases the activity of the sub-G1 peak in AGS cells. G1 peak was measured by using FACScan. The figures show means ± SEMs. * P < 0.01.
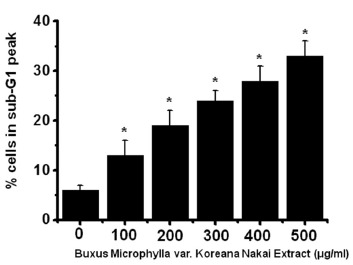
Fig. 3. BMKNE increases the mitochondrial membrane depolarization potentials in AGS cells. The mitochondria membrane depolarization is expressed as a value relative to that of untreated cells which is set to 100%.The figures show means ± SEMs. * P < 0.05, † P < 0.01.
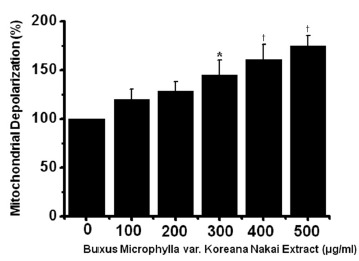
Fig. 4. BMKNE increases the caspase activity in AGS cells. The AGS cells were cultured with BMKNE at the indicated concentrations for 24 h prior to the caspase assays. The caspase activity from untreated cells is expressed as 100%. Pan-caspase inhibitor zVAD-fmk (zVAD) at 20 μM was used to validate the analytical method employed. The figures show means ± SEMs. * P < 0.05, † P < 0.01.
3.3. Involvement of mitogen-activated protein kinases (MAPKs) in BMKNE-induced apoptosis in AGS cells
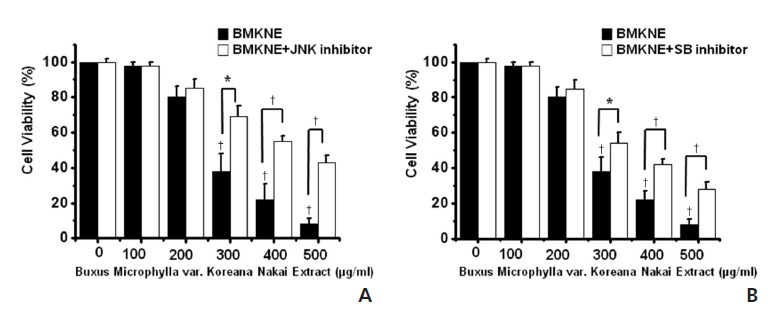
To investigate the signaling pathway of BMKNE-induced apoptosis in AGS cells, we assessed the effect of BMKNE on mitogen-activated protein kinases (MAPKs), because they play critical roles in the apoptosis-related signaling pathway. As shown in Fig 5, exposure to BMKNE with c-jun NH2-terminal kinase (JNK) II inhibitor (Fig 5A) or SB203580 (a p38 MAPK inhibitor) (Fig 5B) resulted in increases in the viable cell populations.
Fig. 5. Effects of JNK II inhibitor and SB203580 on BMKNE-induced AGS cells. MTT–based viability assay. C-jun NH2-terminal kinase (JNK) II inhibitor (A) or SB203580 (a p38 MAPK inhibitor) (B) was administered to the AGS cells pretreated with BMKNE. The figures show means ± SEMs. * P < 0.05, † P < 0.01.
3.4. Effects of BMKNE in TRPM7 currents in AGS- and TRPM7- overexpressed HEK293 cells
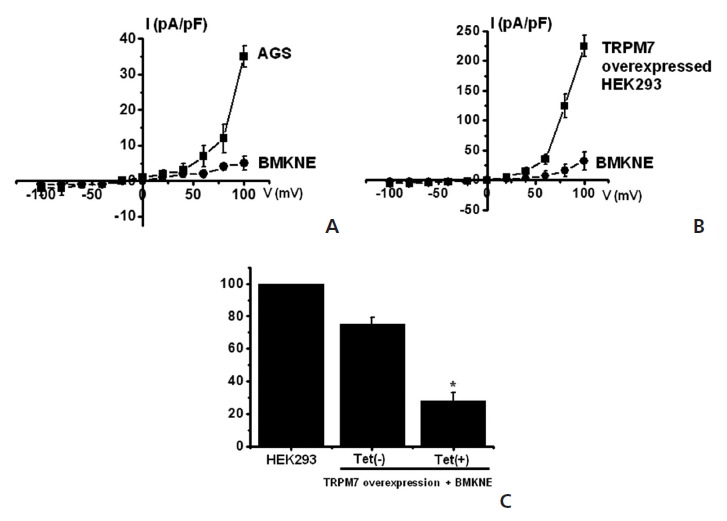
TRPM7 has been proposed to be required for cell survival on the basis of experiments on genetically-engineered DT-40 B-cells [9]. Also, we recently suggested, as in previous reports, that AGS cells expressed the TRPM7 channel and that suppression of the TRPM7 channel induced cell death [2]. Therefore, we investigated whether BMKNE influenced TRPM7 currents in AGS cells. To confirm the effect of BMKNE in TRPM7 currents, we investigated the effects of BMKNE in AGS cells by using patch-clamp techniques. We performed whole cell voltage-clamp recordings to investigate the effect of BMKNE on the TRPM7-like current in AGS cells. A voltage ramp of from +100 mV to -100 mV evoked small inward currents at negative potentials, whereas larger outward currents were evoked at positive potentials, showing that the currents were outward- rectifying cation currents (n = 4; Fig 6A). However, in the presence of 500 μg/ml of BMKNE, the amplitudes of these currents were inhibited outwardly by 92.3 ± 2.4% and inwardly by 95.1 ± 2.1% (n = 4; Fig 6A). Also similar results were obtained in HEK293 cells over-expressing TRPM7 (Fig 6B). To provide additional evidence that supports the contribution of the TRPM7 channel to BMKNE toxicity, we investigated changing expression levels of the TRPM7 channel and its influences on BMKNE-mediated cell death. We used HEK293 cells with inducible TRPM7 channel expression [18]. In the absence of induced TRPM7 channel expression [TRPM7(-) cells, Tet(-)], HEK293 cells incubation with BMKNE induced cell death, as observed on the MTT assay (n = 4; Fig 6C). However, when TRPM7 channel overexpression was induced by adding tetracycline [TRPM7(+) cells, Tet(+)], HEK293 cells incubation with BMKNE induced cell death at an increased rate in the MTT assay, which suggests that increased expression of TRPM7 channels leads to an increased rate of BMKNE-induced cell death.
Fig. 6. Effects of BMKNE on AGS- and TRPM7-overexpressed HEK293 cells. (A) Representative I-V relationships of the effect of BMKNE on TRPM7 currents in AGS cells. (B) Representative I-V relationships of the effect of BMKNE on TRPM7 currents in HEK293 cells. (C) TRPM7 channels were treated or not treated with tetracycline for 1 day. Cells were incubated with BMKNE, followed by the MTT assay. A voltage ramp from +100 to -100 mV was applied from a holding potential of -60 mV. The figures show means ± SEMs. * P < 0.01.
4. Discussion
In this study, we demonstrated that BMKNE suppresses AGS cell growth in culture models. Molecular and cellular analyses showed that the anticancer activity of BMKNE is attributable to cell cycle arrest and/or apoptosis. In addition, we found that BMKNE-induced apoptosis was inhibited by MAPK inhibitors and was induced by the blockade of TRPM7 channels. Our results indicate that BMKNE is a useful agent for pharmacological approaches for future development of anticancer drugs [26]. In addition, our findings suggest that BMKNE would be a useful drug tool for approaches to identify novel therapeutic targets for gastric cancer.
Sophorae radix (SR) and Orostachys japonicas (OJ) inhibited the growth and the survival of gastric and breast cancer cells due to a blockade of the TRPM7 channel activity [27, 28]. Also there are many types ion channels in gastric and breast cancer cells. Therefore, the involvement of another ion channels should be investigated in future. TRPM6 channels have characteristics very similar to those of TRPM7 channels. Therefore, TRPM6 also might have an important role in apoptosis, so the involvement of TRPM6 should be investigated in future. Among the TRP families, TRPC, TRPV and TRPM are mainly related to the growth and the progression in cancer cells. Depending on the type of cancer, regulation of TRP mRNA and protein expression will change. These ion channel changes are related with cell growth and apoptotic-induced cell death in cancer cells. Therefore, the regulations of ion channels in cancer cells are the most promising strategy and should be the subjects of considerable efforts to find ways to fight cancer cells [29]. In line with these studies, our studies show that BMKNE induces apoptosis in human gastric adenocarcinoma cells, which may be due to a blocking of the TRPM7 channel activity and MAPK signaling.
Apoptotic cell death pathways are induced by a variety of signals. One mechanism that is consistently implicated in apoptosis is the activation of a series of cytosolic proteases, the caspases [30]. Caspases are synthesized as inactive proenzymes that are processed in cells undergoing apoptosis by self-proteolysis and/or cleavage by another protein [2]. BMKNE has been used as a folk remedy for several diseases, including malaria and venereal diseases. However, only a few reports have described the effects of BMKNE on gastric cancer.
5. Conclusion
In summary, BMKNE inhibited the growth and the survival of AGS cells. Sub-G1 and caspase-3 and -9 activities were elevated, and the mitochondrial membrane depolarization potentials were increased. MAPK signaling was involved in BMKNE-induced apoptosis. TRPM7 currents were inhibited by BMKNE. Furthermore, overexpression of TRPM7 channels in HEK 293 cells increased the rate of BMKNE-induced cell death. These findings indicate that BMKNE inhibits the growth and the survival of gastric cancer and that BMKNE-induced apoptosis is due to the blockade of the TRPM7 channel’s activity and is involved in MAPK signaling. Therefore, BMKNE may be a potential drug for the treatment of gastric cancer.
Acknowledgments
This study was supported by a grant of the Traditonal Korean Medicine R&D Project, Ministry of Health & Welfare, Republic of Korea (B120008).
References
- 1.Tang P, Huang H, Chang J, Zhao GF, Lu ML, Wang Y. Increased expression of DLX2 correlates with advanced stage of gastric adenocarcinoma. World J Gastroenterol. 2013;19(17):2697–2703. doi: 10.3748/wjg.v19.i17.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99(12):2502–2509. doi: 10.1111/j.1349-7006.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82(2):429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 4.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 5.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291(5506):1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 6.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108(5):595–598. doi: 10.1016/S0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114(2):191–200. doi: 10.1016/S0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96(2):207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 9.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ et al. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411(6837):590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 10.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E et al. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95(4):403–419. doi: 10.1254/jphs.FP0040273. [DOI] [PubMed] [Google Scholar]
- 11.Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD et al. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15(7):667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 12.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115(7):863–877. doi: 10.1016/S0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 13.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52(3):485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Clark K, Langeslag M, van Leeuwen, B, Ran L, Ryazanov AG, Figdor CG et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25(2):290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su D, May JM, Koury MJ, Asard H. Human erythrocyte membranes contain a cytochrome b561 that may be involved in extracellular ascorbate recycling. J Biol Chem. 2006;281(52):39852–39859. doi: 10.1074/jbc.M606543200. [DOI] [PubMed] [Google Scholar]
- 16.Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS et al. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129(5):1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J Immunol. 2007;179(6):4045–4052. doi: 10.4049/jimmunol.179.6.4045. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67(22):10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007;40(6):849–865. doi: 10.1111/j.1365-2184.2007.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Park YH, Cho BH, Kim YJ, Kim JB, Kim CM et al. Effects of Cyclobuxine D on the biosynthesis of prostaglandins in vitro, prostaglandins production and leukocyte migration in vivo. Korean Journal of Pharmacology. 1987;23:51–56. [Google Scholar]
- 21.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–279. doi: 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- 22.Hellein KN, Kennedy EM, Harwood VJ, Gordon KV, Wang SY, Lepo JE. A filter-based propidium monoazide technique to distinguish live from membrane-compromised microorganisms using quantitative PCR. J Microbiol Methods. 2012;89(1):76–78. doi: 10.1016/j.mimet.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Wang BJ, Won SJ, Yu ZR, Su CL. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem Toxicol. 2005;43(4):543–52. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Hotz MA, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994;15(3):237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- 25.Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000;243(1-2):167–190. doi: 10.1016/S0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 27.Kim BJ. Involvement of transient receptor potential melastatin 7 channels in sophorae radix-induced apoptosis in cancer cells. Pharmacopuncture. 2012;15(3):31–38. doi: 10.3831/KPI.2012.15.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang MW, Kim HW, Kim BJ. Involvement of transient receptor potential melastatin 7 channels in orostachys Japonicus-induced apoptosis in cancer cells. International Journal of Pharmacology. 2012;8(7):638–646. [Google Scholar]
- 29.Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11(1):54–67. doi: 10.2174/187153011794982068. [DOI] [PubMed] [Google Scholar]
- 30.Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996;10(5):587–597. doi: 10.1096/fasebj.10.5.8621058. [DOI] [PubMed] [Google Scholar]


