Abstract
Objectives:
The present study investigated the toxic properties of petroleum ether extract of Wattakaka (W.) volubilis in Wistar female rats.
Methods:
An in vitro brine shrimp lethality bioassay was studied in A. Salina nauplii, and the lethality concentrations were assessed for petroleum ether extract of W. volubilis. A water soluble portion of the test extract was used in different concentrations from 100-1000 μg/mL of 1 mg/mL stock solution. A 24-hours incubation with a 1-mL aliquot in 50 mL of aerated sea water was considered to calculate the percentage rate of dead nauplii with test extract administration against a potassium-dichromate positive control. The acute and the sub-acute toxicities of petroleum ether extract of W. volubilis were evaluated orally by using gavage in female Wistar rats. Food and water intake, body weight, general behavioral changes and mortality of animals were noted. Toxicity or death was evaluated following the administration of petroleum ether extract for 28 consecutive days in the female rats. Serum biochemical parameters, such as alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, total cholesterol, triglyceride, total protein, glucose, urea, creatinine, sodium, potassium and α-amylase levels, were measured in the toxicity evaluations. Pathological changes in isolated organs, such as the liver, kidneys, and pancreas, were also examined using hematoxylin and eosin dye fixation after the end of the test extract’s administration.
Results:
The results of the brine-shrimp assay indicate that the evaluated concentrations of petroleum ether extract of W. volubilis were found to be non-toxic. In the acute and the sub-acute toxicity evaluations, no significant differences were observed between the control animals and the animals treated with extract of W. volubilis. No abnormal histological changes were observed in any of the animal groups treated with petroleum ether extract of W. volubilis.
Conclusion:
These results suggest that petroleum ether extract of W. volubilis has a non-toxic effect in Wistar female rats.
Keywords: acute toxicity, brine shrimp, kidney, pancreas, Wattakaka volubilis
1. Introduction
Plants have been considered as valuable sources of medicines for treating a variety of diseases and ailments [1]. Despite the availability of modern medicine throughout all countries, herbal medicines have often remained popular for historical and cultural reasons. Previous experimental results, which were highly encouraging, revealed Wattakaka (W.) volubilis to be a medicinal member of the family Asclepiadaceae, which is well known for its ethnobotanical importance and is widely used in Indian traditional medicines. W. volubilis occurs throughout the hotter parts of India [2] and is used in the ayurvedic system of medicine for treating contusions, fresh wounds, fractures, piles, leucoderma, asthma, tumors, and urinary discharges. It is also used as an emetic, diaphoretic and diuretic. Traditional healers of South India use the leaves to treat inflammatory and painful conditions [3]. The leaves are applied to boils and abscesses to promote suppuration. Leaf extracts of W. volubilis have been highlighted for their benefits in curing snake bites and post-childhood headaches, for their anti-inflammatory and anti-rheumatic effects, and for treating pain, cough, fever and severe cold, and dyspepsia. The dried leaf powder of W. volubilis is taken orally with cow’s milk for anti-diabetic activity [4]. In addition, several studies have proven its therapeutic effects, such as antitumor [5, 6] anti-leishmanial [7], anti-diabetic, anti-hyperlipidemic and antioxidant [6, 8, 9], anti-inflammatory, analgesic and antipyretic [10], anti- leukemic [11], anti-ulcer and anthelmintic [12] effects, indicating that the use of this powder could be an effective therapeutic option. With the increased use of medicinal plants by the population, discussions about the safety and efficacy of these products have also expanded.
The national agency for sanitary vigilance is responsible for the regulation of medications and uses protocols from international regulatory agencies to evaluate the efficacy and the safety of medications. However, no studies have specifically addressed the toxic efficacy of petroleum ether extract of W. volubilis. Therefore, the aim of the present study was to prove the absence of toxicity for petroleum ether extract of W. volubilis through in vitro and in vivo evaluations.
2. Materials and Methods
Around 2 kg of leaves of W. volubilis were powdered and passed through 40-mesh filters to obtain a coarse powder, which was best suited for extraction. The powder from all the leaves was placed with petroleum ether in a container with a stopper and was allowed to react at room temperature over a period of 72 hours with recurrent stirring until all the soluble matter had dissolved. The mixture was then strained, the damp solid material was pressed, the pooled liquids were clarified by filtration after settling, and the extract was concentrated under vacuum by using a rotary vacuum evaporator.
Cytotoxicity tests were performed using the A. Salina nauplii assay development with some modifications [13, 14]. In this method, the eggs of brine shrimp were collected from the Tutucorin Research Centre of the Central Marine Fisheries Research Institute, Tutucorin, Tamil Nadu, India. One gram of A. Salina cysts was allowed to hatch and mature as nauplii in 1000 mL of filtered sea water for 48 hours at 25 — 30℃ under continuous aeration and light regimen for 48 hours [15]. Highly active nauplii were concentrated to a suitable density by placing an artificial light at one end of their incubation chamber (50 mm (L) × 35 mm (W)), and the nauplii-rich water closest to the light was removed with a pipette for the cytotoxicity assay. Petroleum ether extract were dissolved in purified water (by forceful mixing to get maximum solubility) to obtain a final concentration of 1 mg/mL, which was then diluted to obtain different concentrations 100-1000 μg/mL [16].
A 1-mL aliquot of each concentration was transferred, in triplicate, into a clean incubation chamber, and the volume was increased to 50 mL by adding aerated sea water. Ten shrimp nauplii were transferred to each chamber under identical conditions and were maintained throughout the experiment period. Petroleum ether extract in purified water was used as a negative control, and potassium dichromate was used as a positive control. After 24 hours of incubation, the numbers of dead nauplii were observed, and the mortality percentage was calculated. Nauplii were considered dead only if they did not move their appendages for 10 seconds during observation. The concentration that killed 50% of the nauplii (LC50) was determined by using a statistical analysis.
Animal experiments were performed in a Wistar strain of albino female rats weighing approximately between 140 to 180 g. The rats were maintained in a standard environment (temperature: 23 ± 1℃, with 55% ± 5% humidity and a 12-hours/12-hours light/dark cycle) and were supplied with water and a standard laboratory diet. Experiments were conducted in accordance with the guidelines of Committee for Purpose of Control and Supervision of Experimental Animals, New Delhi, India (Registration No: 0367/01/C/CPCSEA), and the study was approved by the institutional ethics committee of the Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India.
The assessment of acute toxicity was performed according to the OECD guidelines NO. 423 [17]. Healthy rats were fasted overnight, but allowed access to water ad libitum. They were randomly divided into five groups (n = 6). The first group (control group) received saline. The other four groups were orally treated with a single dose of petroleum ether extract at 5, 50, 300 and 2000 mg/kg, respectively.
All the treatments were administered by force-feeding. The treated animals were under observation for 72 hours to check the food and water intake, body weight, general behavior observations; also, mortality was checked for 14 days.
The sub-acute toxicity study was performed according to OECD test guidelines [18]. The animals were divided randomly into four groups (n = 6). Group I served as the saline control (0.9% NaCl), and groups II-IV received petroleum ether extract (125, 250 and 500 mg/kg b.w.) daily orally by gavage for 28 days [19]. Toxicity signs and mortality were monitored daily whereas body weight changes and changes in food and water consumption were monitored weekly.
24 hours after the last dose (i.e., 29th day) all the animals were anesthetized with diethyl ether, and cardiac puncture was used to collect blood in non-heparinized tube in order to estimate the serum biochemical parameters. Following dissection, the liver, the kidney and the pancreas were removed and weight immediately.
Blood samples were collected for biochemical analysis (Star 21 Plus, Rapid Diagnostic Pvt. Ltd., New Delhi). The biochemical parameters analyzed to evaluate liver functions were alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, total cholesterol and triglyceride, and total serum protein (TP). Urea, creatinine, sodium and potassium were used to assess the effects on renal functions whereas α-amylase and glucose were used to assess pancreatic functions. Colorimetric estimates were done using commercial diagnostic kits (Span Diagnostics Ltd, Gujarat, India).
Fragments of the liver, kidney and pancreas from female rats were stored immediately in formalin 10% v/v for histopathological analysis. These tissue samples were dehydrated in alcohol, cleared with xylene, and embedded in paraffin. Later, they were sectioned (5 μm), stained with hematoxylin and eosin (H&E), and examined with light microscopy. The samples were analyzed under 40 × magnifications for general structural changes in the organs.
All the studies mentioned above were done in triplicate (except the LD50 study), and the results are expressed as means ± standard deviations (SDs), with each group containing six rats. Graph pad prism version 5.04 was used to determine statistical significance by using ANOVA, followed by Tukey’s multiple comparison tests to assess the mean differences and the significance variation. P < 0.05 was considered significant.
3. Results
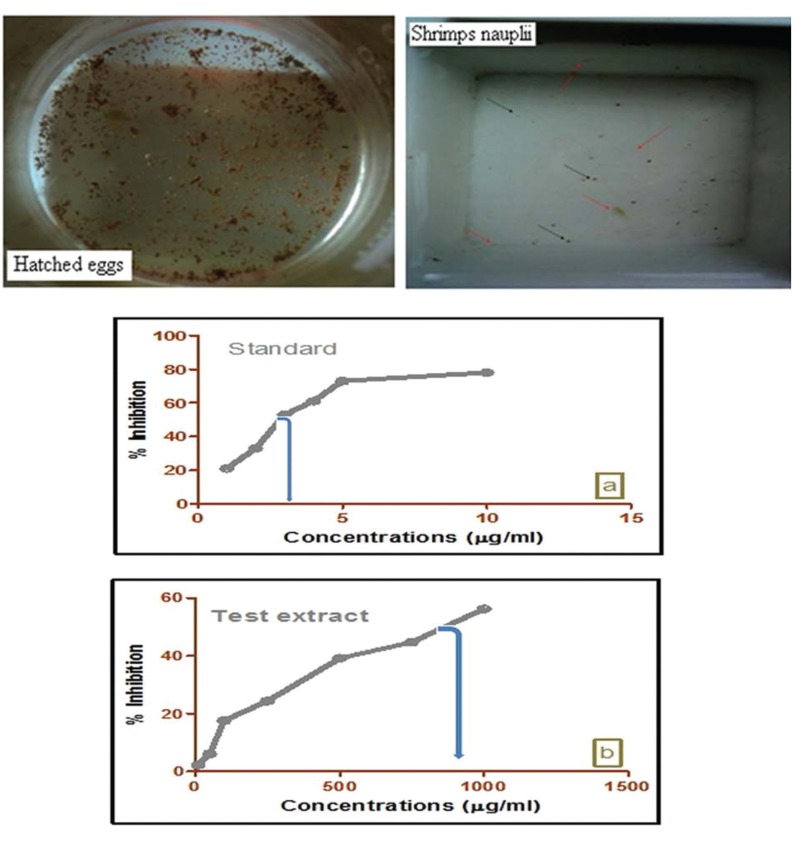
The petroleum ether extract of W. volubilis was lethal for a number of brine shrimp nauplii surviving after 24 hours, which indicated that they were biologically active. The lethality was found to be directly proportional to the concentration of the petroleum ether extract. Mortality was confirmed by a curve plotting concentration vs. percentage inhibition (Fig 1) The percentage inhibitions obtained for the cytotoxic bioassay with standard potassium dichromate were 21 ± 0.32, 33 ± 0.17, 53 ± 0.08, 61 ± 0.14, 73 ± 0.24 and 78 ± 0.18 μg/mL (Fig 1 A) for petroleum ether extracts with concentrations of 2.3 ± 0.52, 6.1 ± 0.08, 17.6 ± 0.43, 24.4 ± 0.19, 39.11 ± 0.16, 44.70 ± 0.38 and 56.24 ± 0.13 μg/mL (Fig 1 B). The 50% lethality concentration of extract was found to be 644.99 μg/mL, which was confirmed by using a plot of the lethality concentration vs. percentage inhibition. The LC50 value for standard potassium dichromate was 3.23.
Fig. 1. In vitro cytotoxicity evaluation of test extract of W. volubilis administered to brine shrimp nauplus. Potassium dichromate was used as a reference standard. Percentage inhibitions of test samples are dose dependent. Each value is expressed as a mean ± standard deviation (n = 3).
For the acute toxicity tests, petroleum ether extract was given as single doses. The food and water intakes, body weights, general behavioral changes and mortalities of the animals for these tests are presented in (Table 1) Observations included changes in skin, fur, eyes, mucous membranes, the respiratory, circulatory, autonomic, and central nervous systems, somatomotor activity, and behavior pattern. Attention was also directed toward observations of tremors, convulsions, salivation, diarrhea and sleep patterns. No signs of toxicity were found. No significant variations were observed in the body weights of the rats treated with petroleum ether extract as a single dose (Table 2) Neither signs of toxicity nor death was observed throughout the study following administration of petroleum ether extract for 28 consecutive days. The treated groups did not show any significant changes in organ weights, as shown in (Table 3).
Table. 1. Variations of mean food and water intakes by and body weights of rats following oral administration of test extract of W. volubilis.
| Group | Dose | Food intake (g) | Water intake (mL) | Body weight (g) | ||||
|---|---|---|---|---|---|---|---|---|
| (mg/kg) | Week 1 | Week 2 | Week 1 | Week 2 | 0 day | Week 1 | Week 2 | |
| 1 | Vehicle | 10.72 ± 1.74 | 13.86 ± 2.28 | 13.24 ± 1.30 | 17.19 ± 2.63 | 140.4 ± 0.83 | 144.4 ± 0.84 | 149.0 ± 0.54 |
| 2 | 5 | 8.78 ± 1.13 | 11.06 ± 1.27 | 14.12 ± 2.04 | 17.04 ± 2.06 | 156.5 ± 0.65 | 160.5 ± 0.63 | 164.0 ± 0.48 |
| 3 | 50 | 7.14 ± 0.94 | 8.52 ± 0.74 | 11.36 ± 1.35 | 11.16 ± 1.13 | 161.2 ± 0.74 | 165.4 ± 0.46 | 169.1 ± 0.62 |
| 4 | 300 | 8.85 ± 1.12 | 8.43 ± 0.86 | 10.17 ± 0.87 | 8.43 ± 0.32 | 174.8 ± 0.66 | 179.3 ± 0.72 | 183.1 ± 0.34 |
| 5 | 2000 | 7.53 ± 0.81 | 6.80 ± 0.63 | 11.47 ± 1.33 | 9.55 ± 0.74 | 169.4 ± 0.93 | 173.2 ± 0.43 | 177.5 ± 0.72 |
Data are expressed as means ± SEMs, n = 6, for each group. No statistical difference was found between the control and the extract-treated groups (P < 0.05).
Table. 2. Effects of different oral single doses of test extract of W. volubilis in rats for acute toxicity.
| Group | Dose (mg/kg) |
Sex | Number of animals that died during day of dosing (D/T) |
Toxicity symptoms observed |
|---|---|---|---|---|
| 1 | 5 | F | 0 / 10 | - |
| 2 | 50 | F | 0 / 10 | - |
| 3 | 300 | F | 0 / 10 | None |
| 4 | 2000 | F | 0 / 10 | - |
All treated rats were carefully examined for up to 14 days after the dose for toxicity and lethality. D, number of rats that died; T, number of rats that were treated; None, no toxicity symptoms observed during the observation period.
Table. 3. Organ weights of rats in the sub-acute toxicity study for the control group and the groups treated with different doses of the test extract of W. volubilis.
| Absolute organ weight (g) | Vehicle | 125 mg/kg | 250 mg/kg | 500 mg/kg |
|---|---|---|---|---|
| Liver | 4.33 ± 0.45 | 4.62 ± 0.53 | 4.29 ± 0.38 | 5.05 ± 0.64 |
| Kidney | 1.17 ± 0.11 | 1.23 ± 0.10 | 1.34 ± 0.15 | 1.38 ± 0.11 |
| Pancreas | 1.04 ± 0.09 | 0.97 ± 0.03 | 1.02 ± 0.08 | 1.12 ± 0.13 |
Data are expressed as means ± SEMs, n = 6, for each group. No statistical difference was found between the control group and the test groups treated with extract of W. volubilis (P < 0.05).
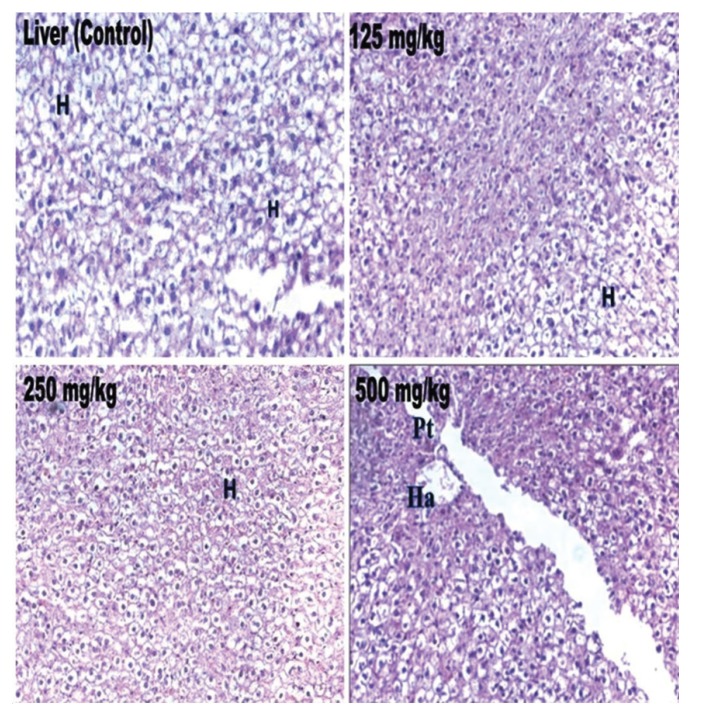
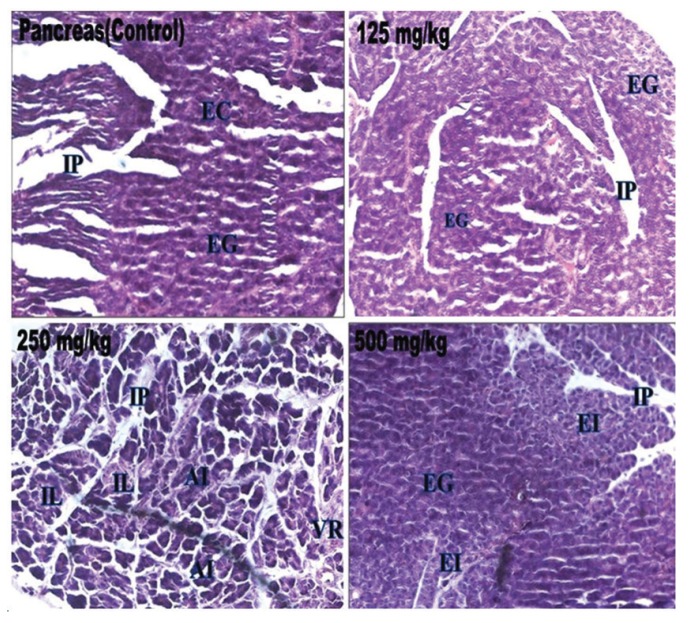
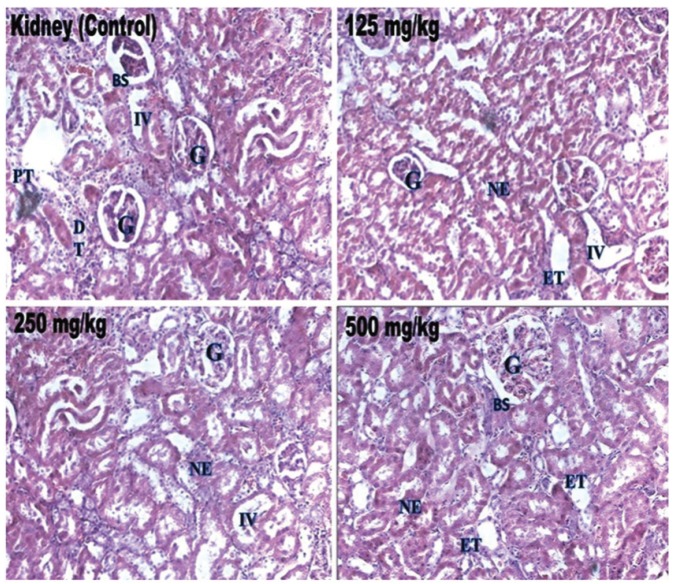
Biochemical parameters such as ALT, ALP, bilirubin, total cholesterol, triglyceride, total protein, glucose, urea, creatinine, sodium, potassium and α-amylase in the treated rats were not significantly different from those in the control group, and all the values remained within the normal limits (Table 4) Histopathological changes were not related to the treatment with petroleum ether extract in any of the analyzed organs. The tissues were morphologically normal. Any changes were slight and similar to those in Wistar rats treated with the vehicle (Figs 2 - 4).
Table. 4. Serum biochemical values for rats in the sub-acute toxicity study for the control group and the groups treated with different doses of the test extract of W. volubilis.
| Parameters | Vehicle | 125 mg/kg | 250 mg/kg | 500 mg/kg |
|---|---|---|---|---|
| ALT | 26.32 ± 0.83 | 27.62 ± 0.13 | 28.12 ± 0.72 | 28.89 ± 0.43 |
| ALP | 26.11 ± 0.17 | 26.87 ± 0.03 | 28.12 ± 0.84 | 29.46 ± 0.83 |
| TP | 6.84 ± 0.14 | 8.14 ± 0.08 | 8.39 ± 0.15 | 8.41 ± 0.18 |
| Bilirubin | 0.33 ± 0.02 | 0.38 ± 0.04 | 0.40 ± 0.01 | 0.58 ± 0.03 |
| Total cholesterol | 49.55 ± 0.28 | 53.85 ± 0.64 | 55.35 ± 1.14 | 59.12 ± 1.52 |
| Triglycerides | 52.16 ± 1.35 | 55.46 ± 1.54 | 57.96 ± 1.26 | 64.16 ± 1.04 |
| Urea | 16.33 ± 0.68 | 18.63 ± 0.97 | 18.83 ± 0.37 | 20.33 ± 0.53 |
| Creatinine | 1.58 ± 0.04 | 1.71 ± 0.05 | 1.42 ± 0.03 | 1.73 ± 0.04 |
| Sodium | 108.42 ± 1.72 | 109.72 ± 1.40 | 112.22 ± 1.13 | 119.42 ± 1.26 |
| Potassium | 1.69 ± 0.08 | 2.99 ± 0.04 | 3.24 ± 0.04 | 3.26 ± 0.06 |
| α-amylase | 113.64 ± 1.87 | 114.94 ± 0.14 | 117.44 ± 2.73 | 125.64 ± 1.23 |
| Glucose | 70.53 ± 0.87 | 71.83 ± 1.13 | 74.33 ± 2.09 | 74.53 ± 0.83 |
Data are expressed as means ± SEMs, n = 6, for each group. No statistical difference was found between the control group and the test groups treated with extract of W. volubilis (P < 0.05). ALT, alanine aminotransferase; ALP, alkaline phosphatase; TP, total serum protein.
Fig. 2. Histopathological examinations of rat liver sections from the control rats and from the test rats treated with extract of W. volubilis. H, hepatocytes; Ha, hepatic artery; Pt, portal track.
Fig. 4. Histopathological examinations of rat pancreas sections from the control rats and from the test rats treated with extract of W. volubilis. EG, exocrine gland; IP, interlobular septae; IL, Islets of Langerhans; AI, unatrophied islets; EI, endocrine islets; VR, vascular regeneration.
4. Discussion
Cytotoxicity studies on brine shrimps are considered to be a useful tool for preliminary assessment of in vitro toxicity and are reported to be an efficient, inexpensive and relative rapid way to detect toxic compounds in low amounts [20]. The brine shrimp toxicity study of the extract indicated that the tested petroleum ether extract of W. volubilis had a LC50 value less than 1000 μg/mL, according to the chemical labeling and classification of acute systemic toxicity based on oral LD50 values as recommended by the Organization for Economic Co-operation and Development for a substance to be labeled as non-toxic [21]. The results of our brine shrimps assay indicate that the evaluated concentrations of the extract might be non-toxic.
The oral route of drug administration is the most convenient and commonly used route for toxicity evaluations in pre-clinical animal models. In vivo acute toxicity of petroleum ether extract of W. volubilis was studied in female Swiss albino rats. The evaluation of food intake is important in any study of the safety of an extract with therapeutic purposes because proper nutritional consumption is crucial to the physiological status of the animals and to obtaining a proper response to the substance tested instead of a false response due to nutritional imbalances. Body weight alterations in the physiological and pathological states of humans and animals are very common indices of adverse effects of drugs/chemicals because of abnormal metabolic toxic reactions. Observations were made to study body weight differences between the control rats and the rats treated with the petroleum ether extract of W. volubilis, no changes in body weights were noted, which was concluded to be due to the regular water and food intakes in the rats treated with the petroleum ether extract of W. volubilis. In the acute toxicity tests, the result shows that the tested petroleum ether extract of W. volubilis did not induce any toxic effect due to its non-toxic properties.
Fig. 3. Histopathological examinations of rat kidney sections from the control rats and from the test rats treated with extract of W. volubilis. G, glomerulus; BS, Bowman space; PT, proximal tubule; DT, distal tubule; IV, interstitial vessel; ET, epithelium of tubules; NE, normal epithelium.
Based on the acute toxicity of the petroleum ether extract of W. volubilis, the doses to be evaluated in the sub-acute toxicity study for 28 days of repeated doses were found to be 125, 250 and 500 mg/kg. The 28-day toxicity test has been accepted in practice for a sub-acute oral toxicity study. Sub-acute oral toxicity studies have been applied in safety assessment studies to provide safety information prior to the commercialization of certain products [22, 23]. In this research, sub-acute toxicity evaluations were made using absolute organ weights, serum biochemical parameters such as ALT, ALP, bilirubin, total protein, total cholesterol, triglycerides, glucose, urea, creatinine, sodium and potassium and α-amylase, and histopathology of isolated organs. The absolute weights of all the isolated organs (liver, kidney and pancreas) between the treated and the control groups remained normal, indicating that the petroleum ether extract of W. volubilis was non-toxic in these organs.
Assessments of liver function are made by estimating the activities of elevated serum alanine transaminase, alkaline phosphatase and bilirubin, because these molecules leak into the blood stream in compliance with the extent of liver damages [24]. Alanine transaminase, which mediates the conversion of alanine to pyruvate and glutamate, is specific to the liver and is a suitable indicator of hepatic injuries. Increased levels of this enzyme are an indicator of cellular infiltration and functional disturbance of liver- cell membranes. Alkaline phosphatase is membrane bound, and its alteration is likely to affect the membrane’s permeability and produce derangement in the transport of metabolites. On the other hand, bilirubin is associated with the function of hepatic cells. In the current study, no significant elevations in markers of liver injury (ALT, ALP and bilirubin) were observed in the rats treated with petroleum ether extract of W. volubilis.
Inefficient scavenging of reactive oxygen species might be implicated in the oxidative inactivation of enzymes, especially the deleterious effects, due to accumulation of superoxide radicals. The generation of hydroxyl radicals from the superoxide-anion Haber-Weiss reaction, resulting in peroxidation of membrane lipids and in protein glycation, leads to oxidative damage in biomolecules such as carbohydrates, proteins, DNA and cellular organelles. A variety of derangements in metabolic and regulatory mechanisms due to insulin deficiency - elevated free fatty acids levels decrease insulin sensitivity because of pancreatic lipotoxicity/inhibition of insulin - stimulated glucose transport (glucose transport plays an essential role in free fatty acid gene expression for carbohydrates and lipids metabolism) - are also responsible for accumulation of lipids, and this may be due to a higher activity of cholesterol biosynthesis enzymes (HMG-COA reductase) or high levels of lipolysis.
Due to lipolysis, the visceral adipose deposition of elevated free fatty acids from adipose tissues to non-adipose tissues (liver, skeletal muscle) may lead to excessive endogenous glucose production. Triglyceridemia is also associated with the metabolic consequences of hyperinsulinemia, insulin resistance and glucose tolerance.
Glucose is the primary and an essential source of energy for the cells in the body. The blood-glucose level in the body is tightly regulated and is maintained at approximately 5 mmol/L. Not maintaining this level leads to chronically high (hyperglycemia) or low (hypoglycemia) glucose levels. Selective destruction of the β-cells of the pancreas by generating excess reactive oxygen species in order to break DNA bases by alkylation leads to diabetogenic activity. Increased total cholesterol and triglyceride levels represent a displayed lipid profile known as an atherogenic profile, uptake of oxidized form of lipids by cellular macrophages results in the formation of foam cells, and vascular endothelial cholesterol accumulation, which promotes the development of characteristic fatty streaks found in atherosclerotic lesions, leads to the development of coronary heart disease. During oxidative stress, reactive oxygen species have a strong oxidizing ability to damage cellular proteins, which causes loss of body weight due to increased muscle destructions and loss of protein content in the tissue, and subsequently changes in serum levels.
The reduction is attributed to the damage produced locally in the endoplasmic reticulum, which results in the loss of cytochrome P 450 and leads to its functional failure, resulting in decreased protein synthesis and increased accumulation of triglycerides during glycation of lipoprotein lipase deficiency and possibly contributing to a significant elevation of triglycerides. This action could be due to microproteinuria and increased protein catabolism, which are important clinical markers in essential organ failure [25, 26]. These results raised and confirmed a number of interesting issues with petroleum ether extract of W. volubilis with respect to hepatic markers, serum cholesterol and glucose. In this study, all the experimental values were in the normal range when compared to the values for the control group.
The kidneys are the primary means for eliminating waste products of metabolism that are no longer needed by the body; these products, non-protein nitrogen’s, include urea (from the metabolism of amino acid) and creatinine (from muscle creatinine). The concentrations of these, particularly of urea, can be as high as 10 times normal for 1 to 2 weeks, and this total condition is called uremia/total renal failure. It would be reasonable to suspect that decreasing the number of functional nephrons, which reduces the glomerular filtration rate (GFR), would also cause major decreases in renal excretion of water and solutes. A reduction in the number of nephrons leads to electrolyte and fluid retention, and death usually ensues when the number of nephrons falls below 5 to 10 percent of normal. In contrast to the electrolytes, many of the waste products of metabolism, such as urea and creatinine, accumulate almost in proportion to the number of nephrons that have been destroyed. The reason for this is that substances such as urea and creatinine depend largely on glomerular filtration for their excretion, and they are not reabsorbed as avidly as the electrolytes. Therefore, if the GFR decreases, the creatinine excretion rate also transiently decreases, causing accumulation of creatinine in the body fluids and raising the plasma concentration until the excretion rate of creatinine returns to normal. However, this normal rate of creatinine excretion occurs at the expense of an elevated plasma creatinine concentration. Effects of petroleum ether extract of W. volubilis with respect to kidney functions, all the experimental values were in normal.
Excretions of excessive cellular inorganic ions (sodium and potassium) are common biological parameters in cases of diabetic ketoacidosis, which leads to tissue dehydration and abnormal levels of electrolytes. Excretion of elevated levels of serum inorganic ions from intra and extra cellular spaces occurs for normal electrolyte equilibrium, but does not in cases of insulin deficiency, hypertonicity and acidemia [27]. Increased fatty acid oxidation is a characteristic of starvation and of diabetes mellitus, leading to ketone body production by the liver (ketosis). Ketone bodies are acidic, and when produced in excess over long periods, as in diabetes, cause ketoacidosis, which is ultimately fatal. In the present study, no significant elevations in cellular inorganic ions were observed with petroleum ether extract of W. volubilis administration.
Elevated levels of pancreatic amylase are common in diabetic ketoacidosis (DKA). Pancreatic acinar cells are responsible for secretion of pancreatic amylase, which is a key enzyme in the digestive system and catalyses the hydrolysis of starch to a mixture of smaller oligosaccharides such as maltose, maltotriose, and oligoglucans [28]. Pancreatic amylase levels were in normal range in the rats treated with petroleum ether extract of W. volubilis. Inhibition of pancreatic amylase delays carbohydrate digestion and protracts the overall carbohydrate digestion time, resulting in a reduction in the glucose absorption rate and, consequently, a dulling of the post-prandial plasma glucose rise. Therefore, a few high-quality studies support the use of amylase inhibition for weight loss in human. The serum biochemical parameters in the rats treated with petroleum ether extract of W. volubilis for 28 days did not exhibit any significant changes as compared with those in the control group, and all the values remained within normal limits.
5. Conclusion
This is the first study to investigate the toxicity of petroleum ether extract of W. volubilis in brine shrimp and Wistar female rats for acute and sub-acute toxicity tests in order to conduct and establish the adverse effects of petroleum ether extract of W. volubilis in a dose-dependent manner. The results suggest that petroleum ether extract of W. volubilis has a non-toxic effect in Wistar female rats.
Acknowledgments
The authors wish to thank the University Grants Commission (UGC), New Delhi, for providing the financial support as UGC major research project for conducting the experiments (file no. F. No. 37-211/2009 (SR). The authors gratefully acknowledged the authorities of Jadavpur University for providing necessary facilities.
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest
References
- 1.Da Costa, JM, Campos AR, Brito SA, Pereira CKB, Souza EO, Rodrigues FFG. Biological screening of araripe basin medicinal plants using artemia salina leach and pathogenic bacteria. Pharmacogn Mag. 2010;6(24):331–334. doi: 10.4103/0973-1296.71792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panda N, Banerjee S, Mandal NB, Sahu NP. Pregnane glycosides. Nat Prod Comm. 2006;3:665–669. [Google Scholar]
- 3.Kirtikar KR, Basu BD. India medicinal plants, Bishen Singh Mahendrapal Singh. Dehradun. 2003;3:1635. [Google Scholar]
- 4.Ayyanar M, Sankara sivaraman, K, Ignacimuthu S. Traditional herbal medicines used for the treatment of diabetes among two major tribal groups in south Tamil Nadu, India. Ethno Botanical Leaflet. 2008;12:276–280. [Google Scholar]
- 5.Yoshimura S, Narita H, Hayashi K, Mitsuhashi H. Studies on the constituents of asclepiadaceae plants. LVI. isolation of new antitumor-active glycosides from dregea volubilis (L.) BENTH. Chem Pharm Bull. 1983;31(11):3971–3983. doi: 10.1248/cpb.31.3971. [DOI] [PubMed] [Google Scholar]
- 6.Biswas M, Bera S, Biswakanth K, Karan TK, Bhattacharya S, Ghosh AK et al. Antitumor effect of dregea volubilis fruit in ehrlich ascites carcinoma bearing mice. Global J Pharmacol. 2010;4:102–106. [Google Scholar]
- 7.Biswas M, Biswas K, Ghosh AK, Haldar PK. A pentacyclic triterpenoid possessing analgesic activity from the fruits of dregea volubilis. Pharmacogn Mag. 2009;5:90–92. [Google Scholar]
- 8.Arun kumar, R, Ali Ahmed, AB, Venkateshvaran, Mani P, John bastin., TMM Anti-hyperlipidemic and hypoglycemic activity of wattakaka volubilis methanol extract in alloxan- induced diabetic rats. J Pharma Res. 2010;3:1913–1915. [Google Scholar]
- 9.Velmani G, Vivekananda M, Mandal SC. A critical biochemical assessment on the antihyperglycemic activity of aqueous fraction of wattakaka volubilis supported by antioxidant defense. Orient Pharm Exp Med. 2014;14(1):15–24. [Google Scholar]
- 10.Hossain E, Sarkar D, Maiti A, Chatterjee M, Mandal SC, Gupta JK. Anti-inflammatory effect of a methanolic extract of leaves of dregea volubilis. J Ethnopharmacol. 2010;132(2):525–528. doi: 10.1016/j.jep.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Nandi D, Elizabeth Besra, S, Vedasiromoni JR, Giri SV, Prince R, Parasuraman J. Anti-leukemic activityof wattakaka volubilis leaf extract against human myeloid leukemia cell lines. J Ethnopharmacol. 2012;144(2):466–473. doi: 10.1016/j.jep.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK. Anthelmintic effect of a methanol extract of leaves of dregea volubilis on paramphistomum explanatum. Parasitol Res. 2012;110(2):809–814. doi: 10.1007/s00436-011-2558-2. [DOI] [PubMed] [Google Scholar]
- 13.Meyer B, Ferrigni N, Putnam J, Jacobsen L, Nichols D, McLaughlin J. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica. 1982;45(5):31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 14.Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using artemia salina. Planta Medica. 1993;59(3):250–252. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 15.Cepleanu F, Hamburger M, Sordat B, Msonthi J, Gupta M, Saadou M et al. Screening of tropical medicinal plants for molluscicidal, larvicidal, fungicidal and cytotoxic activities and brine shrimp toxicity. Pharm Biol. 1994;32(3):294–307. doi: 10.3109/13880209409083007. [DOI] [Google Scholar]
- 16.Suresh Kumar, RB, Puratchikodi A, Angelene Prasanna, Narayan Dolai, Piyali Majumder, Mazumder UK et al. 2011. Pre clinical studies of streblus asper lour in terms of behavioural safety and toxicity. Orient Pharm Exp Med. 2011;11(4):243–249. [Google Scholar]
- 17.OECD. OECD guide lines for the testing of chemicals / section 4: health effects test no 423: Acute oral toxicity–Acute toxic class method. OECD; Paris: 2002. [Google Scholar]
- 18.OECD. Paris. Guide-line for testing of chemicals. Guideline 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. 1995
- 19.Malek, D, Malley L, Slone T, Elliott G, Kennedy G, Mellert W et al. Repeated dose toxicity study (28 days) in rats and mice with N methylpyrrolidone (NMP) Drug Chem Toxicol. 1997;20(1-2):63–77. doi: 10.3109/01480549709011079. [DOI] [PubMed] [Google Scholar]
- 20.Gupta M, Monge A, Karikas G, Lopez de, Cerain, Solis P, De Leon, E et al. Screening of panamanian medicinal plants for brine shrimp toxicity, crown gall tumor inhibition, cytotoxicity and DNA intercalation. Pharm Biol. 1996;34(1):19–27. doi: 10.1076/phbi.34.1.19.13180. [DOI] [Google Scholar]
- 21.Deciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, Castaneda-Corral G, Angeles-Lopez GE, Navarrete A et al. Acute toxicity and mutagenic activity of mexican plants used in traditional medicine. J Ethnopharmacol. 2007;110(2):334–342. doi: 10.1016/j.jep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Arts JH, Muijser H, Appel MJ, Frieke Kuper, C, Bessems JG, Woutersen RA. Subacute (28-day) toxicity of furfural in fischer 344 rats: a comparison of the oral and inhalation route. Food Chem Toxicol. 2004;42(9):1389–1399. doi: 10.1016/j.fct.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Bautista ARPL, Moreira ELT, Batista MS, Miranda MS, Gomes ICS. Subacute toxicity assessment of annatto in rat. Food Chem Toxicol. 2004;42(4):625–629. doi: 10.1016/j.fct.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Wannang NN, Jimam NS, Omale S, Maxwell LPD, Steven SG, Aguiyi JC. Effect of cucumis metuliferus (cucurbitaceae) fruits on enzymes and haematological parameters in albino rats. Afr J Biotechnol. 2007;6(22):2515–2518. [Google Scholar]
- 25.Mauer SM, Steffes MW, Brown DM. The kidney in diabetes. Am J Med. 1981;70(3):603–612. doi: 10.1016/0002-9343(81)90582-9. [DOI] [PubMed] [Google Scholar]
- 26.Almadal TP, Vilstrup H. Strict insulin treatment normalizes the organic nitrogen contents and the capacity of urea–N synthesis in experimental diabetes in rats. Diabetologica. 1988;31(2):114–118. doi: 10.1007/BF00395558. [DOI] [PubMed] [Google Scholar]
- 27.Kerl ME. Diabetic ketoacidosis: pathophysiology and clinical laboratory presentation. Comp Cont Educ Pract Vet. 2001;23(3):220–229. [Google Scholar]
- 28.Tarling CA, Woods K, Zhang R, Brastianos HC, Brayer GD, Andersen RJ et al. The search for novel human pancreatic alpha-amylase inhibitors: high-throughput screening of terrestrial and marine natural product extracts. Chem Bio Chem. 2008;9(3):433–438. doi: 10.1002/cbic.200700470. [DOI] [PubMed] [Google Scholar]