Abstract
To estimate exposures to smokers from cigarettes, smoking topography is typically measured and programmed into a smoking machine to mimic human smoking, and the resulting smoke emissions are tested for relative levels of harmful constituents. However, using only the summary puff data—with a fixed puff frequency, volume, and duration—may underestimate or overestimate actual exposure to smoke toxins. In this laboratory study, we used a topography-driven smoking machine that faithfully reproduces a human smoking session and individual human topography data (n = 24) collected during previous clinical research to investigate if replicating the true puff profile (TP) versus the mathematically derived smoothed puff profile (SM) resulted in differences in particle size distributions and selected toxic/carcinogenic organic compounds from mainstream smoke emissions. Particle size distributions were measured using an electrical low pressure impactor, the masses of the size-fractionated fine and ultrafine particles were determined gravimetrically, and the collected particulate was analyzed for selected particle-bound, semivolatile compounds. Volatile compounds were measured in real time using a proton transfer reaction-mass spectrometer. By and large, TP levels for the fine and ultrafine particulate masses as well as particle-bound organic compounds were slightly lower than the SM concentrations. The volatile compounds, by contrast, showed no clear trend. Differences in emissions due to the use of the TP and SM profiles are generally not large enough to warrant abandoning the procedures used to generate the simpler smoothed profile in favor of the true profile.
Introduction
Despite reductions in the measured “tar” and nicotine content of cigarettes over the last 30 years, there has been no decline in tobacco-related disease,1−5 and cigarette smoking continues to be the single most preventable cause of premature death in the United States, accounting for more than 440,000 deaths annually in the United States.6−8 “Low yield” cigarettes, which were designed to reduce the amounts of “tar,” carbon monoxide, nicotine, and other harmful cigarette smoke constituents inhaled by a smoker, require the addicted smoker to change smoking behavior and compensate for the lower yield by taking more frequent and larger puffs, and inhaling the smoke more deeply into the lungs.9 By the time the public health community understood that smoking machine measurements of cigarette smoke chemical yields did not account for differences in human smoking behavior or reproduce human exposure, the low yield cigarettes were firmly established in the minds of the public as safer alternatives. An understanding of how best to evaluate human smoking exposures requires direct high quality, precise, and objective measurements of human smoking behavior to standard tobacco products and reduced nicotine cigarettes.
An approach some researchers have taken to compare delivered dose among different cigarettes is to conduct human testing in which smoking topography (i.e., puff volume, interpuff interval, puff duration, and air flow) is measured and recorded.10−12 The human puffing behavior data is then averaged and programmed into a smoking machine, and the resulting smoke emissions are measured to determine relative levels of harmful smoke constituents.10,11 Puff topography data frequently used in human behavioral smoking studies has been summary data.11,12 This was largely due to the fact that at the time those studies were conducted, topography devices did not provide a means to access the time-resolved continuous puffing data, but instead produced summary topography data for each puff, i.e., puff volume, duration, and flow. The summary data were averaged, and the smoking machine was programmed with the average puff, with smooth, periodic parabolic waveform, and a fixed puff frequency, volume, and duration.
Because humans smoke differently as the cigarette rod is consumed, individual human puffing behavior is rarely uniform, and combustion chemistry is highly nonlinear. Thus, representing smoking behavior with a smoothed periodic waveform may result in a tobacco smoke aerosol with different chemical composition and physical properties than that actually generated and inhaled by the smoker, and the smoker’s exposure to harmful smoke constituents may be over- or underestimated when relying on machine smoking using averaged smoothed puffs. Commercial puff analyzers currently available (e.g., Sodim SPA-D, http://sodim.hauni.com, and CReSS, http://borgwaldt.hauni.com/en) give researchers access to high-resolution (20–50 Hz) time-resolved puffing data which can be uploaded to directly program smoking machines and more accurately mimic human smoking behavior.11
This study was undertaken to determine if equipment and procedures previously used to generate mainstream smoke constituents should be replaced with equipment that captures each smoker’s true puff profile to more accurately reflect human exposure when testing new tobacco products. In this study, we did not use standard smoking regimes, such as the International Organization for Standardization/U.S. Federal Trade Commission (ISO/FTC)13 or Canadian Intense14 methods, to machine smoke the test cigarettes. Instead, we used actual recorded topography profiles obtained previously from individual smokers,15 which were uploaded unmodified, in the case of the true puffing profile (TP), or averaged and manually entered as a single puff volume, duration, and interpuff interval for the number of puffs taken, in the case of the smoothed profile (SM), to program the smoking machine. These two profiles (TP and SM) were used with a topography-driven smoking machine to investigate if replicating the true puffing topography results in differences in fine (>0.1 μm) and ultrafine (≤0.1 μm) particle size distributions and mainstream smoke emissions of selected volatile and semivolatile compounds compared to machine smoking using averaged, smoothed puff profiles. The machine smoking measurements conducted in both the true and smoothed replication modes used a subset of individual specific smokers’ puff topography profiles collected in an independent human exposure study15 that allowed us to evaluate emissions from 24 different topography profiles. Chemical characterization included quantification of several of the volatile and semivolatile chemicals categorized by the U.S. Food and Drug Administration as harmful and potentially harmful constituents (HPHCs).16 Particle size distributions in mainstream smoke were measured using a real-time particle size classifier/collector of size-resolved particulate matter over the ultrafine and fine ranges, for subsequent gravimetric determination and chemical characterization. The size-fractionated particulate that was collected was extracted and analyzed chemically for selected toxic/carcinogenic tobacco-specific nitrosamines (TSNAs), polycyclic aromatic hydrocarbons (PAHs), and other selected semivolatile organic compounds (SVOCs). We also measured six smoke-related volatile organic compounds (VOCs) on a puff-by-puff basis using the same profiles. Formaldehyde, which could not be measured using this technique, was sampled and quantified separately.
EXPERIMENTAL PROCEDURES
Puff Profiles
The smoking topography profiles used for the comparisons were obtained from a separate within-subject crossover study in which 45 subjects each smoked two conventional cigarettes (GPC Ultra Light and GPC Full Flavor) and two reduced nicotine cigarettes during each of four separate laboratory test visits.15 This laboratory study used only the profiles obtained with the conventional cigarettes. To ensure that sampling order had no effect on smoking behavior, each subject was randomly assigned one of the four designated cigarette types during each session. Only one cigarette type was smoked during any exposure session (four cigarettes per session). Subjects in the previous crossover study were healthy smokers aged 18 to 30 years. All had been screened before they were accepted into the study to ensure that they were established smokers of full flavor or light cigarettes, who smoked at least 20 cigarettes per day every day, and had smoked for at least the previous six months. They were excluded from participation if they had respiratory illnesses, used tobacco in any other form, were or could be pregnant, or were trying to quit smoking. None of the participants had previously used any of the test cigarette types; two were menthol smokers.
During each visit, the subjects smoked the cigarettes through a holder attached to a Sodim SPA-D Smoking Puff Analyzer (Sodim SAS, Fleury-les-Aubrais, France). This is a transducer-based smoking topography data collection device that captures and digitally records true puff profiles at a frequency of 20 Hz and produces summary reports of individual puff volumes, durations, flow rates, and interpuff intervals. The subjects smoked the test cigarettes while their true (full) puff profiles were recorded using the SPA-D Smoking Puff Analyzer. Further details on the operation of the device are available elsewhere.17
Subject-specific puff profiles selected for use in this study were taken from smoking sessions conducted only with high-tar GPC Full Flavor (FF) king-size filter hard-pack (15.2 mg tar, 0.97 mg nicotine, 0% filter ventilation per cigarette) and low-tar GPC Ultra Light (UL) king-size filter soft-pack (5.8 mg tar, 0.51 mg nicotine, 51.5% filter ventilation per cigarette) cigarettes.18,19 Use of the profiles obtained with the GPC cigarettes allowed us (1) to focus on extremes with respect to machine-smoked tar (if these extreme conditions did not result in measurable differences when comparing TP with SM profiles, then intermediate conditions were unlikely to be of interest); and (2) to obtain greater consistency by focusing on nonmenthol cigarettes only. Although GPC is regarded as a “deep discount” cigarette brand,20,21 because modern commercial cigarettes are so highly engineered and uniform in production, the discounted stature of brands may have less to do with the quality of the product than with the image that the manufacturer associates with it.21 Also, a comparison of manufacturer’s measured smoke yields22 for a premium (Marlboro Light) and a deep discount brand (GPC Ultra Light) shows no greater variability in nicotine yields for the latter, as shown in Figure S1 (Supporting Information). Furthermore, the comparative nature of our study required that the same brand be smoked using both the TP and SM profiles, so any variability in the quality of the brand would likely affect both data sets equally.
Profiles collected in the previous study from subjects smoking the FF cigarettes were rank ordered by total puff volume, then the puff volumes were divided into tertiles (High, Medium, Low), and six profiles were randomly sampled from the High and the Low tertiles. The Medium tertile profiles were excluded to concentrate on the extremes with respect to total puff volume. We repeated this procedure for the profiles collected from the subjects smoking the UL cigarettes. The 24 puff profiles selected consisted of six High and six Low tertile profiles obtained with each of the FF and UL cigarettes. Details of the puff profile characteristics are provided as Supporting Information in Table S1.
Protocol and Procedures
Differences in smoke emissions as a function of the true or smoothed puff profile were measured by loading the prerecorded true puff profiles or manually entering their mathematically derived smoothed puff profiles into the linear smoking machine and smoking the FF or UL cigarettes to generate the emissions. The smoke generation procedures and those used to measure particle size distributions and VOCs on a puff-by-puff basis are summarized below. In each case, total emissions were estimated from the areas under the individual concentration/time curves acquired during each run. Size-fractionated smoke aerosol particulate collected with the particle impactor was analyzed chemically for SVOCs. Each of the 24 selected puff profiles was used in three successive machine smoking sessions, five cigarettes per session for particle collection, and three cigarettes per session for VOCs monitoring.
Each previously recorded subject-specific, true puff profile from the Sodim SPA-D puff analyzer was used to drive a Hawktech FP2000 Smoking Machine (Tri-City Machine Works, VA), which replicated the profile in its entirety so that the cigarette, in effect, was “sampled” in the same way that the subject had “sampled” the mainstream smoke when smoking each cigarette. In this way, mainstream smoke was generated exactly as it had been inhaled by the smoker. The process was repeated with the same cigarette brand and style using only the corresponding smoothed measures in the puff replicator, i.e., the total puff volume divided by the number of puffs for an average puff volume, with average puff duration and fixed interpuff intervals, which were mathematically derived from the previously collected full-profile data. The replicator was thus programmed to reproduce the smoothed summary profile with steady, periodic parabolic waveform. Calibrations of the smoking machine and puff analyzer were performed each day before use.
Mainstream smoke from the smoking machine was directed into the sampling inlet of the Electrical Low Pressure Impactor (ELPI, Dekati, Ltd., Finland) and the Compact Proton Transfer Reaction-Mass Spectrometer (PTR-MS, Ionicon, Innsbruck, Austria). In a session with a given brand and topography, one cigarette was smoked at high dilution with the ELPI charger on to determine the particle size distribution and concentration (with the ELPI). Four cigarettes were then smoked with the ELPI charger off, and particles were collected on the ELPI impactor plates for subsequent extraction and quantitation of the SVOCs of interest. In a separate session, three cigarettes were analyzed directly for target VOCs (with the PTR-MS) and formaldehyde (using DNPH cartridges).
Selection of Target Smoke Toxins
To compare mainstream smoke emissions generated with the TP and SM puff profiles, we selected representative VOC and SVOC toxins/carcinogens found in mainstream tobacco smoke, the majority of which are classified as HPHCs. The mainstream smoke produced by a burning cigarette and inhaled by a smoker is an aerosol that contains many suspended smoke particles and an associated gas phase. All of the VOCs and SVOCs found in tobacco smoke tend to partition between the particulate phase and the gas phase.23,24 This partitioning is compound-dependent, as are the mechanisms that govern the deposition of each smoke constituent in the respiratory tract.24 The uptake of the compounds found in mainstream smoke is therefore fundamentally dependent on the gas/particle partitioning process. The vapor pressure of a compound plays a primary role in determining its gas/particle partitioning. Thus, highly volatile compounds, such as acetaldehyde and 1,3-butadiene, exist mostly in the gas phase of mainstream smoke, whereas less volatile compounds, such as the TSNAs and PAHs, occur predominantly in the particle phase.
In light of these factors and other recommendations,25 representative vapor and particle-phase target compounds were selected (1) to cover a wide range of vapor pressures; (2) for their prevalence in mainstream smoke; (3) for their toxicity and potential adverse effects on human health; and/or (4) for their role as tobacco smoke markers. The VOCs were acetaldehyde, acetonitrile, acrylonitrile, benzene, 1,3-butadiene, 2,5-dimethylfuran, and formaldehyde; SVOCs comprised nicotine, cotinine, the PAHs pyrene and benzo[a]pyrene (BaP), quinoline, and the TSNAs N-nitrosoanatabine (NAT), N-nitrosonornicotine (NNN), N-nitrosoanabasine (NAB), and 4-(N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone (NNK).
Particulate and SVOC Measurements
We measured the particle size distributions and concentrations in machine-generated subject-specific mainstream smoke with an ELPI. The principal components of the ELPI include a unipolar corona discharge, a 13-stage low-pressure impactor, with electrically isolated collection stages and a series of electrometers, which together provide real-time particle size classification and quantification of particulate matter in the size range from 7 nm to 4 μm. Particles entering the ELPI are first bombarded with ions created by the corona discharge and the charged particles pass into the low pressure impactor, where their collection at each impactor stage depends on their aerodynamic size. As each particle is collected, its charge is absorbed by the electrically isolated collection plate, which is connected to a highly sensitive electrometer. A full description of the operation of the ELPI and its interface with the smoking machine is available elsewhere.17
Using precleaned, silanized, and preweighed aluminum foil substrates, we divided the four sets of ELPI substrates obtained from each smoking machine session, using a true or smoothed puff profile, into fine (0.093 < aerodynamic diameter ≤3.930 μm, nine substrates) and ultrafine (aerodynamic diameter ≤0.093 μm, four substrates) particulate fractions. We made gravimetric measurements with an analytical microbalance in a humidity- and temperature-controlled room on the fine substrates corresponding to cutpoints at 0.257, 0.375, 0.602 μm, and the remaining six fine cutpoints were combined. Particulate yields were determined gravimetrically from the SM- and TP-derived mainstream smoke samples collected with the ELPI in impactor-only mode (charger OFF).17 To estimate particulate mass from ELPI electronic data, the areas under the curves corresponding to the cutpoints from the ELPI were integrated and multiplied by the sample flow rate to give the mass of particles collected. The limit of detection (LOD), or three times the estimated standard deviation, for the microbalance was 27 μg, as determined using a standard ISO method26 and data from seven sets of three laboratory blanks weighed throughout the study. For each sample set, both the combined ELPI-substrate fine and combined ultrafine samples were individually extracted and analyzed for the target SVOCs. Chemical analysis was based on Soxhlet extraction followed by solid phase extraction and analysis using gas chromatography/mass spectrometry (GC/MS).27,28 The ELPI was used to collect mainstream smoke particulate instead of the more conventional Cambridge filter pad collection method because it allowed us to size-fractionate the particulate material, which is not possible when using a Cambridge filter.
VOC Measurements
The FP2000 smoking machine, the Compact PTR-MS, and the interface between the two units are described in detail elsewhere.29 The test cigarettes were smoked using actual topography profiles from individual smokers that were recorded in an earlier study.15 The interface dilutes the mainstream smoke from each cigarette so that the total concentration of the sample is reduced to a level that is compatible with the detector response range of the PTR-MS.
The PTR-MS uses proton transfer to measure VOCs present in the mainstream smoke directly in real-time on a puff-by-puff basis.29,30 The instrument consists of three main components: an ion source, a drift tube, and a quadrupole mass analyzer attached to an ion detector/amplifier. Pure water vapor within the ion source, a hollow cathode discharge, results in the production of high concentrations of H3O+ primary reactant ions. These primary ions pass into the drift tube, where they undergo mostly nondissociative proton transfer to the VOCs. The mainstream smoke sample is introduced into the drift tube close to its entrance at a typical flow rate of ∼75 mL/min. Trace-level organics can be monitored with the instrument within seconds with a detection limit of a few parts-per-billion by volume (ppbv).
We were unable to measure formaldehyde reliably with the PTR-MS because its proton affinity is only slightly higher than that of water. As a result, the back-reaction between protonated formaldehyde and the water present in a humid mixture is significant and the resulting PTR-MS sensitivity for formaldehyde is low.31,32 To overcome this limitation, we used a standard (“batch”) sample collection and analysis method33 in which the mainstream smoke sample was drawn at a rate of about 1 L/min through a solid sorbent cartridge (Supelco LpDNPH S10, catalog no. 21014) impregnated with acidic 2,4-dinitrophenylhydrazine (DNPH) reagent. After sampling, the cartridges were extracted with acetonitrile, and the DNPH-formaldehyde derivative was analyzed using isocratic reverse-phase high-performance liquid chromatography with an ultraviolet absorption detector operated at 360 nm. Total carbonyl capacity of the cartridges was 75 μg; the detection limit was 0.03 μg.
Data Processing and Analysis
The procedures used to estimate the levels of particle-bound SVOCs in the GC/MS data, and the total yields of the VOCs in the gas phase of the mainstream smoke from the machine smoked cigarettes have been described in detail elsewhere.17,29 Briefly, each SVOC was quantified by comparing the integrated ion current response of the target ion to that of the internal standard using the average response factor of the target analyte generated from a standard calibration curve.28 The average yield and 95% confidence interval for each analyte was expressed in ng or μg per session. For the VOC levels in each cigarette, we integrated and summed the areas under the individual peaks of the ion mass signals from the PTR-MS data.29 Because a purpose of the study was to compare changes in VOC responses resulting from the use of true or smoothed profiles obtained under the same operating conditions, we did not convert the summed peak areas associated with each compound to yield per cigarette.
Mixed models analysis was used to assess the differences between the two profile types (smoothed, SM vs true, TP) for all outcomes. Because nearly all outcomes in this study were not normally distributed, they were first log-transformed to meet the normality assumption. The use of mixed models accounts for the correlated data within the same puff profile. Other factors also included in the models were cigarette type (UL vs FF), tertile classification (High vs Low), session number (1, 2, 3, except for SVOC ultrafine outcomes), and cigarette number (1 to 5, only for topography outcomes). We tested the main effects of profile type as well as the interaction effects between profile type with cigarette type and tertile classification. Interactions between profile type and tertile classification were significant only for four outcomes, namely, puff duration, average peak flow, interpuff interval, and nicotine in fine particulate, and the interaction between profile type and cigarette type was significant only for interpuff interval. For these outcomes, regardless of the interaction terms in the models, the main effect results for the profile type remain the same. In the results that follow, p-values that are significant at the 0.05 significance level are shown with an asterisk. Because this was an exploratory study (not hypothesis testing), we report the p-values without adjustment for multiple testing.
Results
Topographic, Gravimetric, and Size-Specific Particulate Data
The topography values acquired during machine smoking are summarized in Table 1. Table 1 briefly summarizes the geometric mean values (±95% confidence intervals, CI) obtained for the replication of the previously collected behavioral data (TP) and the mathematically derived behavioral data (SM), with geometric mean ratios and associated p-values assessing statistically significant differences between the SM and TP profiles. Supporting Information Table S2 provides a detailed summary of all topography measures obtained during machine smoking.
Table 1. Topography Measures Collected during Machine-Smoking Replication of Human Profiles: Geometric Means (95% CIa).
| geometric
mean (95% CI)b |
||||
|---|---|---|---|---|
| description | TPc | SMc | geo. mean ratio (TP/SM) | p-value |
| puff volume (mL) | 55.57 (53.71, 57.49) | 55.62 (53.75, 57.56) | 1.00 | 0.6767 |
| total puffing volume (mL) | 643.8 (627.2,660.9) | 644.4 (627.8, 661.6) | 1.00 | 0.7856 |
| puff duration (s) | 2.119 (2.042, 2.198) | 2.101 (2.024, 2.180) | 1.01 | <0.0001* |
| interpuff interval (s) | 19.29 (18.48, 20.14) | 18.93 (18.10, 19.80) | 1.02 | <0.0001* |
| total puff duration (s) | 24.55 (23.77, 25.35) | 24.34 (23.55, 25.15) | 1.01 | 0.0095* |
| average flow (mL/s) | 27.20 (26.22, 28.21) | 26.94 (25.98, 27.94) | 1.01 | 0.0024* |
| average peak flow (mL/s) | 41.00 (39.48, 42.58) | 41.39 (39.74, 43.10) | 0.99 | 0.1312 |
95% CI = 95% confidence interval.
Sample size = 706, using all profiles.
TP = true profile; SM = smoothed profile.
Significant at p ≤ 0.05 level.
Table 2 and Supporting Information Table S3 present the geometric mean values (±95% CI) obtained from the size-fractionated fine and ultrafine particulate gravimetric microbalance data as well as the integrated ELPI data. Each fine particulate gravimetric session consisted of five cigarettes: one run at high dilution (to collect ELPI particle size distribution data) and the remaining four run at low dilution with the ELPI charger turned off (to maximize the collection of particulate material that was composited for chemical analysis).17 For the ultrafines, one session consisted of three cigarettes run at high dilution (with ELPI charger on) and three sessions of four cigarettes each run at low dilution (with ELPI charger off). This was to ensure that we obtained detectable levels of all particle-bound target compounds in both the fine and ultrafine particulate fractions. The integrated ELPI data in Tables 2 and S3 were obtained via the ELPI software, which displays and stores individual real-time traces, one for each of the 13 particle size cutpoints. For each cigarette smoked, the area under the curve was integrated and multiplied by the sample flow rate to give the mass of particles collected.17 The masses corresponding to the individual cutpoints and the summed masses are included in Tables 2 and S3, along with the geometric mean ratios of the TP-to-SM masses.
Table 2. Size-Fractionated, Gravimetrically-Determined, and Integrated ELPI Fine and Ultrafine Particulate Mass from Mainstream Smoke: Geometric Means (95% CIa).
| geometric
mean (95% CI) |
|||||
|---|---|---|---|---|---|
| description | cut point (μm)b | TPc | SMc | geo. mean ratio (TP/SM) | p-value |
| gravimetric microbalance datad | |||||
| fine particulate (mg/session) | 0.257 | 11.02 (9.98, 12.17) | 11.92 (10.80, 13.15) | 0.92 | 0.0024* |
| 0.375 | 6.759 (6.293, 7.259) | 7.293 (6.793, 7.830) | 0.93 | 0.0015* | |
| 0.602 | 6.556 (6.130, 7.011) | 6.928 (6.522, 7.338) | 0.95 | 0.0178* | |
| other fine | 5.693 (5.203, 6.230) | 5.965 (5.546, 6.415) | 0.95 | 0.0779 | |
| sum | 30.38 (28.14, 32.81) | 32.50 (30.29, 34.88) | 0.93 | 0.0002* | |
| ultrafine particulate (μg/session) | sum | 154.6 (136.6, 174.9) | 161.5 (145.2, 179.8) | 0.96 | 0.4215 |
| integrated ELPI datae | |||||
| fine particulate (mg/run) | 0.093 | 0.00110 (0.00096, 0.00126) | 0.00120 (0.00105, 0.00136) | 0.92 | 0.1543 |
| 0.153 | 0.00496 (0.00432, 0.00570) | 0.00528 (0.00459, 0.00606) | 0.94 | 0.2547 | |
| 0.257 | 0.0318 (0.0275, 0.0369) | 0.0334 (0.0290, 0.0384) | 0.95 | 0.3697 | |
| 0.375 | 0.124 (0.108, 0.142) | 0.130 (0.114, 0.149) | 0.95 | 0.3404 | |
| 0.602 | 0.111 (0.096, 0.128) | 0.116 (0.101, 0.133) | 0.96 | 0.4565 | |
| 0.932 | 0.096 (0.083, 0.110) | 0.097 (0.085, 0.111) | 0.99 | 0.7692 | |
| 1.57 | 0.095 (0.083, 0.108) | 0.101 (0.089, 0.114) | 0.94 | 0.3543 | |
| sum | 0.467 (0.407, 0.535) | 0.488 (0.429, 0.555) | 0.96 | 0.4490 | |
| ultrafine particulate (mg/run) | 0.007 | 4.51 × 10–5 (3.73 × 10–5, 5.44 × 10–5) | 5.13 × 10–5 (4.40 × 10–5, 5.98 × 10–5) | 0.88 | 0.1455 |
| 0.027 | 6.72 × 10–5 (5.88 × 10–5, 7.70 × 10–5) | 7.19 × 10–5 (6.26 × 10–5, 8.28 × 10–5) | 0.93 | 0.3535 | |
| 0.054 | 2.45 × 10–4 (2.16 × 10–4, 2.78 × 10–4) | 2.66 × 10–4 (2.34 × 10–4, 3.04 × 10–4) | 0.92 | 0.1484 | |
| sum | 3.63 × 10–4 (3.19 × 10–4, 4.13 × 10–4) | 3.94 × 10–4 (3.45 × 10–4, 4.50 × 10–4) | 0.92 | 0.1830 | |
95% CI = 95% confidence interval.
The two highest fine particulate cut points were ignored because the mass of particulate matter at each was negligibly small.
TP = true profile; SM = smoothed profile.
Charger OFF. Sample sizes: Fine Particulate Mass = 144, using all profiles; Ultrafine Particulate Mass = 144, using all profiles.
Charger ON. Sample sizes: Fine Particulate Mass = 144, using all profiles; Ultrafine Particulate Mass = 144, using all profiles.
Significant at p ≤ 0.05 level.
Particle-Bound and Gas-Phase Organic Compounds
The data obtained from the chemical analysis of the size-fractionated fine and ultrafine particulate for TSNAs, PAHs, nicotine, and cotinine, collected with the ELPI, were also evaluated and compared in terms of puff profile type. Tables 3 and S4 list the geometric mean values (±95% CI) obtained for the size-fractionated SVOCs for all profiles examined.
Table 3. Size-Fractionated Fine and Ultrafine Particle-Bound SVOCs: Geometric Means (95% CIa).
| geometric
mean (95% CI)b |
||||
|---|---|---|---|---|
| compound | TPc | SMc | geo. mean ratio (TP/SM) | p-value |
| fine particle-bound SVOCs | ||||
| nicotine (μg/session) | 2099 (1910, 2306) | 2246 (2064, 2444) | 0.93 | 0.0021* |
| cotinine (ng/session) | 17 017 (15 793, 18 337) | 17 627 (16 444, 18 896) | 0.97 | 0.0545 |
| pyrene (ng/session) | 346 (325, 369) | 363 (342, 385) | 0.95 | 0.0065* |
| benzo[a]pyrene (ng/session) | 75.4 (69.4, 81.9) | 78.1 (71.8, 85.8) | 0.97 | 0.1032 |
| quinoline (ng/session) | 204 (178, 234) | 217 (191, 247) | 0.94 | 0.0219* |
| NAT (ng/session)d | 781 (709, 859) | 793 (720, 872) | 0.98 | 0.3517 |
| NNN (ng/session)d | 372 (339, 408) | 376 (344, 411) | 0.99 | 0.5857 |
| NAB (ng/session)d | 444 (405, 486) | 445 (405, 488) | 1.00 | 0.9244 |
| NNK (ng/session)d | 402 (365, 444) | 402 (365, 443) | 1.00 | 0.9975 |
| ultrafine particle-bound SVOCse | ||||
| nicotine (μg/session) | 1157 (1122, 1192) | 1167 (1131, 1204) | 0.99 | 0.5383 |
| cotinine (ng/session) | 1172 (982, 1398) | 1217 (1030, 1438) | 0.96 | 0.1272 |
| pyrene (ng/session) | 23.6 (21.4, 26.1) | 24.7 (22.1, 27.5) | 0.96 | 0.0704 |
| benzo[a]pyrene (ng/session) | 4.20 (3.49, 5.06) | 4.36 (3.57, 5.33) | 0.96 | 0.1115 |
| quinoline (ng/session) | 129 (117, 141) | 136 (124, 149) | 0.95 | 0.0034* |
| NAT (ng/session)d | 82.8 (70.8, 96.8) | 84.2 (71.6, 99.0) | 0.98 | 0.3932 |
| NNN (ng/session)d | 29.5 (24.3, 35.7) | 30.2 (25.2, 36.3) | 0.98 | 0.2918 |
| NAB (ng/session)d | 62.0 (52.4, 73.3) | 61.1 (51.2, 72.9) | 1.01 | 0.5104 |
| NNK (ng/session)d | 38.4 (32.0, 46.1) | 38.04 (31.8, 45.5) | 1.01 | 0.7305 |
95% CI = 95% confidence interval.
Sample sizes: Fine Particle-Bound SVOCs = 144, using all profiles; Ultrafine Particle-Bound SVOCs = 48, using all profiles.
TP = true profile; SM = smoothed profile.
NAT = N-Nitrosoanatabine; NNN = N-Nitrosonornicotine; NAB = N-Nitrosoanabasine; NNK = 4-(N-Nitrosomethylamino)-1-(3-pyridyl)-1-butanone.
Summed over three replicate samples.
Significant at p < 0.05 level.
We used the PTR-MS to make real-time measurements in triplicate of six gas-phase VOCs in the machine-generated mainstream smoke on a puff-by-puff basis. The resulting peak areas (in ppbv·s) for each compound from each cigarette were integrated and summed, as described previously.29 Tables 4 and S5 present a summary of the geometric mean values (±95% CI) obtained for all of the target VOCs as well as formaldehyde, which was measured separately, using all of the profiles.
Table 4. Concentrations in Peak Area Units for Gas-Phase VOCs: Geometric Means (95% CIa).
| geometric
mean (95% CI)b |
||||
|---|---|---|---|---|
| compound | TPc | SMc | geo. mean ratio (TP/SM) | p-value |
| acetaldehyde (ppbv·s) | 1 081 242 (928 674, 1 258 876) | 1 078 627 (930 473, 1 250 370) | 1.00 | 0.9505 |
| acetonitrile (ppbv·s) | 231 320 (193 160, 277 019) | 240 574 (203 115, 284 942) | 0.96 | 0.3981 |
| acrylonitrile (ppbv·s) | 18 404 (15 536, 21 803) | 18 689 (15 904, 21 961) | 0.98 | 0.7148 |
| benzene (ppbv·s) | 23 217 (19 813, 27 206) | 23 954 (20 646, 27 791) | 0.97 | 0.3080 |
| 1,3-butadiene (ppbv·s) | 36 533 (31 109, 42 903) | 37 100 (31 844, 43 223) | 0.98 | 0.6652 |
| 2,5-dimethylfuran (ppbv·s) | 14 526 (12 108, 17 428) | 15 528 (13 048, 18 480) | 0.94 | 0.0184* |
| formaldehyde (μg/cartridge) | 0.416 (0.378, 0.458) | 0.380 (0.346, 0.418) | 1.09 | 0.0546 |
95% CI = 95% confidence interval.
Sample size = 144, using all profiles.
TP = true profile; SM = smoothed profile.
Significant at p ≤ 0.05 level.
Discussion
This study is the first to compare the effects on replicated smoking behavior and exposure of simulating the actual puff profile from cigarette smokers with the more commonly used averaged puff profile. Previous studies11,22 have used averaged puff profiles, with smooth, periodic parabolic waveform and a fixed puff frequency, volume, and duration, to conduct machine smoking measurements of puff topography and mainstream smoke constituents. Because that approach may not accurately reflect the chemical composition and physical properties actually generated and inhaled by the smoker, the results may not truly reproduce the smoker’s exposure to potentially harmful smoke constituents. Using individual smokers’ puff topography profiles to drive a smoking machine, we undertook the study to compare the effects of using the true puff profile and the associated smoothed profile on fine and ultrafine particle size distributions and mainstream smoke emissions of selected compounds.
Topographic, Gravimetric, and ELPI Particulate Data
The results in Tables 1 and S2 show that differences in the geometric mean for puff duration, total puff duration, interpuff interval, and average flow were very small but statistically significant between the true and smoothed profiles. The mean values in the smoothed profile were usually smaller than the mean values in the true profile. This is not surprising, as the mathematical derivation of the smoothed profile involves averaging and smoothing out the detail and extremes inherent in the true profile. However, in all three cases, the geometric mean values obtained with the true profiles were higher by only 1–3% when compared with the results obtained with the smoothed profiles. There were no statistically significant differences for the puff volume, total volume inhaled, and average peak flows. These results indicate that our smoke generation and measurement procedures for both the smoothed and true profile-based machine smoking did not introduce significant bias to either profile type.
Tables 2 and S3 show that, for both the gravimetrically determined fine and ultrafine particulate masses, the geometric means obtained with the true profiles were lower by 4–8% with respect to the results obtained with the smoothed profiles. The differences in the geometric means between the true and smoothed profiles were statistically significant for three of the four cut point ranges considered as well as for the summed values. However, none of the differences in the case of the integrated ELPI data was statistically significant, although, again, all of the geometric means were lower by 1–12% when measured with the true profiles. Compared with the results obtained for the smoking topography measures, measurements of the fine and ultrafine particulate mass show a larger overestimate with the smoothed profiles compared with the results obtained with the true profiles.
Particle-Bound and Gas-Phase Organic Compounds
Tables 3 and S4 show that differences in the geometric means between the true and smoothed profiles were statistically significant for three of the fine particle-bound SVOCs examined (nicotine, pyrene, and quinoline) and one of the ultrafine particle-bound SVOCs (quinoline). For nicotine, the geometric means obtained with the true profiles were lower by 7%. To put the magnitude of this difference into perspective, we can compare it to reported differences between constituent levels generated using established smoking regimes. The ISO/FTC puffing regime provided the initial framework for comparison of tar and nicotine yields in 1967.13,34 Since then, researchers have come to a consensus that this puffing regimen underestimates typical smokers’ exposures, especially for highly ventilated cigarettes.35−37 In an attempt to provide a more meaningful framework within which to estimate human uptake for a given cigarette, the tobacco regulatory community has proposed more intensive variants on the ISO/FTC regime, including the Massachusetts Benchmark (MAB)22 and the Canadian Intense (CI)14 regimes. The difference in per cigarette nicotine yield between these three established smoking regimes is roughly 1:2:4 (ISO/FTC:MAB:CI), shown graphically in Supporting Information Figure S1, which is about 10–20 times larger22,38 than that measured for nicotine in fine particulate matter between the true and smoothed profiles in this study.
Overall, except for NAB and NNK, the geometric means were lower by 1–7% when measured with the true profiles than with the smoothed profiles. For fine particle-bound NAB and NNK, the yields were essentially unaffected by the profile used; in the case of the ultrafine particles, the mean yields were only 1% greater with the TP profiles than with the SM profiles.
Only one of the gas-phase VOCs in Tables 4 and S5, 2,5-dimethylfuran, showed a statistically significant difference in the geometric mean concentrations between the true and smoothed profiles. For acetonitrile, acrylonitrile, benzene, 1,3-butadiene, and 2,5-dimethylfuran, the geometric mean values were lower by 2–6% with the true profiles than with the smoothed profiles. On the other hand, the geometric mean formaldehyde concentration was 9% higher with the true profiles, but this difference was not statistically significant and may have been a reflection, at least in part, of differences in the sampling and analysis procedures used to measure this compound in the mainstream smoke compared with the other VOCs.
Statistical Significance in Multiple Testing
The p-values reported in the tables did not take multiple testing into account. Because we conducted multiple testing using the same data sets (see Supporting Information), it is likely that our results with unadjusted p-values show more significant differences than are warranted. For this exploratory study, the results in Tables 1–4 are presented without the adjustments. Had we used the adjusted p-values with Bonferroni correction, two of the compounds (quinoline in the fine particle-bound SVOCs group (Table 3) and 2,5-dimethylfuran in the gas-phase VOCs group (Table 4) would no longer have been significant at p < 0.05. Using adjusted p-values, one might be able to draw conclusions about statistically significant differences between the overall yields generated using TP vs SM profiles.
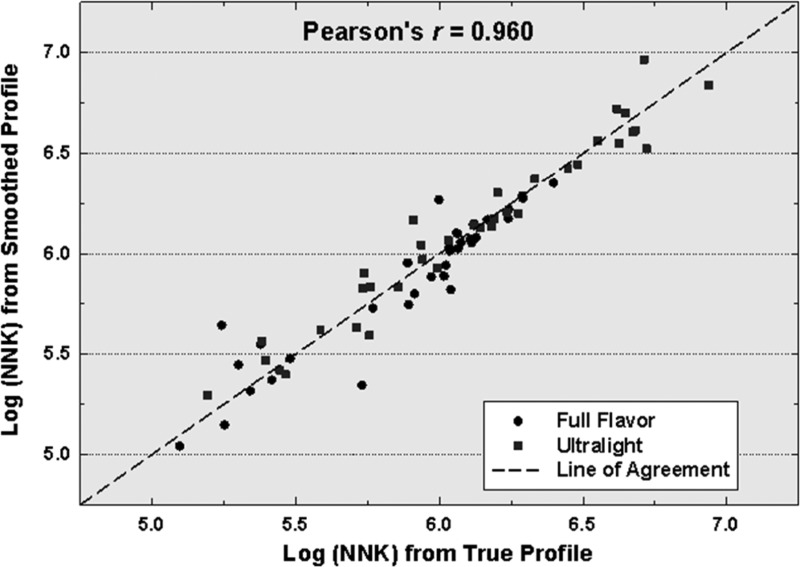
Of the remaining compounds, only fine particle-bound nicotine and pyrene, and ultrafine particle-bound quinoline (Table 3), show relatively small, statistically significant differences (5–7%). The reasons for this are not clear but may arise because the total volume of smoke drawn through the cigarette does not correlate directly with mass of constituent produced for all of the chemicals monitored. More intense puffing (at higher flow rates) has been shown to be consistent with the formation of larger particles and a greater production of some chemical constituents of mainstream smoke but not others.17,39−44
Variation in Analytical Methods Used to Measure SVOCs and VOCs
We calculated the coefficients of variation (CVs) of the measured particulate–bound SVOCs and the VOCs to compare the variability for the compounds in these groups due to the use of the true and smoothed profiles.
For the fine particulate-bound SVOCs, the variation due to the TP profile was slightly greater than that due to the SM profile (7 out of 9 cases), but the differences were very small. The differences in CVs for BaP and NAB between the two profiles were almost identical. The difference in the CVs between the two profiles ranged from 0.1% for BaP to 4.6% for nicotine. The smallest variation was found for pyrene (27.8% for TP vs 25.7% for SM) whereas the largest variation was found for quinoline (64.0% for TP vs 59.7% for SM).
For the ultrafine particulate-bound SVOCs, the variation due to the TP profile was slightly greater than that due to the SM profile for cotinine, quinoline, NNN, and NNK; the variation due to the TP profile was slightly smaller than that due to the SM profile for nicotine, pyrene, BaP, NAT, and NAB. Again, the differences were very small, ranging from 0.2% to 3.8% between the two profiles. The smallest variation occurred with nicotine (7.1% for TP vs 7.4% for SM), whereas the largest variation was found for BaP (46.2% for TP vs 50.0% for SM).
For the VOCs, the variation due to the TP profile was greater than the variation due to the SM profile. The differences in the CVs between the two profiles ranged from 0.6% for formaldehyde to 7.0% for acetonitrile. The smallest variation occurred with formaldehyde (42.9% for TP vs 42.3% for SM), and acetonitrile showed the largest variation (90.7% for TP vs 85.5% for SM).
By and large, there were no substantial differences in variation between the TP and SM profiles for SVOC and VOC measurements. We did, however, observe more variation in the VOC measurements than in the SVOC measurements.
Conclusions
The purpose of this study was to determine if topography measurements made using only the smoothed puff profile to generate mainstream smoke constituents when testing new tobacco products should be replaced with each smoker’s true puff profile to obtain a better measure of human exposure. The study was limited to machine-smoking in the laboratory and did not involve active smokers, only the use of their previously collected true puffing topography profiles that were obtained in a separate study. The topography measures employed indicate that the behavioral outcomes are essentially independent of the form of the profile used (SM vs TP). With few exceptions, the results obtained for fine and ultrafine particulate matter masses as well as particle-bound and gas-phase organic chemical emissions from mainstream smoke show that the use of the SM profile in machine smoking measurements leads to only a modest overestimate of these levels with respect to the TP profile. The overestimate is between 5 and 8% for the individual cut points used to fractionate the gravimetrically determined fine particulate mass and is 7% for the sum of the fine particulate mass. These differences are statistically significant for three of the four cut points and the sum. Similarly, with two exceptions, the use of the SM profile overestimates the fine and ultrafine particle-bound SVOCs by between 1 and 7%, but the overestimate is statistically significant in only three of the fine particle cases and one of the ultrafine-bound SVOCs. The gas-phase VOCs provide a more mixed picture, with five of the seven target compounds resulting in slightly higher concentrations with the SM profile, and only one exhibiting statistical significance.
For machine-generated human topography replications, use of the smoothed profile should suffice because overall differences in mainstream smoke constituents using either method are small. However, when there is a specific need to know the detailed shape of the true profile, the use of the more sophisticated technique that produces the true profile may be warranted.
Acknowledgments
We acknowledge receipt of the 24 full human topography profiles that were used in this study and were previously acquired by Battelle as part of a separate project under Grant Number R01CA129511 with funding from the National Cancer Institute of the National Institutes of Health. We thank Carrie J. Marshall for her important technical contributions to the project. We also thank the reviewers for their helpful comments and suggestions.
Glossary
Abbreviations
- BaP
benzo(a)pyrene
- CI
confidence interval
- DNPH
2,4-dinitrophenylhydrazine
- ELPI
Electrical Low Pressure Impactor
- FF
full flavor
- FTC
U.S. Federal Trade Commission
- GC/MS
gas chromatography/mass spectrometry
- ISO
International Organization for Standardization
- LOD
limit of detection
- NAB
N-nitrosoanabasine
- NAT
N-nitrosoanatabine
- NNK
4-(N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone
- NNN
N-nitrosonornicotine
- PAH
polycyclic aromatic hydrocarbon
- PTR-MS
Proton Transfer Reaction-Mass Spectrometer
- SM
smoothed puff profile
- SPA-D
Smoking Puff Analyzer
- SVOC
semivolatile organic compound
- TP
true puff profile
- TSNA: tobacco-specific nitrosamine
- UL
ultralight
- VOC
volatile organic compound
Supporting Information Available
Supplementary tables of puff profile characteristics, topography measures, fine and ultrafine particulate masses, and SVOCs and VOCs. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written with contributions from all the authors. All authors have given approval to the final version of the manuscript.
The project described was supported by Award Number R21CA133893 from the National Cancer Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- National Cancer Institute. (August 2001) Report of the Lung Cancer Progress Review Group. NIH Publication No. 01–5025. p70.
- Stratton K., Shetty P., Wallace R., Bondurant S. (Eds.) (2001) Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Institute of Medicine (IOM)., National Academy Press, Washington, DC. [PubMed] [Google Scholar]
- Burns D. M., Major J. M., Shanks T. G., Thun M. J., and Samet J. (2001) Smoking lower-yield cigarettes and disease risk. Smoking and Tobacco Control Monograph No. 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine, pp 65–158, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, Washington, DC [Google Scholar]
- Hoffmann D., Hoffmann I. (2001) The changing cigarette: Chemical studies and bioassays. Smoking and Tobacco Control Monograph No. 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine, pp 159–191, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, Washington, DC [Google Scholar]
- Health Canada. (1999) A National Strategy, Health Canada, Ottawa, Ontario: (Accessed at http://www.hc-sc.gc.ca/hc-ps/pubs/tobac-tabac/ns-sn/index-eng.php). [Google Scholar]
- Mokdad A. H.; Marks J. S.; Stroup D. F.; Gerberding J. L. (2004) Actual causes of death in the United States, 2000. JAMA 291, 1238–1245. [DOI] [PubMed] [Google Scholar]
- (2007) (2008) Morbidity and Mortality Weekly Reports (MMWR) 57, 1221–1228. [PubMed] [Google Scholar]
- Woloshin S.; Schwartz L. M.; Welch H. G. (2008) The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J. Natl. Cancer Inst. 100, 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2001) Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine, Bethesda, MD. [Google Scholar]
- Melikian A. A.; Djordjevic M. V.; Chen S.; Richie J. Jr.; Stellman S. D. (2007) Effect of delivered dosage of cigarette smoke toxins on the levels of urinary biomarkers of exposure. Cancer Epidemiol., Biomarkers Prev. 16, 1408–1415. [DOI] [PubMed] [Google Scholar]
- Marian C.; O’Connor R. J.; Djordjevic M. V.; Rees V. W.; Hatsukami D. K.; Shields P. G. (2009) Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol., Biomarkers Prev. 18, 3305–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M. D.; Disharoon S.; Eissenberg T. (2009) Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob. Res. 11, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1967) Cigarettes: Testing for Tar and Nicotine Content. Federal Register 32(147), 11178. [Google Scholar]
- Health Canada (1999) Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke - Official Method, Ottawa, Ontario. [Google Scholar]
- Brinkman M. C., Kim H., Gordon S. M., Chuang J. C., Kroeger R. R., and Clark P. I. (April 27–30, 2009) Smokers and PREPs: Measurement of Inhaled and Exhaled Tobacco Smoke Particulate. Presented at the Joint Conference of the Society for Research on Nicotine and Tobacco and SRNT-Europe, Dublin, Ireland. [Google Scholar]
- U.S. Food and Drug Administration (March 2012) Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List, Silver Spring, MD: (Accessed at http://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm). [Google Scholar]
- Brinkman M. C.; Chuang J. C.; Gordon S. M.; Kim H.; Kroeger R. R.; Polzin G. M.; Richter P. A. (2012) Exposure to and deposition of fine and ultrafine particles in smokers of menthol and nonmenthol cigarettes. Inhal. Toxicol. 24, 255–269. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health (January 16, 1998) 1997 Cigarette Nicotine Disclosure Report (Accessed at http://archives.lib.state.ma.us/bitstream/handle/2452/50461/ocm40773686.pdf?sequence=1).
- U.S. Federal Trade Commission. (2000) Report of Tar, Nicotine, and Carbon Monoxide of the Smoke of 1294 Varieties of Domestic Cigarettes for the Year 1998.
- Chaloupka F. J.; Cummings K. M.; Morley C. P.; Horan J. K. (2002) Tax, price and cigarette smoking: evidence from the tobacco documents and implications for tobacco company marketing strategies. Tob. Control 11(Suppl 1), I62–I72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius M. E.; Driezen P.; Fong G. T.; Chaloupka F. J.; Hyland A.; Bansal-Travers M.; Carpenter M. J.; Cummings K. M. (2014) Trends in the use of premium and discount cigarette brands: findings from the ITC US Surveys (2002–2011). Tob. Control 23(Suppl 1), i48–i53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgerding M. F., Bodnar J. A., and Wingate D. E. (July 24, 2000) The 1999 Massachusetts Benchmark Study: Final Report. Brown & Williamson Tobacco Corporation; Lorillard Tobacco Company; R.J. Reynolds Tobacco Company, Philip Morris USA. [Google Scholar]
- Pankow J. F. (2001) A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol. 14, 1465–1481. [DOI] [PubMed] [Google Scholar]
- Pankow J. F.; Luo W.; Tavakoli A. D.; Chen C.; Isabelle L. M. (2004) Delivery levels and behavior of 1,3-butadiene, acrylonitrile, benzene, and other toxic volatile organic compounds in mainstream tobacco smoke from two brands of commercial cigarettes. Chem. Res. Toxicol. 17, 805–813. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2007) The Scientific Basis of Tobacco Product Regulation; Report of a WHO Study Group, Geneva, Switzerland. [Google Scholar]
- International Organization for Standardization (2009) Workplace Atmospheres - Controlling and Characterizing Errors in Weighing Collected Aerosols, ISO 15767:2009(E), 2nd edition, 2003-06-01.
- Chuang J. C.; Kuhlman M. R.; Wilson N. K. (1990) Evaluation of methods for simultaneous collection and determination of nicotine and polynuclear aromatic hydrocarbons in indoor air. Environ. Sci. Technol. 24, 661–665. [Google Scholar]
- Chuang J. C.; Wilson N. K.; Lewis R. G. (1999) Methodology of ambient air monitoring for polycyclic aromatic hydrocarbons. Fresenius Environ. Bull. 8, 547–556. [Google Scholar]
- Gordon S. M.; Brinkman M. C.; Meng R. Q.; Anderson G. M.; Chuang J. C.; Kroeger R. R.; Reyes I. L.; Clark P. I. (2011) Effect of cigarette menthol content on mainstream smoke emissions. Chem. Res. Toxicol. 24, 1744–1753. [DOI] [PubMed] [Google Scholar]
- Hansel A.; Jordan A.; Holzinger R.; Lindinger W. (1995) Proton transfer reaction-mass spectrometry: on-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Process 149–150, 609–619. [Google Scholar]
- de Gouw J.; Warneke C. (2007) Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 26, 223–257. [DOI] [PubMed] [Google Scholar]
- Smith D.; Spanel P. (2005) Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 24, 661–700. [DOI] [PubMed] [Google Scholar]
- Winberry W. T. Jr., Forehand L., Murphy N. T., Ceroli A., Phinney B., and Evans A. (1992) Determination of formaldehyde and other aldehydes in indoor air using a solid adsorbent cartridge. Compendium Method IP-6A. Methods for the Determination of Indoor Air Pollutants: EPA Methods, pp 473–508, Noyes Data Corporation, Park Ridge, NJ. [Google Scholar]
- International Organization for Standardization (2000) Routine Analytical Cigarette-Smoking Machine - Definitions and Standard Conditions, ISO Standard 3308, 4th ed.
- Benowitz N. L. (1996) Biomarkers of Cigarette Smoking, The FTC Cigarette Test Method for Determining Tar, Nicotine, and Carbon Monoxide Yields of U.S. Cigarettes. Report of the NCI Expert Committee. Smoking and Tobacco Control Monograph No. 7, Chapter 7. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda. MD.
- Benowitz N. L. (2001) Compensatory Smoking of Low-Yield Cigarettes. Risks Associated with Smoking Cigarettes with Low Machine Yields of Tar and Nicotine, pp 39–63, U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda. MD. [Google Scholar]
- Kozlowski L. T.; Mehta N. Y.; Sweeney C. T.; Schwartz S. S.; Vogler G. P.; Jarvis M. J.; West R. J. (1998) Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob. Control 7, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.; Wiebel F.; Kozlowski L. T.; Borland R.; Cummings K. M.; O’Connor R. J.; McNeill A.; Connolly G. N.; Arnott D.; Fong G. T. (2007) Revising the machine smoking regime for cigarette emissions: implications for tobacco control policy. Tob. Control 16, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam T.; McAughey J.; McGrath C.; Mocker C.; Zimmermann R. (2009) Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal. Bioanal. Chem. 394, 1193–1203. [DOI] [PubMed] [Google Scholar]
- Adam T.; McAughey J.; Mocker C.; McGrath C.; Zimmermann R. (2010) Influence of filter ventilation on the chemical composition of cigarette mainstream smoke. Anal. Chim. Acta 657, 36–44. [DOI] [PubMed] [Google Scholar]
- Armitage A. K.; Dixon M.; Frost B. E.; Mariner D. C.; Sinclair N. M. (2004) The effect of inhalation volume and breath-hold duration on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Beitr. Tabakforsch. Int. 21, 240–249. [DOI] [PubMed] [Google Scholar]
- Dickens C.; McGrath C.; Warren N.; Biggs P.; McAughey J. (2009) Puffing and inhalation behavior in cigarette smoking: implications for particle diameter and dose. J. Phys.: Conf. Ser. 151, 012019. [Google Scholar]
- Gowadia N.; Oldham M. J.; Dunn-Rankin D. (2009) Particle size distribution of nicotine in mainstream smoke from 2R4F, Marlboro Medium, and Quest1 cigarettes under different puffing regimens. Inhal. Toxicol. 21, 435–446. [DOI] [PubMed] [Google Scholar]
- Ishizu Y.; Kaneki K.; Okada T. (1987) A new method to determine the relation between the particle size and chemical composition of tobacco smoke particles. J. Aerosol Sci. 18, 123–129. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.