Abstract
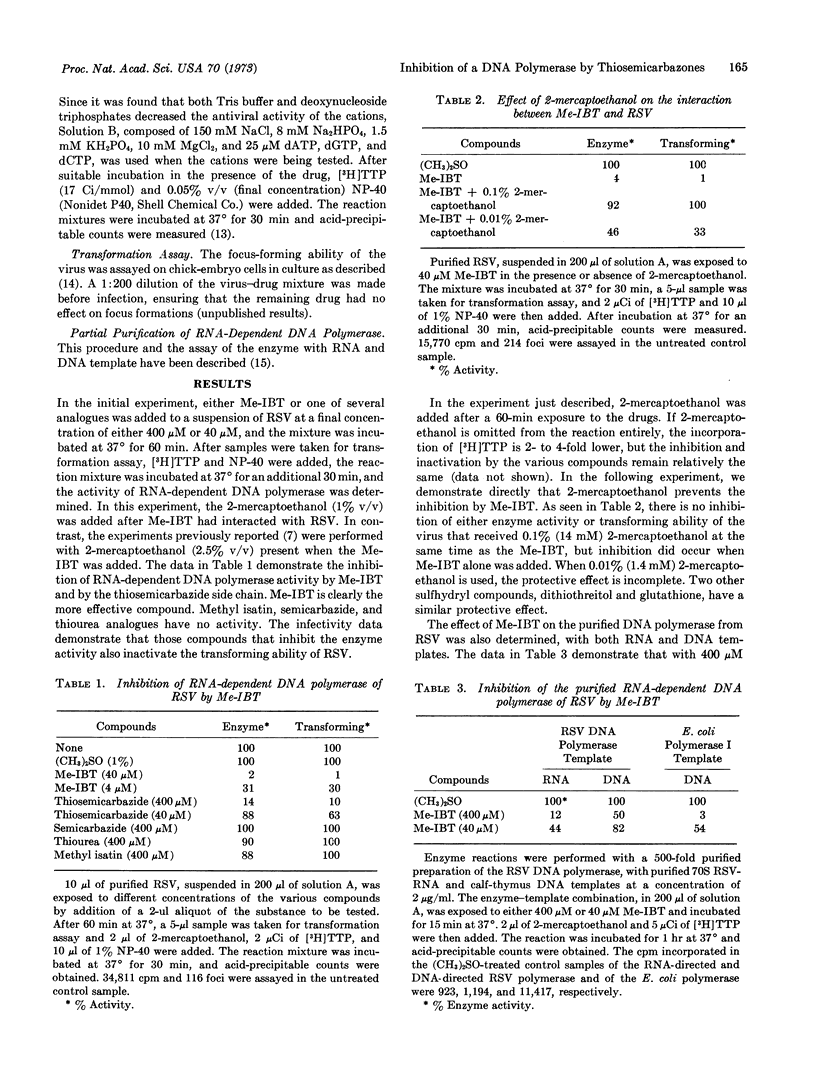
The RNA-dependent DNA polymerase of Rous sarcoma virus is inhibited by N-methyl isatin β-thiosemicarbazone and by thiosemicarbazide, but not by semicarbazide. These inhibitors also inactivate, upon contact with the virion, the transforming ability of Rous sarcoma virus. Sulfhydryl donors, such as 2-mercapto-ethanol, can prevent these effects. The RNA-directed activity of the purified polymerase is inhibited to a greater degree than is the DNA-directed activity.
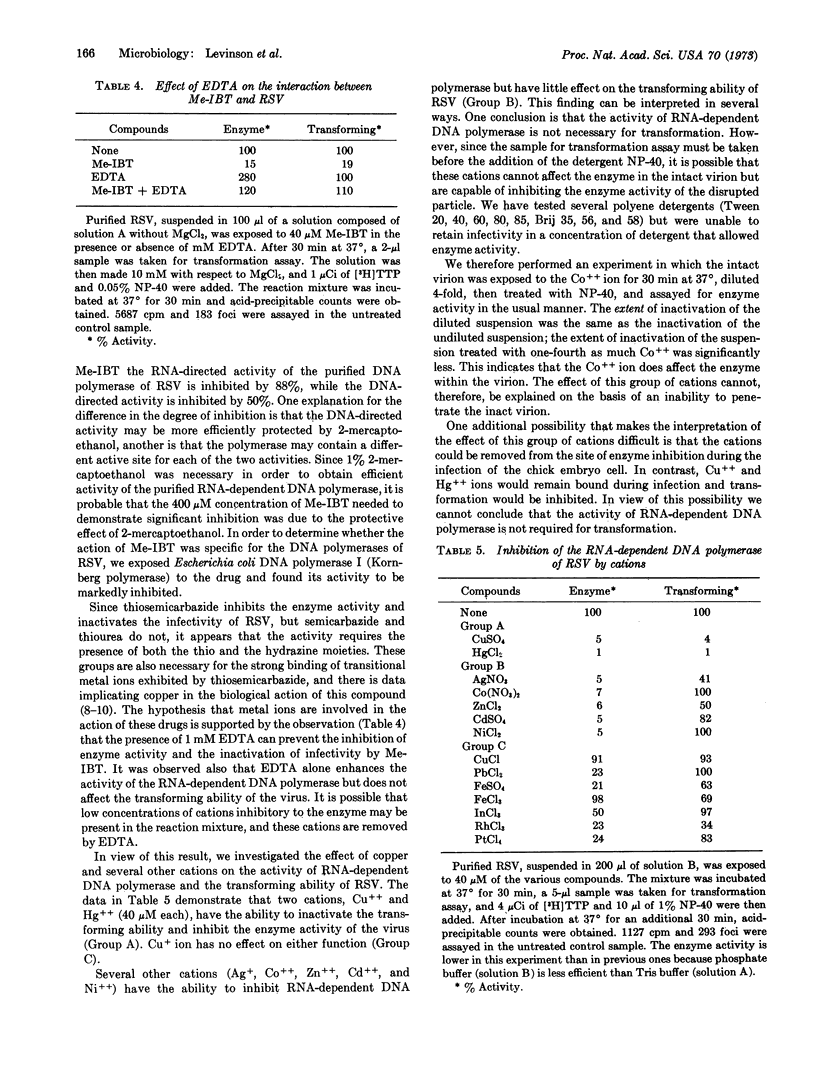
Two cations, Cu++ and Hg++, can inhibit RNA-dependent DNA polymerase and inactivate the transforming ability of the virus. Synergism between N-methyl isatin β-thiosemicarbazone and Cu++ occurs, since treatment of the virus with a low dose of either N-methyl isatin β-thiosemicarbazone or Cu++ has little effect; however, when the two compounds are mixed together, significant inactivation occurs. This observation supports the hypothesis that the antiviral action of thiosemicarbazones is a function of their ability to act as a ligand for metallic ions.
Several cations (Ag+, Co++, Zn++, Cd++, and Ni++) significantly inactivate the RNA-dependent DNA polymerase, but have little effect on the transforming ability. In view of this result, the conclusion that the enzyme activity is required for transformation remains open to question.
Keywords: RNA tumor viruses, DNA synthesis, viral inactivation, chelation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER D. J., DUMBELL K. R., FOX-HULME P., SADLER P. W. The chemotherapy of variola major infection. Bull World Health Organ. 1962;26:727–732. [PMC free article] [PubMed] [Google Scholar]
- BAUER D. J., SADLER P. W. The structure-activity relationships of the antiviral chemotherapeutic activity of isatin beta-thiosemicarbazone. Br J Pharmacol Chemother. 1960 Mar;15:101–110. doi: 10.1111/j.1476-5381.1960.tb01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER D. J., STVINCENT L., KEMPE C. H., DOWNIE A. W. PROPHYLACTIC TREATMENT OF SMALL POX CONTACTS WITH N-METHYLISATIN BETA-THIOSEMICARBAZONE (COMPOUND 33T57, MARBORAN). Lancet. 1963 Sep 7;2(7306):494–496. doi: 10.1016/s0140-6736(63)90230-7. [DOI] [PubMed] [Google Scholar]
- BAUER D. J. The chemotherapeutic activity of compounds of copper, rhodium and certain other metals in mice infected with neurovaccinia and ectromelia viruses. Br J Exp Pathol. 1958 Oct;39(5):480–489. [PMC free article] [PubMed] [Google Scholar]
- Bauer D. J., Apostolov K. Adenovirus multiplication: inhibition by methisazone. Science. 1966 Nov 11;154(3750):796–797. doi: 10.1126/science.154.3750.796. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Butzow J. J., Eichhorn G. L. Interactions of metal ions with polynucleotides and related compounds. IV. Degradation of polyribonucleotides by zinc and other divalent metal ions. Biopolymers. 1965;3(1):95–107. doi: 10.1002/bip.360030110. [DOI] [PubMed] [Google Scholar]
- EICHHORN G. L., CLARK P. INTERACTIONS OF METAL IONS WITH POLYNUCLEOTIDES AND RELATED COMPOUNDS. V. THE UNWINDING AND REWINDING OF DNA STRANDS UNDER THE INFLUENCE OF COPPER (II) IONS. Proc Natl Acad Sci U S A. 1965 Mar;53:586–593. doi: 10.1073/pnas.53.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Structure and infectivity of tobacco mosaic virus. Harvey Lect. 1957;53:56–68. [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- French F. A., Blanz E. J., Jr, DoAmaral J. R., French D. A. Carcinostatic activity of thiosemicarbazones of formyl heteroaromatic compounds. VI. 1-Formylisoquinoline derivatives bearing additional ring substituents, with notes on mechanism of action. J Med Chem. 1970 Nov;13(6):1117–1124. doi: 10.1021/jm00300a024. [DOI] [PubMed] [Google Scholar]
- French F. A., Blanz E. J., Jr The carcinostatic activity of thiosemicarbazones of formyl heteroaromatic compounds. 3. Primary correlation. J Med Chem. 1966 Jul;9(4):585–589. doi: 10.1021/jm00322a032. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIAI S. EFFECTS OF CUPRIC IONS ON THERMAL DENATURATION OF NUCLEIC ACIDS. J Mol Biol. 1965 Apr;11:672–690. doi: 10.1016/s0022-2836(65)80025-0. [DOI] [PubMed] [Google Scholar]
- Levinson W. Fragmentation of the nucleus in Rous sarcoma virus-infected chick embryo cells. Virology. 1967 May;32(1):74–83. doi: 10.1016/0042-6822(67)90254-1. [DOI] [PubMed] [Google Scholar]
- Levinson W., Woodson B., Jackson J. Inactivation of Rous sarcoma virus on contact with N-ethyl isatin -thiosemicarbazone. Nat New Biol. 1971 Jul 28;232(30):116–118. doi: 10.1038/newbio232116a0. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Booth B. A., Sartorelli A. C. Inhibition of deoxyribonucleotide synthesis by pyridine carboxaldehyde thiosemicarbazones. Cancer Res. 1971 Mar;31(3):235–238. [PubMed] [Google Scholar]
- Moore E. C., Zedeck M. S., Agrawal K. C., Sartorelli A. C. Inhibition of ribonucleoside diphosphate reductase by 1-formylisoquinoline thiosemicarbazone and related compounds. Biochemistry. 1970 Nov 10;9(23):4492–4498. doi: 10.1021/bi00825a005. [DOI] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. DNA polymerase of chicken embryo: purification and properties. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1781–1785. doi: 10.1073/pnas.69.7.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaralingam M., Carrabine J. A. Stereochemistry of nucleic acids and their constituents. XIX. Copper binding sites and mechanism of G-C selective denaturation of DNA. Crystal and molecular structures of guanine-copper(II) chloride and cytosine-copper(II) chloride complexes. J Mol Biol. 1971 Oct 28;61(2):287–309. doi: 10.1016/0022-2836(71)90381-0. [DOI] [PubMed] [Google Scholar]
- TURNER W., BAUER D. J., NIMMO-SMITH R. H. Eczema vaccinatum treated with n-methylisatin beta-thiosemicarbazone. Br Med J. 1962 May 12;1(5288):1317–1319. doi: 10.1136/bmj.1.5288.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. R., Gabe E. J., Glusker J. P., Minkin J. A., Patterson A. L. The crystal structures of compounds with antitumor activity. 2-keto-3-ethoxybutyraldehyde bis(thiosemicarbazone) and its cupric complex. J Am Chem Soc. 1966 Apr 20;88(8):1845–1846. doi: 10.1021/ja00960a067. [DOI] [PubMed] [Google Scholar]
- Ting R. C., Yang S. S., Gallo R. C. Reverse transcriptase, RNA tumour virus transformation and derivatives of rifamycin SV. Nat New Biol. 1972 Apr 12;236(67):163–166. doi: 10.1038/newbio236163a0. [DOI] [PubMed] [Google Scholar]



