Abstract
Patients with schizophrenia are at an increased risk for the development of depression. Overlap in the symptoms and genetic risk factors between the two disorders suggests a common etiological mechanism may underlie the presentation of comorbid depression in schizophrenia. Understanding these shared mechanisms will be important in informing the development of new treatments. Rodent models are powerful tools for understanding gene function as it relates to behavior. Examining rodent models relevant to both schizophrenia and depression reveals a number of common mechanisms. Current models which demonstrate endophenotypes of both schizophrenia and depression are reviewed here, including models of CUB and SUSHI multiple domains 1, PDZ and LIM domain 5, glutamate Delta 1 receptor, diabetic db/db mice, neuropeptide Y, disrupted in schizophrenia 1, and its interacting partners, reelin, maternal immune activation, and social isolation. Neurotransmission, brain connectivity, the immune system, the environment, and metabolism emerge as potential common mechanisms linking these models and potentially explaining comorbid depression in schizophrenia.
Keywords: mouse, schizophrenia, depression, animal model, genetics
Introduction
Schizophrenia and depression are devastating mental illnesses that contribute substantially to the global burden of disease (1–3). Moreover, schizophrenia patients have an elevated risk for developing depressive symptoms compared to the already high lifetime prevalence of depression in the general population (4). Depression has been reported during all stages of the course of schizophrenia (5–8), and depressive symptoms are associated with an increased risk of suicide (9, 10). Methodological differences in diagnosis and time course of evaluation mean that there is a wide variance of depressive symptoms reported by patients with schizophrenia in the literature, with prevalence rates as high as 61% (11). Nevertheless, reviews of the literature convincingly show that depression is elevated in schizophrenia (4).
Conversely, depressed patients have also been shown to be at a higher risk of developing psychosis, and depression is often seen in people at high risk for schizophrenia prior to the emergence of psychotic symptoms (12–17). Furthermore, the emergence of psychotic symptoms in depression, considered as a distinct clinical subtype of depression called psychotic depression or depression with psychotic features, is associated with increased severity of depressive symptoms (18, 19). This mutual relationship of risk between schizophrenia and depression suggests potential overlap in the pathophysiology and/or etiology of the two disorders.
The relationship between psychotic and affective symptoms has been a controversial issue within psychiatric nosology for years. A central question in the debate is reflected by the discussion surrounding schizoaffective disorder, which currently remains a distinct diagnosis characterized by the presence of a major mood episode (depressive or manic) concurrent with schizophrenia (20). Low diagnostic reliability has led some to question the inclusion of schizoaffective disorder as a separate condition (21, 22). It remains unclear whether depressive symptoms should be considered as a symptom of schizophrenia, comorbid symptoms, or unrelated epiphenomena (23).
There is an overlap between certain negative symptoms of schizophrenia and depressive symptoms; for example, anhedonia, abulia, alogia, amotivational and avolitional states, and social withdrawal (24). Hence, some argue depressive symptoms should be part of the schizophrenia syndrome (25–28). This view is supported by the high prevalence of depressive symptoms in schizophrenia and the association between trait depression and other trait-like features of schizophrenia. Alternatively, depressive symptoms in schizophrenia could partly be a side-effect of neuroleptics, secondary to other comorbidities such as substance abuse, or an understandable reaction to the consequences of the disorder (29–34). Regardless of the status of depressive symptoms as core or comorbid with schizophrenia, there is clearly some overlap in the presentation of both disorders.
There is increasing evidence of shared genetic risk factors for both schizophrenia and depression. A genome wide association study (GWAS) of five major psychiatric disorders found that SNPs within chromosomal regions 3p21 and 10q24, and calcium channel subunit genes CACNA1C and CACNB2 were significantly associated with schizophrenia, depression, bipolar, attention deficit-hyperactivity (ADHD), and autism spectrum disorders (ASD) (35). Additionally, the subgroup of schizophrenia patients who also suffer from depression has proved useful in finding genetic associations. The NMDA receptor subunit gene GRIN1, the hippocampal stress modulating glycoprotein gene GPM6A, and chromosomal regions 4q28.3 and 20q11.21 were associated with depression comorbidity in schizophrenia patients (36–38). These studies hint at potential shared molecular pathways that underlie both schizophrenia and depression.
The etiology and pathophysiology of both schizophrenia and depression remain poorly understood. It is clear that there is some relationship between the two disorders, which affects the risk and severity of disability for both disorders. Understanding the neurobiology linking schizophrenia and depression could provide great insight into both disorders. Animal models are some of our best tools for understanding the complex pathways that connect genes and behavior. Many excellent reviews have been written on the use of animal models in studying neuropsychiatric disorders and outlining many of the current models (39–42). Rather than reviewing the literature on neuropsychiatric disorders as a whole or focusing specifically on one disorder, this review will focus on what animal models can teach us about schizophrenia and depression comorbidity. Here, we provide a broad overview of the rodent models that express phenotypes resembling comorbid schizophrenia and depression, and what they reveal about the neurobiology of comorbidity in psychiatric illness.
Finding Animal Models for Neuropsychiatric Disorders
When modeling neuropsychiatric disorders in animals, it is desirable that the criteria for the three types of validity are fulfilled: face (i.e., similar symptoms), construct (i.e., similar etiology or genetic/environmental cause), and predictive (i.e., responds to relevant drug treatments) (39, 43). Given that depression and particularly schizophrenia are defined by complex multidimensional sets of symptoms that can be highly heterogeneous between patients, it has been proposed that these disorders may be approached by examining endophenotypes, which are easier to measure, and may be more proximal to the underlying genetic and biological mechanisms (44). Therefore, the typical approach for modeling these disorders in mice or rats is to manipulate some genetic or environmental factor, which has a plausible etiological link to either schizophrenia or depression, and then examine the animal for endophenotypes that resemble those seen in either disorder. Models typically will display only a subset of all the endophenotypes, which define either disorder, which is expected given the heterogeneous and polygenetic nature of both schizophrenia and depression.
Behavioral endophenotypes have been particularly useful for studying neuropsychiatric illness in rodent models. For instance, certain features of schizophrenia have behavioral correlates that are measurable in rodents. Pre-pulse inhibition (PPI) is a phenomenon in which the response to a stimulus is inhibited by a preceding similar stimulus. For example, the startle response to a loud noise is less intense if a quieter preceding warning noise is played. PPI deficits are seen in schizophrenia patients and their unaffected relatives, and are measurable in rodents (45–48). PPI is framed as a measure of sensorimotor gating, which is known to be affected in schizophrenia (49). Sensorimotor gating deficits in rodents can also be exhibited as sustained hyperactivity in a novel environment caused by a failure to habituate to novel stimuli (50).
Cognition is another area in which many sophisticated rodent tests have been developed. Even complex cognitive processes such as executive function are measurable in rodents. For instance, a rat version of the Wisconsin-card-sorting test used to measure the ability learn rules and adapt to change in humans has been developed (51). Rats must switch between learned scent and texture cues to locate hidden food rewards. Schizophrenia patients are known to have deficits in executive function, and have impaired performance in the Wisconsin-card-sorting test (52–55). Animal behavioral tests that can predict clinical drug effects in humans are also important. For example, antidepressants can reduce immobility in the forced swim test (FST) and tail suspension test (TST). Therefore, these behavioral tests have been used to screen potential antidepressant medications (56, 57). Increased immobility in these tests has been suggested to indicate behavioral despair or the inability to cope with stress, but the meaning of these tests and how they translate to behavior in humans remains unclear (39). Table 1 provides a few examples of rodent behavioral paradigms that are relevant for neuropsychiatric disorders.
Table 1.
Mouse behavioral phenotypes related to neuropsychiatric disorders.
| Mouse behavioral test | Ethological correlate | Disease associations |
|---|---|---|
| Elevated plus/0 maze | Decreased time in open arms of a maze with open and enclosed arms models state anxiety (58) | BPD, GAD, OCD, panic disorder, phobias, PTSD (59) |
| Forced swim/tail suspension test | Increased immobility possibly related to behavioral despair or coping with stress (55) | Related to antidepressant activity, depression (39) |
| Latent inhibition | The effectiveness of conditioning in mice previously exposed vs. not exposed to a stimulus. Related to the ability to ignore irrelevant stimuli during learning (60) | SCZ (61) |
| Morris water maze (MWM) | Latency to find a hidden platform in a pool of murky water and memory for platform position tests spatial learning and memory (62) | AD, OCD, SCZ (63–65) |
| MWM reversal learning | Ability to learn a new position when the platform is moved from its previous position tests cognitive flexibility (66) | ADHD, ASD, Huntington’s, OCD, SCZ (67–69) |
| Novel object recognition test | Preference for exploring new vs. familiar objects tests recognition memory, episodic memory, and visual attention (70, 71) | ADHD, ASD, learning disability, PTSD, SCZ (72–74) |
| Open field test | Tests exploration and motor activity (75) | Altered motor activity linked to ADHD, BPD, depression, SCZ (53, 76) |
| Willingness to enter the center of the field measures anxiety (75) | BPD, GAD, OCD, panic disorder, phobias, PTSD (59) | |
| Pre-pulse inhibition | Magnitude of the startle response to a loud noise in the presence and absence of a preceding noise, tests sensorimotor gating (74) | ASD, Huntington’s, OCT, SCZ, Tourette’s (77) |
| Psychostimulant-induced locomotor activity | Excess hyperactivity after injection with a psychostimulant tests sensitivity. Connected to the functioning of brain reward circuits (78) | Drug addiction, psychostimulant-induced mania, SCZ (39, 79, 80) |
| Set-shifting test | Ability to switch between different cues to locate a food reward tests cognitive flexibility (54) | ADHD, ASD, Huntington’s, OCD, SCZ (68, 69) |
| Sucrose preference test | Mouse preference for sugar vs. normal water tests anhedonia (81) | Alcohol dependence, depression, hysteria (82, 83) |
| T-maze/Y-maze | Alternation of entry onto the arms of the maze during reward retrieval (T-maze)/exploration (Y-maze) tests working memory (84, 85) | SCZ (68) |
| Three-chamber social interaction test | Time spent with mouse vs. object tests social motivation, time spent with familiar vs. new mouse tests social memory (86) | ASD, BPD, depression, SCZ (87–91) |
AD, Alzheimer’s disease; ASD, autism spectrum disorder; ADHD, attention deficit-hyperactivity disorder; BPD, bipolar disorder; GAD, generalized anxiety disorder; OCD, obsessive–compulsive disorder; PTSD, posttraumatic stress disorder.
Disorders of the human brain are complex, and while animal models are useful tools in understanding neurobiology and gene function, caution must be exercised when using animal models for the translational study of human psychiatric disorders. Despite high conservation of gene structure and function, there can be large interspecies differences in gene expression patterns, gene regulation, and protein translation between human and mouse or rat. Furthermore, the correlations between genes, biology, and behavior we measure in animals may not map perfectly to the symptoms in humans we wish to model. Behavioral findings are sometimes idiosyncratic and specific to particular laboratories (92), so it is sometimes difficult for models developed in one lab to be replicated and used by others without careful attention to environmental and test conditions (93).
Nevertheless, animal models have greatly contributed to the understanding and treatment of neuropsychiatric disorders. For instance, latent inhibition (LI) is a class of cognitive tests that measure a learning effect in which exposure to conditioned stimuli alone in associative learning paradigms can interfere with subsequent learning. LI is an established model of attentional deficits in schizophrenia (61). Assessing mouse LI has been productive in validating new candidate genes for schizophrenia (94). Additionally, animal behavioral testing has been integral in assessing the efficacy of new compounds with potential antidepressant and antipsychotic effects (95–97).
Models
Many animal models have been generated to explore various factors associated with both depression and schizophrenia. The presence of both schizophrenia and depression-related endophenotypes in a single model may be useful in understanding comorbidity and the shared symptomology between the two disorders. Hence, this review will focus exclusively on rodent models that display endophenotypes relevant for both schizophrenia and depression. Investigating the common elements between such models may provide clues about the shared pathways which lead to comorbidity for these two disorders.
CUB and SUSHI multiple domains 1
Human genetic studies have found significant links between CSMD1 and schizophrenia, with nominally significant links reported for depression and bipolar disorder (98–102). Furthermore, risk variants in CSMD1 were shown to have effects on cognition and brain activation in healthy participants (103, 104). CUB and SUSHI multiple domains 1 (CSMD1) is a complement control-related protein that inhibits C3 in vitro (105, 106). Complement is tightly regulated in the CNS as it is involved in microglia-dependent synaptic pruning and phagocytosis. For this reason, it is hypothesized that CSMD1 could play a role in aberrant synaptic elimination in neurodegenerative disorders (107). Hence, both immune and synaptic regulation may mediate the effects of Csmd1 in the development of both schizophrenia and depression-related phenotypes.
Steen et al. generated a Csmd1 knockout (KO) by deleting a 1 kb sequence from exon/intron1 (108). These mice developed an anxiety-like phenotype in the open field test (OFT) and elevated plus maze (EPM). The mice also had increased exploratory activity in the novel object recognition test (NORT). However, this did not affect working memory, recognition, or preference, so it may simply be a reflection of increased anxiety. The mice had a potential depression-like phenotype in the TST, but no changes were seen in PPI. Finally, the KO mice had a significant increase in body weight accumulation over time, and increased glucose tolerance.
Interestingly, a previous study tested an exon1 deletion Csmd1-KO mouse on schizophrenia endophenotypes (109). This group found no significant changes in schizophrenia-related behaviors: PPI, social interaction test (SIT), sucrose preference test (SPT), or sensitivity to amphetamine in the amphetamine-induced locomotor activity test (amphetamine-ILAT). This agrees with the results of Steen et al.; however, this group did not test for depressive endophenotypes. The lack of a significant effect on classical schizophrenia endophenotypes may be due to mouse–human differences in CSMD1. Nevertheless, Steen et al. suggest that CSMD1 may play a role in the common symptoms between bipolar disorder, depression, and schizophrenia. Steen et al. found that depletion of Csmd1 had very little effect on the whole transcriptome, and identified a Csmd1 promoter-associated lncRNA, possibly responsible for brain-specific promoter activity in the CNS. This suggests that Csmd1 was directly responsible for the manifestation of schizophrenia and depression-like behavior in these mice (108).
PDZ and LIM domain 5
PDZ and LIM domain 5 (PDLIM5) has been associated with schizophrenia, depression, and bipolar disorder in human genetic and expression studies (110–117). PDLIM5 encodes the enigma homolog (ENH), of which five protein isoforms have been identified in humans (118). PDLIM5 is known to interact with protein kinase C (PKC), and may be involved in the regulation of intracellular calcium levels through PKC epsilon (PCKE) and Ca2+ channel interactions (119). In the nervous system, PDLIM5 is localized in presynaptic terminals and the postsynaptic density; furthermore, PDLIM5 was shown to interact with spine-associated RapGAP (SPAR, SIPA1L1), and to stimulate the shrinkage of dendritic spines (120, 121). Combined with the genetic results implicating L-type calcium channel genes CACNA1C and CACNB2 in schizophrenia, depression, bipolar disorder, autism, and ADHD, this model further implicates Ca2+ channels in neuropsychiatric illness (35). Hence, Pdlim5 may affect schizophrenia and depression-related behaviors through regulation of Ca2+ channels as well as synapse regulation.
Horiuchi et al. generated a Pdlim5 KO using a gene trap embryonic stem cell line (122). Homozygotes for the Pdlim5-KO were embryonic lethal; however, heterozygotes were viable with normal weight and brain size. Pdlim5 deficiency in heterozygotes had a protective effect on schizophrenia-like phenotypes in chronic and acute methamphetamine-induced locomotor hyperactivity in the open field (METH-ILAT) and methamphetamine impairment of PPI. Furthermore, the effects on PPI and METH-ILAT were replicated when Pdlim5 was inhibited with PKCE-TIP in wild type mice. Pdlim5-deficient heterozygotes expressed a depression-like phenotype in the TST that was rescued by the antidepressant imipramine. Pdlim5 expression was shown to increase in the prefrontal cortex (PFC) of mice with chronic methamphetamine injection and in the brains of mice with chronic imipramine injection, but did not change with acute dosing or with injection of the classical antipsychotic haloperidol. This study is limited by the lack of data from complete KO mice; furthermore, PKCE-TIP is not specific to Pdlim5. Nevertheless, the data suggest that increased Plim5 levels may cause schizophrenia-like behavioral phenotypes, whereas decreased Pdlim5 may result in depression.
Glutamate Delta 1 receptor
Human GWAS has associated GRID1 with schizophrenia, bipolar disorder, and depression (123–127). Glutamate Delta 1 receptor (GluD1) is a member of the orphan family of delta ionotropic glutamate receptors (iGluRs), and has widespread neuronal expression in adult mice particularly in the forebrain, with diffuse expression in the CNS during development (128–131). While typical iGluR ligands fail to generate current responses in the GluD1 receptor, there is evidence that the NMDA receptor allosteric activator d-serine binds to GluD1 receptors (132). d-serine binding is hypothesized to affect receptor function indirectly; for instance, through alteration of dimer stability. Studies in vitro suggest GluD1 may be involved in the induction of presynaptic differentiation and synapse formation (133–136). Synaptic regulation and glutamate signaling mediated by GluD1 may influence the development of schizophrenia and depression-related symptoms.
Yadav et al. used targeted disruption to delete exons 11 and 12 of the GluD1 gene Grid1 in mice (137). GluD1-KO mice showed hyperactivity in the OFT, decreased anxiety-like behavior in the EPM and marble burying tests, depression-like behavior and anhedonia in the FST and SPT, and increased aggressive behavior. GluD1-KO mice had deficits in social interaction, which could be rescued by treatment with the GluN1 NMDA receptor subunit agonist d-cycloserine. The GluD1-KO mice also had enhanced working memory in the Y-maze and radial arm maze, but they had deficits in reversal learning in the Morris water maze (MWM) with no changes in spatial learning, and deficits in cue and contextual fear conditioning, but no changes in LI (138). The authors found significantly higher expression of GluA1, GluK2 (ionotropic GluR subunits), and PSD95, and a trend for higher expression of GAD67 (inhibitory neuron marker) in the amygdala of GluD1-KO mice. They also found decreased expression of GluA1 and GluA2 in the PFC and hippocampus of the KO mice, as well as decreased GluK2 and GAD67 and elevated GluN2B and PSD95 in the hippocampus. Decreased GluA1 levels could be rescued by d-cycloserine treatment. While this mouse lacks schizophrenia-associated deficits in PPI and LI, violence has been associated with schizophrenia (139, 140); therefore, the hyper-aggression seen in these mice could be relevant to schizophrenia. Furthermore, changes in working memory, reversal learning, and anhedonia could reflect cognitive and negative symptoms of schizophrenia.
Given the known role of GluD1 in synaptic regulation, synaptic deficits are likely to underlie the changes in behavior seen in the GluD1-KO mice. However, the exact nature of the effects of GluD1 on the synapses in these mice remains to be thoroughly explored. The expression data also hints at an inhibitory–excitatory imbalance in the synapses of the KO mice. This type of imbalance has also been seen in other animal models of schizophrenia (141–143). Certainly, alterations in synaptic regulation and function are becoming a common theme among animal models showing schizophrenia and depression-like behaviors.
Diabetic db/db mice
The leptin receptor-deficient db/db mouse is an established mouse model of metabolic conditions such as diabetes mellitus, obesity, and dyslipidemia. The db/db mice were shown to have impaired spatial learning in the MWM accompanied by deficits in long-term potentiation (LTP) (144). Dinel et al. showed that db/db mice have increased anxiety-like behaviors in the OFT and EPM, and impaired spatial working memory at long stimulus intervals in the Y-maze with no impairments in working or recognition memory in the NORT (145). These behavioral deficits were associated with hippocampal inflammation. These authors found the db/db genotype did not associate with depression-like behavior in the FST and TST. A more recent study reconfirmed previous results of impaired memory and anxiety-like behaviors; however, this group found increased immobility time of both juvenile and adult db/db mice in the FST and impaired PPI of adult, but not juvenile mice, suggesting both schizophrenia-like and depression-like phenotypes (146). The contradictory results of the two studies in the FST may be explained by differences in experimental procedures, as increased immobility in the FST is likely to occur with increasing stress, which could be affected by the order and number of tests in each paper.
An analysis of CNS protein expression in db/db mice found overlap in protein expression in shared pathways in neuropsychiatric disorders; notably, decreased peptide YY, which is seen in drug free cerebrospinal fluid of schizophrenia patients, and inflammatory and Ca2+ regulatory molecules, which share pathways with cognitive disorders, depression, Alzheimer’s, and schizophrenia (147). Metabolic conditions such as obesity and diabetes are frequently comorbid with depression and schizophrenia (148–151). Furthermore, there is considerable evidence that antipsychotics and possibly schizophrenia itself may disrupt important metabolic pathways (152). The db/db mouse could be useful for linking schizophrenia and depression to important metabolic pathways, which also increase susceptibility to obesity and diabetes.
Neuropeptide Y
The 36 amino-acid peptide neuropeptide Y (NPY) is widely distributed in the CNS and recognized to play a role in eating behavior, energy balance, and cardiovascular functions (153, 154). The NPY system has been implicated in schizophrenia by post-mortem human studies, which found decreased NPY in the cortex of schizophrenia and bipolar disorder patients (155, 156). The Y2 receptor is also known to interact with the dopamine (DA) system in humans and rodents, providing a further link to schizophrenia (157, 158). The NPY system has been implicated in depression via its role in modulating stress response, mood, and affective behaviors (159–161). Hence, disruptions in the NPY system could potentially be involved in both schizophrenia and depression, while also linking both disorders to metabolic conditions, appetite changes, and obesity.
Multiple rodent models of the NPY system have been used to investigate the role of NPY in depression and schizophrenia. Stadlbauer et al. administered NPY receptor agonist peptide YY (PYY3–36) intraperitoneally in mice (162). This treatment caused deficits in social interaction with no significant increase in anxiety-like behaviors in the EPM. Schizophrenia-like deficits in LI and PPI were also induced by PYY administration. PYY-induced PPI deficits could be reversed by haloperidol, but not the atypical antipsychotic clozapine. Additionally, PYY injection impaired spatial learning in the MWM. Y2 receptor-deficient male (but not female) mice displayed hyperactivity in the OFT, increased social interaction, and moderately improved PPI, suggesting a protective effect against schizophrenia-associated behaviors (163). Y2 deficiency also caused decreased anxiety-like behavior in the EPM and OFT (164). Implicating NPY in depression-like phenotypes, administration of the NPY Y1 receptor agonist NPY (Leu31, Pro34) had anxiolytic and antidepressant effects on cholecystokinin-4 (CCK-4)-induced anxiety-like behavior in the SIT and depression-like behavior in the FST (165). Furthermore, it was found that PYY3–36 administration increased the immobility time of olfactory bulbectomized rats in the FST (166). These models are part of a larger body of work implicating the NPY system in stress-related depressive disorders (161). These models suggest that activation of the NPY system via Y2 receptors may cause schizophrenia-like behavior while exerting an antidepressant-like effect.
Disrupted in schizophrenia 1
A chromosomal translocation intersecting DISC1 was first found in a Scottish pedigree with a high frequency of severe psychiatric disorders, including schizophrenia, depression, and bipolar disorder (167, 168). Additional genetic associations between DISC1 and neuropsychiatric illness were found in other populations (169–171). Disrupted in schizophrenia 1 (DISC1) is a scaffolding protein implicated in multiple downstream functions, including embryonic and adult neurogenesis; and neuronal proliferation, differentiation, and migration (169, 172–174). DISC1 interacts with many other proteins involved in synaptic function, neurodevelopment, the cytoskeleton, and centrosomal pathways, some of which are also associated with schizophrenia and depression (e.g., AKT, DPYSL2, GSK-3β, PDE4, CREB, and β-arrestin) (175–181). The distinct pathways by which DISC1 mediates its effects have been intensely studied, and a detailed discussion is beyond the scope of this review. Essentially, Disc1 may affect behavior via its roles in neurodevelopment, synaptic transmission, and synaptic plasticity mediated through multiple downstream interacting partners such as PDE4, Ndel1, GSK-3, and Dixdc1.
The numerous animal models that have been generated to investigate the role of DISC1 in the neurobiology of mental illness have been reviewed in considerable detail elsewhere (174). Cognitive, schizophrenia-like, and depression-like deficits are common in the various models, but not all are present simultaneously in every model. Table 2 provides a summary of the behavioral phenotypes of relevant Disc1 mouse models. Disruptions in Disc1 caused alterations in neurodevelopment, such as changes in brain structure, abberant formation of cortical layers, reductions in GABAergic interneurons, and altered neuronal morphology, maturation, neurite growth, and axonal targeting (182–190). Disc1 alterations also caused reductions in dopaminergic and hippocampal synaptic transmission, and short-term plasticity but not LTP (185–188). Changes were also seen in the activity of downstream Disc1 interacting partners, notably in the PDE4 family of phosphodiesterases and glycogen synthase kinase 3 (GSK-3) pathways (181, 182, 186). This implicates Disc1 in both neurodevelopment and synaptic transmission through its interactions with multiple downstream pathways.
Table 2.
Behavioral phenotypes of Disc1 genetic mouse models.
| Name of mouse line | Behavioral phenotypes |
Reference | ||
|---|---|---|---|---|
| SCZ-like | Depression-like | Cognitive | ||
| CaMK-DN-DISC1 tg | Hyperactivity, PPI deficits | ↑Immobility in FST | Working memory deficit in Y-maze | (184) |
| CaMK-DISC1-cc tg at PND 7 | ↑Immobility in FST; ↓sociability | Working memory deficit | (187) | |
| DN-DISC1 tg | Hyperactivity | ↑Aggression | Spatial memory deficit in MWM | (189) |
| Pre- and post-natal Tet-off DN-DISC1 tg | ↑Immobility in TST; ↓sociability; ↑aggression | (191) | ||
| DISC1 KD (transient in utero cortical) | PPI deficits | Impaired long-term but normal short-term operant conditioning; working memory deficit in T-maze | (188) | |
| DISC1tr | ↑Immobility in FST/TST | Fear memory deficit | (190) | |
| DISC1-129 | PPI deficits | Working and fear memory deficits | (185, 192) | |
| DISC1-Q31L | PPI and LI deficits | ↑Immobility in FST; social anhedonia | Working memory deficit in T-maze | (181, 182) |
| DISC1-L100P | Hyperactivity, PPI, and LI deficits | Working memory deficit in T-maze | (182, 193) | |
| Gene × environment models | ||||
| DN-DISC1 tg × polyl:C at E9 | Hyperactivity | ↑Immobility in FST; ↑anxiety; ↓ sociability | (194) | |
| DISC1-L100P+/− × polyl:C | PPI and LI deficits | ↓Sociability | Spatial operant conditioning deficit | (195) |
| DN-DISC1-Tg-PrP × social isolation | Hyperactivity; PPI deficit | ↑Immobility in FST | (196) | |
CaMK-DN-DISC1 tg, transgenic mice expressing dominant-negative C-terminal truncated human DISC1 under control of the α-calmodulin kinase II promoter; CaMK-DISC1-cc tg, transgenic mice expressing C-terminal portion of the human DISC1 under control of the-calmodulin kinase II promoter; DN-DISC1 tg, transgenic mice with inducible expression of dominant-negative C-terminal truncated human DISC1 (hDISC1) limited to forebrain regions, including cerebral cortex, hippocampus, and striatum, using the Tet-off system under the regulation of the CAMKII promoter; DISC1 KD, DISC1 knockdown; DISC1tr, transgenic mice expressing two copies of the truncated human DISC1 encoding the first eight exons using a bacterial artificial chromosome; DISC1-129, 129S6/SvEv inbred mouse strain carries a termination codon at exon 7 of DISC1 gene, which abolishes production of the full-length DISC1 protein; DISC1-Q31L, point mutation in the second exon of DISC1 leading to the substitution of glycine on leucine at 31 amino acid of DISC1 protein; DISC1-L100P, point mutation in the second exon of DISC1 leading to the substitution of leucine on proline at 100 amino acid of DISC1 protein. Table adapted from Lipina and Roder copyright (174), with permission from Elsevier.
DISC1 interacting partners
Mouse models for a number of DISC1 interacting partners also display behaviors relevant to both schizophrenia and depression. Mice deficient in fasciculation and elongation protein zeta 1 (Fez1) displayed a schizophrenia-like hypersensitivity to psychostimulants and antidepressant-like reduced immobility in the FST (197). These changes were associated with increased DA transmission in the nucleus accumbens. Mice deficient in the phosphodiesterase PDE4B not only showed a similar behavioral phenotype to the Fez1-KO mice but also had increased anxiety and deficits in PPI (198, 199). A GSK-3α KO mouse model actually had facilitated PPI, reduced immobility in the FST, and reduced aggression, suggesting a protective effect against both schizophrenia and depression-like behaviors; however, these mice also had increased anxiety, reduced locomotion, and deficits in fear memory (200). Mice with diminished serine racemase (Srr) activity were found to have deficits in sociability and PPI (201). Kalirin (Kalrn) KO mice not only showed a similar phenotype to the Srr model but also showed increased anxiety, deficits in spatial learning and memory, and deficits in working memory (202). Given that deficits in sociability are seen in both depression and schizophrenia, the Srr and Kalrn mouse models could be interpreted as models for schizophrenia only.
Fez1 is involved in intracellular transport and has functions in neurodevelopment (172, 203). PDE4B and Srr are involved in cAMP and NMDA neurotransmission, respectively, and therefore affect diverse aspects of neuronal functioning (204, 205). GSK-3α is a serine–threonine kinase, and has been implicated in neurodevelopment, neurotransmitter function, neuroinflammation, and synaptic plasticity (206–209). Kalrn is a brain-specific guanine nucleotide exchange factor (GEF) that is a known regulator of spine morphogenesis (202). In addition to their link through DISC1, many of these molecules have their own links to schizophrenia and depression. Human genetic studies and expression studies have associated PDE4B, FEZ1, SRR, and KALRN with schizophrenia (177, 210–213). PDE4 and GSK-3 are associated with the action of antipsychotics and antidepressants (207, 214). Additionally, SRR metabolite d-serine is known to be beneficial in schizophrenia (215). These molecules regulate schizophrenia and depression-associated pathways downstream of DISC1 and further implicate neurodevelopment, synaptic processes such as spine regulation, and cAMP and NMDA signaling in schizophrenia and depression-associated behaviors.
Reelin
Reelin (RELN) has been identified as a top candidate gene for schizophrenia in genetic association studies (216–219). Reelin levels were also shown to be decreased in schizophrenia and bipolar disorder (220–222), and altered with antipsychotic, antidepressant, and mood stabilizing medications (223). Reelin is a glycoprotein that is critical for development. The characteristically disorganized cortex of reeler mice demonstrates the importance of reelin in neuronal migration (224–226). Reelin is also important in synapse formation and plasticity, neuronal development, glutamatergic neurotransmission, and adult neurogenesis (225, 227–230). The intricacies of reelin signaling have been intensively studied and go beyond the scope of this review (231). Briefly, the effects of reelin on behavior and its connections to schizophrenia and depression may be realized through multiple pathways, from alterations in glutamate signaling and synapse regulation to widespread neurodevelopmental effects related to neuronal migration. Further work is needed to understand the specific contributions of these different pathways in mediating the effects of reelin on behavior, as well as their relationship to specific neuropsychiatric disorders such as schizophrenia and depression.
Reelin-deficient “reeler” mice are not suitable for behavioral testing due to disruptions in motor activity (225). However, heterozygous reeler mice have been used as a model for schizophrenia, although their validity in this context has been questioned (232). Some studies found that heterozygous reeler mice have cognitive deficits in operant conditioning and executive function (233–235), deficits in PPI, LI, and fear conditioning (236–238), male-specific hyperactivity in the MK-801-ILAT (239), and anxiety-like behavior in the EPM and OFT (237, 240). Others groups found no significant effects in these mice (232, 235, 241). Heterozygous reeler mice were also found to have altered LTP, and brain region-specific alterations in NMDA receptor subunit levels and ratios (233, 239, 242). Teixeira et al. found that reelin overexpressing mice had normal behavior under basal conditions; however, the mice had reduced immobility time in the FST after chronic corticosterone treatment, and reduced cocaine sensitization in the cocaine-ILAT (243). Furthermore, reelin overexpression prevented ketamine-induced PPI deficits. This group found no significant differences in heterozygous reelin-deficient mice, adding to the controversial findings associated with this model. Reelin models demonstrate the difficulties that can be encountered when attempting to replicate behavioral effects across different labs. Minute differences in environmental and test conditions can have consequences on behavior. Nevertheless, there are multiple lines of evidence supporting a role for reelin in neuropsychiatric disorders.
Maternal immune activation
Maternal infection during pregnancy has consistently been associated with increased schizophrenia risk (244, 245). Maternal immune activation (MIA) was shown to affect DNA methylation (246). Hence, epigenetic changes caused by immune challenge during critical periods of development may perturb important schizophrenia-related pathways, which interact with underlying genetic susceptibility and lead to the development of symptoms. Interestingly, MIA has not been associated with depression in humans. A recent study of over 6,000 subjects failed to find an association between prenatal viral infection and the development of non-psychotic depression (247). Nevertheless, animal models have demonstrated behaviors relevant to both schizophrenia and depression.
Multiple rodent models have been used to examine the effects of prenatal immune challenge, primarily in relation to schizophrenia (248). MIA models have been less thoroughly explored for endophenotypes of depression. Nevertheless, certain models have shown phenotypes relevant for both schizophrenia and depression. Maternal viral infection modeled by challenge with polyriboinosinic–polyribocytidilic acid (poly I:C) caused: schizophrenia-like deficits in PPI, LI, and psychostimulant hypersensitivity in adult, but not adolescent animals, abnormal hippocampal–prefrontal synchrony (an electrophysiological endophenotype for schizophrenia), and changes in DA metabolism and receptor binding (249–263). Adult poly I:C exposed mice had impairments in recognition memory that were rescued with clozapine, but not haloperidol, deficits in spatial working learning and memory, and increased anxiety-like behavior (250, 254–256, 264, 265). Reversal learning was either impaired or improved depending on the timing of poly I:C exposure (264). The mice also showed anhedonia in the SPT; although only offspring of mothers that lost weight as a result of poly I:C injection displayed this effect (266). Additionally, MIA has been used in conjunction with genetic models to study gene × environment (G × E) interactions. For example, poly I:C MIA exacerbated the schizophrenia-like phenotype in Disc1-L100P heterozygotes (Table 1) (195). Interestingly, poly I:C MIA at embryonic day 9 in DN-DISC1 tg mice caused the development of anxiety-like and depression-like behaviors that were not seen in untreated DN-DISC1 tg mice (194). These changes were associated with enlarged ventricles, reduced hippocampal serotonin (5-HT), and reduced reactivity in the hypothalamic–pituitary–adrenal (HPA) axis.
The aberrant neuroanatomy and DA signaling seen in MIA models has long been associated with schizophrenia (267, 268). While MIA has not yet been associated with depression in humans, MIA interacted with DISC1 mouse models to generate depression-like behaviors. It may be that MIA is linked specifically to comorbid depression, but does not affect the development of major depressive disorder on its own. The effects of MIA on gene expression, DA and 5-HT signaling, neuroanatomy, and the HPA axis may interact with other genetic risk factors such as disruptions in DISC1, which leads to the development of schizophrenia and depression comorbidity. Future research is needed to explore the effects of MIA in conjunction with genetic risk factors on comorbid depression.
Social isolation stress
Increased feelings of loneliness and social isolation, and decreased family and social support were associated with an increased risk depression and suicidality in schizophrenia patients (269). This is consistent with the long-standing hypothesis that environmental stressors such as social isolation can trigger depression in genetically susceptible individuals (270–272). The stress associated with social isolation may therefore be a factor in triggering comorbid depression in schizophrenia patients.
Social isolation is known to cause a number of behavioral changes related to neuropsychiatric disorders (273). Isolation during a critical period of post-natal development after weaning in rats was shown to cause hyperactivity in the OFT, schizophrenia-like deficits in PPI, but not LI, increased social interaction and aggression, and increased anxiety-like behavior (274–284). Isolation-reared rats showed increased responses to rewarding stimuli, including increases in sucrose and ethanol preference, operant responding for ethanol, and initiation of drug self-administration (285–291). Additionally, social isolation was shown to cause cognitive deficits in executive function, reversal learning, and spatial learning (291–296); although, spatial learning deficits were not universally seen, with some groups seeing no change and others seeing improvements (293, 297, 298). Isolation stress has also been used in conjunction with genetic mouse models. Social isolation in DN-DISC1-Tg-PrP mice induced schizophrenia-like deficits in PPI and depression-like behavior in the FST that were not seen in the socially isolated wild type mice or DN-DISC1-Tg-PrP control groups (Table 2) (196). These changes were linked to glucocorticoid and epigenetic control of genes related to DA signaling.
Social isolation causes brain region-specific alterations in DA and 5-HT activity in rodents; for example, heightened dopaminergic activity in the nucleus accumbens and ventral striatum, but reduced DA function in the PFC (273). Isolation was also shown to cause alterations in the expression and localization of glutamate receptor subunits, decrease numbers of GABAergic interneurons in the hippocampus, and decrease hippocampal brain-derived neurotrophic factor (BDNF) (50, 299–302). Additionally, isolation affected dendritic spine density and morphology in the PFC, striatum, and hippocampus (303–306). Post-weaning social isolation may therefore influence the development of schizophrenia and depression-related behavior via epigenetic changes – possibly through the HPA axis – that cause alterations in neurosignaling. These alterations may also interact with genetic risk factors such as DISC1, resulting in the development of symptoms. Isolation stress demonstrates that environmental input can induce schizophrenia and depression-related behavioral deficits in animals and is an excellent tool to use in conjunction with genetic models to test G × E interactions.
Honorable mentions
This review has focused on animal models that display both schizophrenia and depression-relevant endophenotypes. However, due to the large amount of overlap between risk factors for schizophrenia and depression, numerous genes show convergent evidence from human data for a shared association between these disorders. Animal models of these genes do not necessarily display endophenotypes for both schizophrenia and depression. Nevertheless, below are some of the genes relevant to both disorders despite less phenotypic relevance to comorbidity than the previous animal models.
Neuregulin 1
An association between neuregulin 1 (NRG1) and schizophrenia is strongly supported by human genetic studies (307–313). NRG1 SNPs have also been associated with depression and bipolar disorder (314–318). NRG1 is a member of a family of epidermal growth factor-like proteins, which interact with the ErbB family of receptor tyrosine kinases to play a role in neurodevelopment, neuronal migration, Schwann cell growth, and brain activity homeostasis (319, 320).
While homozygous mice are embryonic lethal, heterozygous KO of NRG1, and its various isoforms display multiple schizophrenia-related behavioral deficits, including impaired PPI and LI, hyperactivity in the OFT, deficits in fear conditioning, impaired working memory, and/or abnormal social behavior (321–330). Overexpression of NRG1 also results in deficits in PPI, hyperactivity, and impairments in working memory, contextual fear conditioning, and social interaction (331–335). Additionally, heterozygous NRG1-KO mice show an increased sensitivity to the cannabinoid delta9-tetrahydrocannabinol (THC) and altered behavior in response to chronic social defeat stress (336, 337). Notably, social defeat caused impaired working memory and decreased aggression in NRG1 mice, but reduced deficits in sucrose preference relative to wild type mice (337). The relevance of NRG1 models to depression is weaker. While cognitive and social deficits and the altered response to psychosocial stress overlap as endophenotypes for schizophrenia and depression, there is much less research examining NRG1-KO mice in the specific context of depression. Future research and improvements in depression-related endophenotypes will reveal the utility of NRG1 models in studying comorbidity directly. Nevertheless, these models remain as excellent tools for understanding a pathway that shows convergent evidence for both depression and schizophrenia associations in humans.
Catechol-O-methyltransferase
Catechol-O-methyltransferase (COMT) is a primary DA metabolizing enzyme in the PFC and amygdala (338). COMT has been of particular interest in human studies because of a functional polymorphism (Val158Met), which is associated with a three to fourfold reduction in enzymatic activity and increased synaptic DA activity (339). While the COMT gene is located in a region associated with high schizophrenia risk (22q11), associations between the gene itself and schizophrenia have been inconsistent (340, 341). Likewise, COMT genetic variation does not appear to be associated with depression diagnosis or severity, and there is conflicting evidence for an association with response to antidepressants (342, 343). Nevertheless, COMT – particularly the Val158Met allele – is associated with a number of human endophenotypes, which are important in schizophrenia and depression, including PFC-mediated cognition, variations in brain structure, and anxiety traits (344–350). COMT has also been associated with violent behavior in schizophrenia patients (351, 352). COMT is therefore a potentially important player in linking certain cognitive and neuroanatomical symptom domains of depression and schizophrenia to the DA system.
COMT-KO mice exhibited an attenuated response to inhibition of DA transporter (DAT) and amphetamine in the ILAT, female-specific increases in anxiety-like behavior, male-specific increases in aggression, altered exploration and habituation in the OFT, and increased vulnerability to the disruptive effects of THC (353–358). Male COMT-KO mice displayed mild improvements in spatial and working memory (359, 360). Additionally, pharmacological inhibition of COMT improved attentional set-shifting performance in rats (361). Transgenic mice expressing the human COMT Val variant had impairments in attentional set-shifting, recognition memory, and working memory (360). Also, pain sensitivity and stress reactivity were decreased in transgenic mice and increased in COMT-KO mice. While COMT mice lack classical depression and schizophrenia-related endophenotypes, the models demonstrate relevance for cognitive function, which is an important aspect of both disorders. These models are therefore excellent tools for examining the role of DA function in cognition.
Brain-derived neurotrophic factor
Brain-derived neurotrophic factor is the most well studied and characterized neurotrophin in the CNS; we can only briefly touch upon the considerable literature here (362–365). BDNF and its high affinity tropomyosin-related kinase B (TrkB) receptor are involved in many important neuronal processes, including neurodevelopment, axon targeting, neuronal growth and survival, and synaptic plasticity (362). Evidence for altered brain and serum BDNF levels in schizophrenia is controversial, with studies finding both increased and decreased levels in various brain regions (366–372). However, the BDNF Val66Met polymorphism has been associated with increased schizophrenia risk (373). Furthermore, the BDNF Val66Met allele was shown to interact with childhood trauma to decrease blood BDNF mRNA levels and hippocampal subfield volumes in schizophrenia and bipolar disorder patients, suggesting a G × E interaction that may have consequences on brain development and function in psychosis (374). Decreased BDNF levels have been consistently reported in depression, particularly in suicidal patients (375–382). Indeed, a role for BDNF in the pathophysiology and treatment of depression and schizophrenia is strongly supported.
Many mouse lines have been developed with various mutations in BDNF. Homozygous mice possessing a BDNF null mutation are not viable. However, heterozygotes display many relevant phenotypes, including hyperactivity, hyperphagia causing excess weight gain, potentiated response to amphetamine in the ILAT, aggression, impaired contextual fear conditioning, extinction learning deficits, and sex-specific vulnerability to the behavioral effects of THC and corticosterone (383–392). Heterozygous BDNF-KO mice showed baseline PPI deficits only in paradigms involving chronic injection, suggesting that this may be a stress-induced effect (386, 391, 392). Supporting the susceptibility of PPI to environmental factors in heterozygous BDNF-KO mice is the finding that cannabinoid and methamphetamine treatment in young-adult mice caused sex-specific changes in PPI response to acute cannabinoid and amphetamine challenge, respectively, in adult heterozygous mice relative to both wild type and untreated heterozygotes (386, 392). Depression-related endophenotypes such as learned helplessness, anhedonia, and vulnerability to stress were not seen in heterozygous BDNF-KO mice (393–395). However, the response to amine-based antidepressants is attenuated in this model (396). Conditional fetal, post-natal, hippocampal, and forebrain-inducible BDNF-KO mice displayed depression-like behaviors in certain tests (397–399). Forebrain-specific BDNF-KO mice displayed learning and memory deficits (400). Additionally, a mouse model of the human Val66Met allele displayed increased aggression, anxiety, and deficits in contextual fear conditioning (401). BDNF overexpressing mice showed improved learning and memory in the MWM and reduced immobility in the FST (402, 403).
Mice lacking BDNF receptor TrkB in the brain demonstrated a similar phenotype, displaying hyperactivity, and increased impulsivity in the NORT, but not depression-like or anxiety-like behaviors in the FST or EPM (404). Conversely, mice overexpressing TrkB show improvements in spatial learning and memory, contextual fear conditioning, and reduced anxiety in the EPM (405). Finally, the importance of environmental factors to BDNF is supported by the finding that maternal separation and adolescent/young-adult corticosterone treatment caused sex and brain region-specific changes in BDNF and TrkB function coupled with male-specific deficits in working memory and female-specific anhedonia in the SPT (406).
While related endophenotypes of depression and schizophrenia are seen in various BDNF models, they are not seen simultaneously in the same model. More research on BDNF models in the context of both schizophrenia and depression is needed. Nevertheless, various disruptions in the BDNF pathway do lead to both schizophrenia and depression-related behavioral deficits. This suggests a role for BDNF in a shared pathway between the two disorders. Parsing the differences that lead to specific disruptions in behavior will greatly aid in elucidating the contributions of the BDNF pathway to depression and schizophrenia.
Bringing the Picture Together
The emerging picture of the genetic architecture of schizophrenia is revealing that hundreds of genes with small effect sizes influence the disorder (407). The genetic picture of depression is far less clear, with heritability estimates predicting a much greater contribution of environmental effects than in schizophrenia (408, 409). Hence, it is not surprising that the array of factors that influence depression and schizophrenia-related phenotypes in rodent models is diverse. However, a number of common elements between these models are becoming evident. The emerging pathways that are shared between these models are represented in Figure 1.
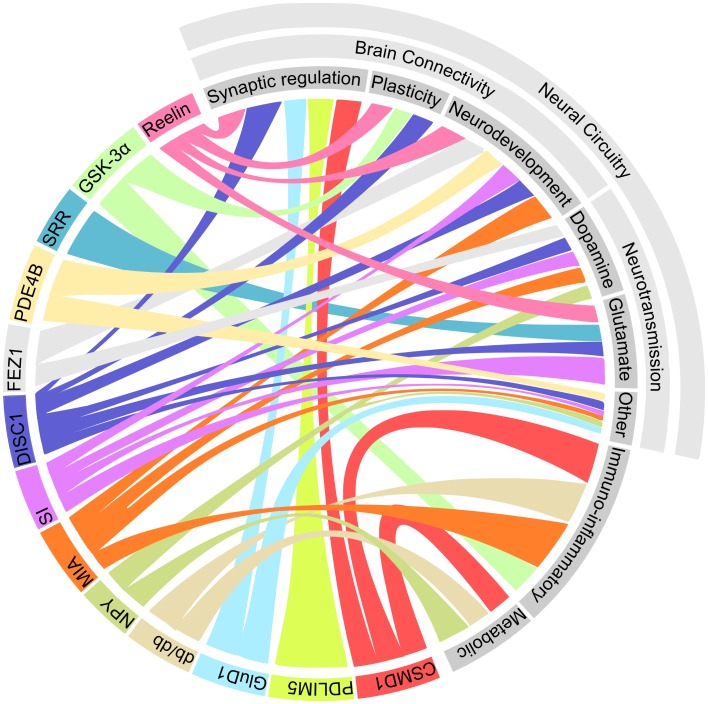
Figure 1.
Multiple shared pathways between rodent models which display both schizophrenia and depression-related phenotypes. This diagram illustrates the connections between each of the models (represented in color on the bottom right) and the biological processes which potentially underlie the observed phenotypes (represented in gray at the top left). Abbreviations: CSMD1, CUB and SUSHI multiple domains 1; DISC1, disrupted in schizophrenia 1; FEZ1, fasciculation and elongation protein zeta 1; GluD1, glutamate receptor delta 1; GSK-3α, glycogen synthase kinase 3α; MIA, maternal immune activation; NPY, neuropeptide Y; PDE4B, phosphodiesterase 4B; PDLIM5, PDZ and LIM domain 5; SRR, serine racemase; SI, social isolation.
Neurotransmission: The familiar suspects
Most current antipsychotics and antidepressants affect neurotransmitters in the synapse. A number of the models mentioned have demonstrated links to various neurotransmitter systems. NPY, DISC1, and Fez1 pathways interact with the DA system (157, 158, 188, 197, 410, 411). Altered DA and 5-HT activity was seen after MIA and social isolation. Furthermore, the increased sensitivity to psychostimulants seen in a number of the aforementioned models is thought to be related to DA activity (412). A role for glutamate is also implicated in many of these models. Srr, DISC1, and reelin are all involved in glutamatergic signaling (205, 413, 414). Social isolation also affected NMDA receptor localization (299). Many antipsychotics have been used in the treatment of depressive disorders (415). It is possible that the shared involvement of certain neurotransmitter systems in schizophrenia and depression underlies both the increased risk of comorbid depression in schizophrenia and the antidepressant activity of these antipsychotics.
Involvement of monoamine systems in schizophrenia and depression is by no means a new hypothesis (267, 416). DA, in particular, is strongly implicated in schizophrenia. Furthermore, it is easy to see how the mesocortical and mesolimbic DA reward circuits could be involved in anhedonia and amotivational states associated with both depression and schizophrenia. While these models provide additional support for this hypothesis, a number of questions remain regarding the role of monoamine systems in generating the phenotypes seen in these models. DISC1, NPY, and Fez1 are all involved in multiple pathways. Future experiments are needed to determine if the DA system alone is necessary and sufficient to account for specific observed phenotypes, or if it is peripheral or supplementary to the development of certain schizophrenia and depression-related endophenotypes. Likewise, MIA and social isolation affect more than just neurotransmitter systems. Some work has been done to uncover the molecular pathways by which these environmental factors cause perturbations in neurotransmitter systems; for example, social isolation caused DNA methylation of the promoter region of tyrosine hydroxylase in the ventral tegmental area of DN-DISC1-Tg-PrP mice. More similar studies are needed to uncover the complex G × E interactions which lead to altered neurotransmission in these models.
There is mounting evidence that glutamate plays a major role in psychiatric illness. The link between glutamate and schizophrenia was first proposed based on the observation that NMDA receptor antagonists phencyclidine (PCP) and ketamine can induce schizophrenia-like symptoms in healthy individuals (417–419). The hypothesis has since gained supporting evidence from human genetic and imaging studies, as well as animal models of NMDA receptor hypofunction (419–421). A relationship between the glutamate system and depression is suggested by the rapid and long-lasting antidepressant effects of ketamine (422). Early evidence is showing that compounds targeting the glutamate system may have efficacy in treating positive, negative, and cognitive symptoms of schizophrenia (423). The efficacy of these compounds in treating negative symptoms such as anhedonia and social withdrawal, which overlap with depression, may indicate potential antidepressant activity. Hence, the glutamate system is of particular interest in treating comorbid depression in schizophrenia. The current animal models will be useful for investigating the efficacy of new compounds targeting the glutamate system in treating symptoms of schizophrenia and depression.
Connectivity is the key
Disruptions in processes related to brain connectivity are a common theme among the many of the models outlined here. Almost all of the models mentioned demonstrate links to synaptic processes such as synapse formation, regulation, and plasticity. Pdlim5, GluD1, reelin, Disc1, Kalrn, and social isolation affect synaptic spine morphology and/or formation, and GSK-3, Disc1, and reelin affect synaptic plasticity (107, 121, 134, 135, 202, 209, 231, 424, 425). Dynamic changes in synaptic spine morphology and formation, both developmentally during the establishment of neuronal circuits and as the result of activity or experience-dependent remodeling of existing circuits, are thought to be intimately linked to cognitive development and function (426–429). Disruptions in neurodevelopment may also interfere with brain connectivity through the “miswiring” of neuronal circuits. Disc1 and reelin are both important for neuronal migration and the formation of cortex layers, and PDE4B is involved in axon guidance and dendritic growth (183, 186, 224). Miswiring of neuronal circuits, whether at the level of brain structure from abnormal neurodevelopment or from dysregulation at the level of the synapse, possibly cause maladaptive alterations in brain connectivity leading to altered stimulus processing and cognition. This may be a common mechanism underlying the cognitive and behavioral symptoms of both schizophrenia and depression.
Broad constructs such as connectivity, synaptic regulation, and plasticity are far too general to lead to significant advancement in the mechanistic understanding or treatment of neuropsychiatric illness (430). Furthermore, these mechanisms are implicated in a myriad of disorders in addition to depression and schizophrenia; for example, neuronal circuit dysfunction is also implicated in intellectual disability, ASD, and Alzheimer’s (431). Future research should examine the precise changes in specific neural circuitry and synaptic processes in these and forthcoming models of schizophrenia and depression. Models such as the Pdlim5-KO and Fez1-KO mice are of particular interest as they display endophenotypes of one disorder while being protective against endophenotypes of the other. Hence, these molecules may represent points in the shared pathway where schizophrenia and depression diverge. Examining these mechanisms could hint at the subtle changes that can cause the emergence of disparate symptoms in disorders with shared genetic susceptibility. Research correlating precise changes in neural circuitry and synapse function to specific disease-related endophenotypes in these animal models will be important in completing the picture linking genetic changes to pathophysiology and ultimately behavior.
Immune and environmental factors
Dysregulation of the immune system, cytokines, and oxidative and nitrositive stress have been proposed as important factors in both schizophrenia and depression (432, 433). This hypothesis is supported by a myriad of evidence from the study of immuno-inflammatory markers in humans, genetic association studies, and animal models (434, 435). Immune and inflammatory processes were implicated in schizophrenia and depression comorbidity by a number of models, including Csmd1, db/db, GSK-3, and MIA (145, 208, 248). Furthermore, Csmd1 provides a link between the immune system and neuronal processes such as synaptic pruning (107). It has been proposed that schizophrenia is immunologically primed for the expression of depression (434). This is supported by the aforementioned models, particularly by findings such as the interaction between MIA and Disc1 in mice to illicit depression and anxiety-like behavior (194).
However, a number of key questions remain in understanding the role of immune and inflammatory processes. Future research should reveal the extent to which these processes are responsible for the observed phenotypes. MIA-induced locomotor changes could be rescued by maternal treatment with non-steroidal anti-inflammatory drugs or adolescent treatment with the COX-2 inhibitor celecoxib (436, 437). Similar investigations could be done using other models; for instance, using the db/db mice in which hippocampal inflammation is thought to be an important factor in the observed phenotype. Furthermore, determining the sensitivity of treatment with anti-inflammatory drugs at different time points will reveal if immune insult leads to permanent changes in brain structure and function. This will be important for developing new therapeutic strategies for schizophrenia and depression.
Models of MIA and social isolation stress demonstrate how environmental factors can cause broad changes in neurobiology and behavior. Heritability is estimated at 81% for schizophrenia and 37% for depression (408, 438). This means almost 1/5th of the estimated variance in liability for schizophrenia and 3/5ths for depression is due to non-genetic factors. Clearly, environmental factors play an important role in influencing brain function, and are modulated by both genetic and epigenetic factors (439). Several environmental stressors such as psychosocial stress, drug abuse, nutrition, and MIA influence schizophrenia and depression in humans, as well as related endophenotypes in mice (440, 441). Environmental factors have already been combined with Disc1 genetic models to explore the complex and synergistic G × E interactions, which trigger the development of pathological endophenotypes (194–196, 442, 443). Additionally, two-hit models have been used combining factors such as acute and chronic response to THC, psychosocial stress, and chronic unpredictable stress with models of NRG1, COMT, BDNF, and other genes (336, 337, 358, 444). Future research should apply this type of hybrid G × E approach to other combinations of genes and environmental factors to improve our understanding of how genes modulate sensitivity to environmental stressors and lead to mental illness. Additionally, correlating these effects with changes in the epigenome will improve our understanding of the sequence of molecular events which lead to the emergence of symptoms in depression and schizophrenia.
Metabolic syndrome: Culprit, accomplice, or bystander?
Patients with both schizophrenia and depression are at an increased risk for components of metabolic syndrome, including obesity, hypertension, atherogenic dyslipidemia, hyperglycemia, and diabetes (445–447). Metabolic syndrome in schizophrenia patients can be partially explained as a side-effect of antipsychotic medications (448). Nevertheless, a common mechanism between these conditions is hinted at by the db/db, Csmd1, and NPY models (108, 145, 161–163). Research linking metabolic syndrome and mental illness is still relatively new. Systemic inflammation and immune activation are features of schizophrenia, depression, and metabolic syndrome (434, 447). Hence, immune dysregulation could be a causal factor in all three disorders. This is supported by fact that knocking out the immune molecule Csmd1 led to the development of glucose tolerance as well as schizophrenia and depression endophenotypes in mice (108). Alternatively, disruptions in systems such as leptin and NPY, which are involved in hunger and satiety, may cause schizophrenia and depression, while simultaneously predisposing patients of these disorders to behavioral risk factors for metabolic syndrome such as poor diet and sedentary lifestyle.
Whether metabolic syndrome is a causal factor, a consequence, or simply a marker of mental illness is subject to controversy (449). Future studies should determine if treating metabolic deficits in models such as the Csmd1-KO and db/db mice rescues schizophrenia and depression-related endophenotypes. This would help to elucidate the causal status of metabolic conditions in schizophrenia and depression endophenotypes. A detailed discussion of the other links between depression, schizophrenia, and metabolic disorder goes beyond the scope of this review (446, 447). More research is needed to reveal the relationship between these disorders.
Whole Picture and Future Directions
Ultimately, all of the factors mentioned here are intimately interconnected. Dopaminergic, glutamatergic, and GABAergic neurotransmitter systems interact as neural circuits and are influenced by inputs from multiple other systems (267, 420, 450). Synaptic processes are regulated by neurotransmitters and immune molecules, and in turn affect neurotransmission (451–453). Finally, neurodevelopmental processes wire the machinery necessary for all this to occur, and are influenced by each of these factors (454, 455). Future research should then focus on differentiating precise mechanisms and their relationships to these highly integrated systems. Advancing technologies such as optogenetics and light sheet microscopy should aid in deciphering the roles of specific neural circuitry (456). Neural circuits can be further interrogated through the use of genetic approaches in simple model organisms such as Drosophila larvae and Caenorhabditis elegans (457, 458). Conversely, applying genetic techniques to more complex organisms such as the rat will allow for the assessment of more sophisticated cognitive and social behaviors (174).
While this review specifically focused on schizophrenia and depression, Table 1 emphasizes that the behavioral endophenotypes used in these studies are linked to multiple disorders. Furthermore, many of the genes targeted in these models have multiple associations; for example, DISC1 is associated with bipolar disorder, major depression, social anhedonia, chronic fatigue syndrome, anxiety, neuroticism, emotional stability, schizophrenia, schizoaffective disorder, and ASD (179, 459, 460). Given this level of complexity, it is unrealistic to assume that disorders such as schizophrenia and depression can be sufficiently approximated and recognized in rodent models, especially considering the controversy in psychiatric nosology itself regarding the definition of discrete boundaries between disorders (461). Hence, it may be useful to focus on correlating specific molecular pathways with certain endophenotypes rather than attempting to interpret models as holistic representations of mental illness. Considering the multidimensional nature of both depression and schizophrenia, understanding causal mechanisms as they relate to certain dimensions of symptomology reflected in specific endophenotypes may provide insight into the genetic origins of the heterogeneous and multidimensional nature of these disorders.
Lastly, if we desire to use animal models for translational research in drug discovery, we will need models with greater etiological validity. Schizophrenia and depression are highly polygenetic, likely resulting from the contribution of multiple low-effect genetic risk factors combined with environmental stressors (407, 409). Less severe and more specific genetic manipulations such as point mutation models, genetic hypomorphs, and RNAi gene knockdown methods cause more subtle changes that better mimic the types of genetic factors seen in human populations (174). Furthermore, multi-hit models, which combine multiple mutations and environmental factors, more closely model the polygenetic nature of these disorders, and allow for the interrogation of both gene × gene and G × E interactions. New methods such as the CRISPR/Cas system allow for the one step generation of multiple mutations to greatly accelerate the development of such models (462).
Conclusion
What has emerged so far from the study of animal models exhibiting both depression and schizophrenia-related phenotypes is a number of broadly defined mechanisms, which may underlie a shared pathophysiology between the two disorders. Given the complex, heterogeneous nature of these disorders, it is likely that neurotransmission, brain connectivity, immune, and environmental factors all contribute to their pathophysiology. As more models are discovered, the emerging picture of the shared pathophysiological mechanisms between schizophrenia and depression will become increasingly coherent. Interrogating precise molecular and neural substrates as they relate to specific endophenotypes, and carefully examining gene × gene and G × E interactions will contribute to a better understanding of the neurobiological mechanisms of comorbidity in mental illness. This understanding will inform future efforts in developing treatments for neuropsychiatric comorbidity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AW was partly supported by a Mid-Career Fellowship from the Ontario Mental Health Foundation (OMHF).
References
- 1.Ekman M, Granstrom O, Omerov S, Jacob J, Landen M. The societal cost of schizophrenia in Sweden. J Ment Health Policy Econ (2013) 16:13–25. 10.1016/s1098-3015(10)72536-5 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; (2008). [Google Scholar]
- 3.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (2013) 382:1575–86. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 4.Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull (2009) 35:383–402. 10.1093/schbul/sbn135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häfner H, Maurer K, An der Heiden W. Schizophrenia – a disorder in its own right? Results from 25 years of the ABC study. Nervenarzt (2013) 84:1093–4. 10.1007/s00115-013-3788-6 [DOI] [PubMed] [Google Scholar]
- 6.Häfner H, Maurer K, An der Heiden W. ABC schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol (2013) 48:1021–31. 10.1007/s00127-013-0700-4 [DOI] [PubMed] [Google Scholar]
- 7.Sönmez N, Romm KL, Andreasssen OA, Melle I, Røssberg JI. Depressive symptoms in first episode psychosis: a one-year follow-up study. BMC Psychiatry (2013) 13:106. 10.1186/1471-244X-13-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlashan T, Carpenter WT., Jr Postpsychotic depression in schizophrenia. Arch Gen Psychiatry (1976) 33:231–9 10.1001/archpsyc.1976.01770020065011 [DOI] [PubMed] [Google Scholar]
- 9.Upthegrove R, Birchwood M, Ross K, Brunett K, McCollum R, Jones L. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr Scand (2010) 122:211–8. 10.1111/j.1600-0447.2009.01506.x [DOI] [PubMed] [Google Scholar]
- 10.Andriopoulos I, Ellul J, Skokou M, Beratis S. Suicidality in the “prodromal” phase of schizophrenia. Compr Psychiatry (2011) 52:479–85. 10.1016/j.comppsych.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Gozdzik-Zelazny A, Borecki L, Pokorski M. Depressive symptoms in schizophrenic patients. Eur J Med Res (2011) 16:549–52 10.1186/2047-783X-16-12-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson J, Horwath E, Weissman MM. The validity of major depression with psychotic features based on a community study. Arch Gen Psychiatry (1991) 48:1075–81. 10.1001/archpsyc.1991.01810360039006 [DOI] [PubMed] [Google Scholar]
- 13.Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry (2002) 159:1855–61. 10.1176/appi.ajp.159.11.1855 [DOI] [PubMed] [Google Scholar]
- 14.Häfner H, Maurer K, Trendler G, An der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases – a controlled study of schizophrenia, depression and healthy controls. Schizophr Res (2005) 77:11–24. 10.1016/j.schres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Häfner H, Löffler W, Maurer K, Hambrecht M, An der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand (1999) 100:105–18. 10.1111/j.1600-0447.1999.tb10831.x [DOI] [PubMed] [Google Scholar]
- 16.Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res (2003) 60:21–32. 10.1016/S0920-9964(02)00167-6 [DOI] [PubMed] [Google Scholar]
- 17.Schothorst PF, Emck C, van Engeland H. Characteristics of early psychosis. Compr Psychiatry (2006) 47:438–42 10.1016/j.comppsych.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Park S, Lee H, Sakong J, Jun T, Lee M, Kim J, et al. Distinctive clinical correlates of psychotic major depression: the CRESCEND study. Psychiatry Investig (2014) 11:281–9. 10.4306/pi.2014.11.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gournellis R, Oulis P, Howard R. Psychotic major depression in older people: a systematic review. Int J Geriatr Psychiatry (2014) 29:784–96. 10.1002/gps.4065 [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association DSM-5, Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; (2013). [Google Scholar]
- 21.Raymond Lake C. Disorders of thought are severe mood disorders: the selective attention defect in mania challenges the Kraepelinian dichotomy a review. Schizophr Bull (2008) 34:109–17. 10.1093/schbul/sbm035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heckers S. Diagnostic criteria for schizoaffective disorder. Expert Rev Neurother (2012) 12:1–3 10.1586/ern.11.179 [DOI] [PubMed] [Google Scholar]
- 23.Möller H. Occurrence and treatment of depressive comorbidity/cosyndromality in schizophrenic psychoses: conceptual and treatment issues. World J Biol Psychiatry (2005) 6:247–63. 10.1080/15622970500316674 [DOI] [PubMed] [Google Scholar]
- 24.Siris SG, Adan F, Cohen M, Mandeli J, Aronson A, Casey E. Postpsychotic depression and negative symptoms: an investigation of syndromal overlap. Am J Psychiatry (1988) 145:1532–7. 10.1176/ajp.145.12.1532 [DOI] [PubMed] [Google Scholar]
- 25.Birchwood M, Iqbal Z, Chadwick P, Trower P. Cognitive approach to depression and suicidal thinking in psychosis. 1. Ontogeny of post-psychotic depression. Br J Psychiatry (2000) 177:516–28. 10.1192/bjp.177.6.522 [DOI] [PubMed] [Google Scholar]
- 26.Johnson DA. Studies of depressive symptoms in schizophrenia. Br J Psychiatry (1981) 139:89–101 10.1192/bjp.139.2.89 [DOI] [PubMed] [Google Scholar]
- 27.An der Heiden W, Könnecke R, Maurer K, Ropeter D, Häfner H. Depression in the long-term course of schizophrenia. Eur Arch Psychiatry Clin Neurosci (2005) 255:174–84. 10.1007/s00406-005-0585-7 [DOI] [PubMed] [Google Scholar]
- 28.Chiappelli J, Kochunov P, DeRiso K, Thangavelu K, Sampath H, Muellerklein F, et al. Testing trait depression as a potential clinical domain in schizophrenia. Schizophr Res (2014) 159:243–8. 10.1016/j.schres.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrow M, Yonan CA, Sands JR, Marengo J. Depression in schizophrenia: are neuroleptics, akinesia, or anhedonia involved? Schizophr Bull (1994) 20:327–38. 10.1093/schbul/20.2.327 [DOI] [PubMed] [Google Scholar]
- 30.Prosser ES, Csernansky JG, Kaplan J, Thiemann S, Becker TJ, Hollister LE. Depression, parkinsonian symptoms, and negative symptoms in schizophrenics treated with neuroleptics. J Nerv Ment Dis (1987) 175:100–5. 10.1097/00005053-198702000-00006 [DOI] [PubMed] [Google Scholar]
- 31.Westermeyer J. Comorbid schizophrenia and substance abuse: a review of epidemiology and course. Am J Addict (2006) 15:345–55. 10.1080/10550490600860114 [DOI] [PubMed] [Google Scholar]
- 32.Turkington A, Mulholland CC, Rushe TM, Anderson R, McCaul R, Barrett SL, et al. Impact of persistent substance misuse on 1-year outcome in first-episode psychosis. Br J Psychiatry (2009) 195:242–8. 10.1192/bjp.bp.108.057471 [DOI] [PubMed] [Google Scholar]
- 33.Clarke DM, Kissane DW. Demoralization: its phenomenology and importance. Aust N Z J Psychiatry (2002) 36:733–42. 10.1046/j.1440-1614.2002.01086.x [DOI] [PubMed] [Google Scholar]
- 34.Kudo J, Mori H, Gomibuchi T. Loneliness as expressed by schizophrenic patients in the early remission phase. Nagoya J Med Sci (2002) 65:115–26. [PubMed] [Google Scholar]
- 35.Cross-Disorder Group of the Psychiatric Genomics Consortium. Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet (2013) 381:1371–9. 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgi A, Jamra RA, Klein K, Villela AW, Schumacher J, Becker T, et al. Possible association between genetic variants at the GRIN1 gene and schizophrenia with lifetime history of depressive symptoms in a German sample. Psychiatr Genet (2007) 17:308–10. 10.1097/YPG.0b013e3280c1e5fb [DOI] [PubMed] [Google Scholar]
- 37.Boks MPM, Hoogendoorn M, Jungerius BJ, Bakker SC, Sommer IE, Sinke RJ, et al. Do mood symptoms subdivide the schizophrenia phenotype? Association of the GMP6A gene with a depression subgroup. Am J Med Genet B Neuropsychiatr Genet (2008) 147B:707–11. 10.1002/ajmg.b.30667 [DOI] [PubMed] [Google Scholar]
- 38.Hamshere ML, Williams NM, Norton N, Williams H, Cardno AG, Zammit S, et al. Genome wide significant linkage in schizophrenia conditioning on occurrence of depressive episodes. J Med Genet (2006) 43:563–7. 10.1136/jmg.2005.035345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci (2010) 13:1161–9 10.1038/nn.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron (2006) 52:179–96. 10.1016/j.neuron.2006.09.023 [DOI] [PubMed] [Google Scholar]
- 41.Desbonnet L, Waddington JL, O’Tuathaigh CMP. Mice mutant for genes associated with schizophrenia: common phenotype or distinct endophenotypes? Behav Brain Res (2009) 204:258–73. 10.1016/j.bbr.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 42.O’Tuathaigh CMP, Moran PM, Waddington JL. Genetic models of schizophrenia and related psychotic disorders: progress and pitfalls across the methodological “minefield”. Cell Tissue Res (2013) 354:247–57. 10.1007/s00441-013-1652-4 [DOI] [PubMed] [Google Scholar]
- 43.Willner P. The validity of animal models of depression. Psychopharmacology (Berl) (1984) 83:1–16 10.1007/BF00427414 [DOI] [PubMed] [Google Scholar]
- 44.McGue M, Gottesman II. Genetic linkage in schizophrenia: perspectives from genetic epidemiology. Schizophr Bull (1989) 15:453–64. 10.1093/schbul/15.3.453 [DOI] [PubMed] [Google Scholar]
- 45.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology (1978) 15:339–43 10.1111/j.1469-8986.1978.tb01390.x [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow NR, Sprock J, Light GA, Cadenhead K, Calkins ME, Dobie DJ, et al. Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the consortium on the genetics of schizophrenia. Schizophr Res (2007) 92:237–51. 10.1016/j.schres.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naïve schizophrenic patients. Biol Psychiatry (2002) 52:863–73. 10.1016/S0006-3223(02)01409-9 [DOI] [PubMed] [Google Scholar]
- 48.Oranje B, Geyer MA, Bocker KBE, Leon Kenemans J, Verbaten MN. Prepulse inhibition and P50 suppression: commonalities and dissociations. Psychiatry Res (2006) 143:147–58. 10.1016/j.psychres.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 49.During S, Glenthoj BY, Andersen GS, Oranje B. Effects of dopamine D2/D3 blockade on human sensory and sensorimotor gating in initially antipsychotic-naive, first-episode schizophrenia patients. Neuropsychopharmacology (2014) 39:3000–8. 10.1038/npp.2014.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine JB, Youngs RM, MacDonald ML, Chu M, Leeder AD, Berthiaume F, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience (2007) 145:42–55 10.1016/j.neuroscience.2006.11.063 [DOI] [PubMed] [Google Scholar]
- 51.Birrell J, Brown V. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci (2000) 20:4320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bozikas VP, Kosmidis MH, Kiosseoglou G, Karavatos A. Neuropsychological profile of cognitively impaired patients with schizophrenia. Compr Psychiatry (2006) 47:136–43. 10.1016/j.comppsych.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 53.Elliott R, McKenna P, Robbins T, Sahakian B. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med (1995) 25:619–30. 10.1017/S0033291700033523 [DOI] [PubMed] [Google Scholar]
- 54.Haut MW, Cahill J, Cutlip WD, Stevenson JM, Makela EH, Bloomfield SM. On the nature of Wisconsin card sorting test performance in schizophrenia. Psychiatry Res (1996) 65:15–22. 10.1016/0165-1781(96)02940-X [DOI] [PubMed] [Google Scholar]
- 55.Ruiz JC, Soler MJ, Fuentes I, Tomas P. Intellectual functioning and memory deficits in schizophrenia. Compr Psychiatry (2007) 48:276–82 10.1016/j.comppsych.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 56.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci (2002) 23:238–45. 10.1016/S0165-6147(02)02017-5 [DOI] [PubMed] [Google Scholar]
- 57.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) (1985) 85:367–70. 10.1007/BF00428203 [DOI] [PubMed] [Google Scholar]
- 58.Wall PM, Messier C. Ethological confirmatory factor analysis of anxiety-like behaviour in the murine elevated plus-maze. Behav Brain Res (2000) 114:199–212. 10.1016/S0166-4328(00)00229-1 [DOI] [PubMed] [Google Scholar]
- 59.Sokolowska E, Hovatta I. Anxiety genetics – findings from cross-species genome-wide approaches. Biol Mood Anxiety Disord (2013) 3:9. 10.1186/2045-5380-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiner I. Latent inhibition. Curr Protoc Neurosci (2001) 16:8.13.1–24 10.1002/0471142301.ns0813s16 [DOI] [PubMed] [Google Scholar]
- 61.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) (2003) 169:257–97. 10.1007/s00213-002-1313-x [DOI] [PubMed] [Google Scholar]
- 62.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods (1984) 11:47–60 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 63.Bublak P, Redel P, Finke K. Spatial and non-spatial attention deficits in neurodegenerative diseases: assessment based on Bundesen’s theory of visual attention (TVA). Restor Neurol Neurosci (2006) 24:287–301. [PubMed] [Google Scholar]
- 64.Wilkins LK, Girard TA, King J, King MJ, Herdman KA, Christensen BK, et al. Spatial-memory deficit in schizophrenia spectrum disorders under viewpoint-independent demands in the virtual courtyard task. J Clin Exp Neuropsychol (2013) 35:1082–93. 10.1080/13803395.2013.857389 [DOI] [PubMed] [Google Scholar]
- 65.Morein-Zamir S, Craig KJ, Ersche KD, Abbott S, Muller U, Fineberg NA, et al. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology (Berl) (2010) 212:357–67. 10.1007/s00213-010-1963-z [DOI] [PubMed] [Google Scholar]
- 66.Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, et al. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology (2013) 64:65–73. 10.1016/j.neuropharm.2012.06.027 [DOI] [PubMed] [Google Scholar]
- 67.Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci (2010) 30:14552–9. 10.1523/JNEUROSCI.2631-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol (2005) 1:321–53 10.1146/annurev.clinpsy.1.102803.143959 [DOI] [PubMed] [Google Scholar]
- 69.Morton JB, Bosma R, Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage (2009) 46:249–56. 10.1016/j.neuroimage.2009.01.037 [DOI] [PubMed] [Google Scholar]
- 70.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res (1988) 31:47–59. 10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- 71.Cohen SJ, Stackman RW, Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res (2014). 10.1016/j.bbr.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu C, Wick FA, Pomplun M. Guidance of visual attention by semantic information in real-world scenes. Front Psychol (2014) 5:54. 10.3389/fpsyg.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin I, Fan S, Huang T, Wu W, Li S. The associations between visual attention and facial expression identification in patients with schizophrenia. Psychiatry Investig (2013) 10:393–8. 10.4306/pi.2013.10.4.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blumenthal TD, Levey BJ. Prepulse rise time and startle reflex modification: different effects for discrete and continuous prepulses. Psychophysiology (1989) 26:158–65. 10.1111/j.1469-8986.1989.tb03148.x [DOI] [PubMed] [Google Scholar]
- 75.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol (2003) 463:3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- 76.Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev (2010) 34:1296–306. 10.1016/j.neubiorev.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) (2008) 199:331–88. 10.1007/s00213-008-1072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shilling PD, Kuczenski R, Segal DS, Barrett TB, Kelsoe JR. Differential regulation of immediate-early gene expression in the prefrontal cortex of rats with a high vs low behavioral response to methamphetamine. Neuropsychopharmacology (2006) 31:2359–67. 10.1038/sj.npp.1301162 [DOI] [PubMed] [Google Scholar]
- 79.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev (1993) 18:247–91. 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- 80.Kegeles LS, Abi-Dargham A, Frankle G, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry (2010) 67:231–9. 10.1001/archgenpsychiatry.2010.10 [DOI] [PubMed] [Google Scholar]
- 81.Strekalova T, Couch Y, Kholod N, Boyks M, Malin D, Leprince P, et al. Update in the methodology of the chronic stress paradigm: internal control matters. Behav Brain Funct (2011) 7:9. 10.1186/1744-9081-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smutzer G, Patel JY, Stull JC, Abarintos RA, Khan NK, Park KC. A preference test for sweet taste that uses edible strips. Appetite (2014) 73:132–9. 10.1016/j.appet.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov-Polevoy A, Swift RM, et al. Role of feeding-related pathways in alcohol dependence: a focus on sweet preference, NPY, and ghrelin. Alcohol Clin Exp Res (2011) 35:194–202. 10.1111/j.1530-0277.2010.01334.x [DOI] [PubMed] [Google Scholar]
- 84.Deacon RMJ, Rawlins JN. T-maze alternation in the rodent. Nat Protoc (2006) 1:7–12 10.1038/nprot.2006.2 [DOI] [PubMed] [Google Scholar]
- 85.Jackson L. VTE on an elevated T-maze. J Comp Psychol (1943) 32:99–107 10.1037/h0058536 [DOI] [Google Scholar]
- 86.Sato A, Mizuguchi M, Ikeda K. Social interaction test: a sensitive method for examining autism-related behavioral deficits. Protocol Exch (2013). 10.1038/protex.2013.046 [DOI] [Google Scholar]
- 87.Chelnokova O, Laeng B, Eikemo M, Riegels J, Loeseth G, Maurud H, et al. Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry (2014) 19:746–7 10.1038/mp.2014.149 [DOI] [PubMed] [Google Scholar]
- 88.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology (2013) 38:1883–94. 10.1016/j.psyneuen.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 89.Li S, Weerda R, Milde C, Wolf OT, Thiel CM. Effects of acute psychosocial stress on neural activity to emotional and neutral faces in a face recognition memory paradigm. Brain Imaging Behav (2014) 8:598–610. 10.1007/s11682-013-9287-3 [DOI] [PubMed] [Google Scholar]
- 90.Tseng H, Chen S, Liu C, Howes O, Huang Y, Hsieh MH, et al. Facial and prosodic emotion recognition deficits associate with specific clusters of psychotic symptoms in schizophrenia. PLoS One (2013) 8:e66571. 10.1371/journal.pone.0066571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weigelt S, Koldewyn K, Kanwisher N. Face recognition deficits in autism spectrum disorders are both domain specific and process specific. PLoS One (2013) 8:e74541. 10.1371/journal.pone.0074541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science (1999) 284:1670–2. 10.1126/science.284.5420.1670 [DOI] [PubMed] [Google Scholar]
- 93.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron (2008) 57:809–18. 10.1016/j.neuron.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 94.Lipina T, Roder J. A new model of the disrupted latent inhibition in C57BL/6J mice after bupropion treatment. Psychopharmacology (Berl) (2010) 208:487–98. 10.1007/s00213-009-1749-3 [DOI] [PubMed] [Google Scholar]
- 95.Koch M. Clinical relevance of animal models of schizophrenia. Suppl Clin Neurophysiol (2013) 62:113–20 10.1016/B978-0-7020-5307-8.00007-7 [DOI] [PubMed] [Google Scholar]
- 96.Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Rev Bras Psiquiatr (2013) 35(Suppl 2):S112–20 10.1590/1516-4446-2013-1098 [DOI] [PubMed] [Google Scholar]
- 97.Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res (2009) 204:369–86. 10.1016/j.bbr.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 98.Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, et al. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry (2011) 70:35–42. 10.1016/j.biopsych.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 99.Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry (2009) 14:359–75. 10.1038/mp.2008.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry (2008) 13:558–69. 10.1038/sj.mp.4002151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry (2008) 13:197–207. 10.1038/sj.mp.4002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet (2014) 15:2. 10.1186/1471-2350-15-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koiliari E, Roussos P, Pasparakis E, Lencz T, Malhotra A, Siever LJ, et al. The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr Res (2014) 154:42–7. 10.1016/j.schres.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 104.Rose EJ, Morris DW, Hargreaves A, Fahey C, Greene C, Garavan H, et al. Neural effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Am J Med Genet B Neuropsychiatr Genet (2013) 162B:530–7. 10.1002/ajmg.b.32182 [DOI] [PubMed] [Google Scholar]
- 105.Kraus DM, Elliott GS, Chute H, Horan T, Pfenninger KH, Sanford SD, et al. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J Immunol (2006) 176:4419–30. 10.4049/jimmunol.176.7.4419 [DOI] [PubMed] [Google Scholar]
- 106.Escudero-Esparza A, Kalchishkova N, Kurbasic E, Jiang WG, Blom AM. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J (2013) 27:5083–93. 10.1096/fj.13-230706 [DOI] [PubMed] [Google Scholar]
- 107.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia (2013) 61:24–36. 10.1002/glia.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steen VM, Nepal C, Ersland KM, Holdhus R, Naevdal M, Ratvik SM, et al. Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PLoS One (2013) 8:e79501. 10.1371/journal.pone.0079501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Distler MG, Opal MD, Dulawa SC, Palmer AA. Assessment of behaviors modeling aspects of schizophrenia in Csmd1 mutant mice. PLoS One (2012) 7:e51235. 10.1371/journal.pone.0051235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horiuchi Y, Arai M, Niizato K, Iritani S, Noguchi E, Ohtsuki T, et al. A polymorphism in the PDLIM5 gene associated with gene expression and schizophrenia. Biol Psychiatry (2006) 59:434–9. 10.1016/j.biopsych.2005.07.041 [DOI] [PubMed] [Google Scholar]
- 111.Kato T, Iwayama Y, Kakiuchi C, Iwamoto K, Yamada K, Minabe Y, et al. Gene expression and association analyses of LIM (PDLIM5) in bipolar disorder and schizophrenia. Mol Psychiatry (2005) 10:1045–55. 10.1038/sj.mp.4001719 [DOI] [PubMed] [Google Scholar]
- 112.Li C, Tao R, Qin W, Zheng Y, He G, Shi Y, et al. Positive association between PDLIM5 and schizophrenia in the Chinese Han population. Int J Neuropsychopharmacol (2008) 11:27–34. 10.1017/S1461145707007687 [DOI] [PubMed] [Google Scholar]
- 113.Zain MA, Roffeei SN, Zainal NZ, Kanagasundram S, Mohamed Z. Nonsynonymous polymorphisms of the PDLIM5 gene association with the occurrence of both bipolar disorder and schizophrenia. Psychiatr Genet (2013) 23:258–61. 10.1097/YPG.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 114.Shi J, Badner JA, Liu C. PDLIM5 and susceptibility to bipolar disorder: a family-based association study and meta-analysis. Psychiatr Genet (2008) 18:116–21. 10.1097/YPG.0b013e3282fa184b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Squassina A, Manchia M, Manconi F, Piccardi M, Ardau R, Chillotti C, et al. A case-control association study of the PDLIM5 gene and bipolar disorder in a Sardinian sample. Psychiatr Genet (2008) 18:128–32. 10.1097/YPG.0b013e3282fb003d [DOI] [PubMed] [Google Scholar]
- 116.Liu Z, Liu W, Xiao Z, Wang G, Yin S, Zhu F, et al. A major single nucleotide polymorphism of the PDLIM5 gene associated with recurrent major depressive disorder. J Psychiatry Neurosci (2008) 33:43–6. [PMC free article] [PubMed] [Google Scholar]
- 117.Wong M, Dong C, Andreev V, Arcos-Burgos M, Licinio J. Prediction of susceptibility to major depression by a model of interactions of multiple functional genetic variants and environmental factors. Mol Psychiatry (2012) 17:624–33. 10.1038/mp.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, et al. Protein-protein interaction of zinc finger LIM domains with protein kinase C. J Biol Chem (1996) 271:31029–32. 10.1074/jbc.271.49.31029 [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Lai M, Maeno-Hikichi Y, Zhang JF. Essential role of the LIM domain in the formation of the PKCepsilon-ENH-N-type Ca2+ channel complex. Cell Signal (2006) 18:215–24. 10.1016/j.cellsig.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 120.Maeno-Hikichi Y, Chang S, Matsumura K, Lai M, Lin H, Nakagawa N, et al. A PKCε-ENH-channel complex specifically modulates N-type Ca2+ channels. Nat Neurosci (2003) 6:468–75. 10.1038/nn1041 [DOI] [PubMed] [Google Scholar]
- 121.Herrick S, Evers DM, Lee JY, Udagawa N, Pak DT. Postsynaptic PDLIM5/enigma homolog binds SPAR and causes dendritic spine shrinkage. Mol Cell Neurosci (2010) 43:188–200. 10.1016/j.mcn.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horiuchi Y, Ishikawa M, Kaito N, Iijima Y, Tanabe Y, Ishiguro H, et al. Experimental evidence for the involvement of PDLIM5 in mood disorders in hetero knockout mice. PLoS One (2013) 8:e59320. 10.1371/journal.pone.0059320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet (2005) 77:918–36. 10.1086/497703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo SZ, Huang K, Shi YY, Tang W, Zhou J, Feng GY, et al. A case-control association study between the GRID1 gene and schizophrenia in the Chinese Northern Han population. Schizophr Res (2007) 93:385–90. 10.1016/j.schres.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 125.Treutlein J, Muhleisen TW, Frank J, Mattheisen M, Herms S, Ludwig KU, et al. Dissection of phenotype reveals possible association between schizophrenia and glutamate receptor Delta 1 (GRID1) gene promoter. Schizophr Res (2009) 111:123–30. 10.1016/j.schres.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 126.Orsetti M, Di Brisco F, Canonico PL, Genazzani AA, Ghi P. Gene regulation in the frontal cortex of rats exposed to the chronic mild stress paradigm, an animal model of human depression. Eur J Neurosci (2008) 27:2156–64. 10.1111/j.1460-9568.2008.06155.x [DOI] [PubMed] [Google Scholar]
- 127.Orsetti M, Di Brisco F, Rinaldi M, Dallorto D, Ghi P. Some molecular effectors of antidepressant action of quetiapine revealed by DNA microarray in the frontal cortex of anhedonic rats. Pharmacogenet Genomics (2009) 19:600–12. 10.1097/FPC.0b013e32832ee573 [DOI] [PubMed] [Google Scholar]
- 128.Safieddine S, Wenthold RJ. The glutamate receptor subunit delta1 is highly expressed in hair cells of the auditory and vestibular systems. J Neurosci (1997) 17:7523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayat E, Petralia RS, Wang YX, Wenthold RJ. Immunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor delta subunits form novel postsynaptic receptor complexes. J Neurosci (1995) 15:2533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett (1993) 315:318–22. 10.1016/0014-5793(93)81186-4 [DOI] [PubMed] [Google Scholar]
- 131.Hepp R, Hay YA, Aguado C, Lujan R, Dauphinot L, Potier MC, et al. Glutamate receptors of the delta family are widely expressed in the adult brain. Brain Struct Funct (2014). 10.1007/s00429-014-0827-4 [DOI] [PubMed] [Google Scholar]
- 132.Yadav R, Rimerman R, Scofield MA, Dravid SM. Mutations in the transmembrane domain M3 generate spontaneously open orphan glutamate delta1 receptor. Brain Res (2011) 1382:1–8. 10.1016/j.brainres.2010.12.086 [DOI] [PubMed] [Google Scholar]
- 133.Uemura T, Mishina M. The amino-terminal domain of glutamate receptor delta2 triggers presynaptic differentiation. Biochem Biophys Res Commun (2008) 377:1315–9. 10.1016/j.bbrc.2008.10.170 [DOI] [PubMed] [Google Scholar]
- 134.Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, et al. Trans-synaptic interaction of GluRdelta2 and neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell (2010) 141:1068–79. 10.1016/j.cell.2010.04.035 [DOI] [PubMed] [Google Scholar]
- 135.Kuroyanagi T, Yokoyama M, Hirano T. Postsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc Natl Acad Sci U S A (2009) 106:4912–6. 10.1073/pnas.0900892106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science (2010) 328:363–8. 10.1126/science.1185152 [DOI] [PubMed] [Google Scholar]
- 137.Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM. Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PLoS One (2012) 7:e32969. 10.1371/journal.pone.0032969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yadav R, Hillman BG, Gupta SC, Suryavanshi P, Bhatt JM, Pavuluri R, et al. Deletion of glutamate delta-1 receptor in mouse leads to enhanced working memory and deficit in fear conditioning. PLoS One (2013) 8:e60785. 10.1371/journal.pone.0060785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Witt K, van Dorn R, Fazel S. Risk factors for violence in psychosis: systematic review and meta-regression analysis of 110 studies. PLoS One (2013) 8:e55942. 10.1371/journal.pone.0055942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med (2009) 6:e1000120. 10.1371/journal.pmed.1000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci (2008) 28:6055–67. 10.1523/JNEUROSCI.0032-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci (2011) 261:150–4. 10.1007/s00406-011-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature (2011) 477:171–8. 10.1038/nature10360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience (2002) 113:607–15. 10.1016/S0306-4522(02)00162-8 [DOI] [PubMed] [Google Scholar]
- 145.Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One (2011) 6:e24325. 10.1371/journal.pone.0024325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav (2010) 101:381–8. 10.1016/j.physbeh.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ernst A, Sharma AN, Elased KM, Guest PC, Rahmoune H, Bahn S. Diabetic db/db mice exhibit central nervous system and peripheral molecular alterations as seen in neurological disorders. Transl Psychiatry (2013) 3:e263. 10.1038/tp.2013.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care (2001) 24:1069–78 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- 149.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med (2006) 23:1165–73. 10.1111/j.1464-5491.2006.01943.x [DOI] [PubMed] [Google Scholar]
- 150.Dixon L, Weiden P, Delahanty J, Goldberg R, Postrado L, Lucksted A, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull (2000) 26:903–12. 10.1093/oxfordjournals.schbul.a033504 [DOI] [PubMed] [Google Scholar]
- 151.De Hert M, Mauri M, Shaw K, Wetterling T, Doble A, Giudicelli A, et al. The METEOR study of diabetes and other metabolic disorders in patients with schizophrenia treated with antipsychotic drugs. I. Methodology. Int J Methods Psychiatr Res (2010) 19:195–210. 10.1002/mpr.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab (2014) 11:593–600. 10.1016/j.tem.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 153.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature (1982) 296:659–60 10.1038/296659a0 [DOI] [PubMed] [Google Scholar]
- 154.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med (2010) 2:429–39. 10.1002/emmm.201000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ikeda K, Ikeda K, Iritani S, Ueno H, Niizato K. Distribution of neuropeptide Y interneurons in the dorsal prefrontal cortex of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2004) 28:379–83. 10.1016/j.pnpbp.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 156.Kuromitsu J, Yokoi A, Kawai T, Nagasu T, Aizawa T, Haga S, et al. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns (2001) 1:17–21. 10.1016/S1567-133X(01)00003-5 [DOI] [PubMed] [Google Scholar]
- 157.Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature (2007) 450:106–9. 10.1038/nature06212 [DOI] [PubMed] [Google Scholar]
- 158.Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y-induced enhancement of the evoked release of newly synthesized dopamine in rat striatum: mediation by Y2 receptors. Neuropharmacology (2007) 52:1396–402. 10.1016/j.neuropharm.2007.01.018 [DOI] [PubMed] [Google Scholar]
- 159.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides (2004) 38:213–24 10.1016/j.npep.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 160.Morales-Medina J, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res (2010) 1314:194–205. 10.1016/j.brainres.2009.09.077 [DOI] [PubMed] [Google Scholar]
- 161.Wu G, Feder A, Wegener G, Bailey C, Saxena S, Charney D, et al. Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin Ther Targets (2011) 15:1317–31. 10.1517/14728222.2011.628314 [DOI] [PubMed] [Google Scholar]
- 162.Stadlbauer U, Langhans W, Meyer U. Administration of the Y2 receptor agonist PYY3-36 in mice induces multiple behavioral changes relevant to schizophrenia. Neuropsychopharmacology (2013) 38:2446–55. 10.1038/npp.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Karl T, Chesworth R, Duffy L, Herzog H. Schizophrenia-relevant behaviours in a genetic mouse model for Y2 deficiency. Behav Brain Res (2010) 207:434–40. 10.1016/j.bbr.2009.10.029 [DOI] [PubMed] [Google Scholar]
- 164.Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res (2003) 141:251–5. 10.1016/S0166-4328(02)00374-1 [DOI] [PubMed] [Google Scholar]
- 165.Desai SJ, Borkar CD, Nakhate KT, Subhedar NK, Kokare DM. Neuropeptide Y attenuates anxiety- and depression-like effects of cholecystokinin-4 in mice. Neuroscience (2014) 277C:818–30. 10.1016/j.neuroscience.2014.07.062 [DOI] [PubMed] [Google Scholar]
- 166.Morales-Medina JC, Dumont Y, Benoit CE, Bastianetto S, Flores G, Fournier A, et al. Role of neuropeptide Y Y(1) and Y(2) receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology (2012) 62:200–8. 10.1016/j.neuropharm.2011.06.030 [DOI] [PubMed] [Google Scholar]
- 167.St Clair D, Blackwood D, Muir W, Walker M, Carothers A, Spowart G, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet (1990) 336:13–6. 10.1016/0140-6736(90)91520-K [DOI] [PubMed] [Google Scholar]
- 168.Millar J, Christie S, Semple C, Porteous D. Chromosomal location and genomic structure of the human translin-associated factor X gene (TRAX; TSNAX) revealed by intergenic splicing to DISC1, a gene disrupted by a translocation segregating with schizophrenia. Genomics (2000) 67:69–77. 10.1006/geno.2000.6239 [DOI] [PubMed] [Google Scholar]
- 169.Song W, Li W, Feng J, Heston LL, Scaringe WA, Sommer SS. Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem Biophys Res Commun (2008) 367:700–6. 10.1016/j.bbrc.2007.12.117 [DOI] [PubMed] [Google Scholar]
- 170.Green EK, Grozeva D, Sims R, Raybould R, Forty L, Gordon-Smith K, et al. DISC1 exon 11 rare variants found more commonly in schizoaffective spectrum cases than controls. Am J Med Genet B Neuropsychiatr Genet (2011) 156B:490–2. 10.1002/ajmg.b.31187 [DOI] [PubMed] [Google Scholar]
- 171.Moens L, De Rijk P, Reumers J, Van den Bossche M, Glassee W, De Zutter S, et al. Sequencing of DISC1 pathway genes reveals increased burden of rare missense variants in schizophrenia patients from a northern Swedish population. PLoS One (2011) 6:e23450. 10.1371/journal.pone.0023450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, et al. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell (2007) 130:1146–58. 10.1016/j.cell.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med (2011) 17:699–706. 10.1016/j.molmed.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lipina TV, Roder JC. Disrupted-in-schizophrenia-1 (DISC1) interactome and mental disorders: impact of mouse models. Neurosci Biobehav Rev (2014) 45:271–94. 10.1016/j.neubiorev.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 175.Soares DC, Carlyle BC, Bradshaw NJ, Porteous DJ. DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem Neurosci (2011) 2(11):609–32. 10.1021/cn200062k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Camargo LM, Collura V, Rain J, Mizuguchi K, Hermjakob H, Kerrien S, et al. Disrupted in schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry (2007) 12:74–86. 10.1038/sj.mp.4001880 [DOI] [PubMed] [Google Scholar]
- 177.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science (2005) 310:1187–91. 10.1126/science.1112915 [DOI] [PubMed] [Google Scholar]
- 178.Bradshaw NJ, Soares DC, Carlyle BC, Ogawa F, Davidson-Smith H, Christie S, et al. PKA phosphorylation of NDE1 is DISC1/PDE4 dependent and modulates its interaction with LIS1 and NDEL1. J Neurosci (2011) 31:9043–54. 10.1523/JNEUROSCI.5410-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bradshaw NJ, Porteous DJ. DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology (2012) 62:1230–41. 10.1016/j.neuropharm.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee FHF, Kaidanovich-Beilin O, Roder JC, Woodgett JR, Wong AHC. Genetic inactivation of GSK3α rescues spine deficits in DISC1-L100P mutant mice. Schizophr Res (2011) 129:74–9. 10.1016/j.schres.2011.03.032 [DOI] [PubMed] [Google Scholar]
- 181.Lipina TV, Fletcher PJ, Lee FH, Wong AH, Roder JC. Disrupted-in-schizophrenia-1 Gln31Leu polymorphism results in social anhedonia associated with monoaminergic imbalance and reduction of CREB and beta-arrestin-1,2 in the nucleus accumbens in a mouse model of depression. Neuropsychopharmacology (2013) 38:423–36. 10.1038/npp.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, et al. Behavioral phenotypes of DISC1 missense mutations in mice. Neuron (2007) 54:387–402. 10.1016/j.neuron.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 183.Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, et al. DISC1 point mutations in mice affect development of the cerebral cortex. J Neurosci (2011) 31:3197–206. 10.1523/JNEUROSCI.4219-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A (2007) 104:14501–6. 10.1073/pnas.0704774104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kvajo M, McKellar H, Alexander Arguello P, Drew LJ, Moore H, MacDermott AB, et al. A mutation in mouse DISC1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A (2008) 105:7076–81. 10.1073/pnas.0802615105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kvajo M, McKellar H, Drew LJ, Lepagnol-Bestel A, Xiao L, Levy RJ, et al. Altered axonal targeting and short-term plasticity in the hippocampus of DISC1 mutant mice. Proc Natl Acad Sci U S A (2011) 108:E1349–58. 10.1073/pnas.1114113108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li W, Zhou Y, Jentsch JD, Brown RAM, Tian X, Ehninger D, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A (2007) 104:18280–5. 10.1073/pnas.0706900104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron (2010) 65:480–9. 10.1016/j.neuron.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry (2008) 13:173–86. 10.1038/sj.mp.4002079 [DOI] [PubMed] [Google Scholar]
- 190.Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan S, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated DISC1. J Neurosci (2008) 28:10893–904. 10.1523/JNEUROSCI.3299-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry (2011) 16:293–306 10.1038/mp.2009.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koike H, Alexander Arguello P, Kvajo M, Karayiorgou M, Gogos JA. DISC1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A (2006) 103:3693–7. 10.1073/pnas.0511189103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lipina T, Roder J. The genetic component of latent inhibition: studies of inbred and mutant mice. In: Lubow RE, Weiner I, editors. Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia. Cambridge: Cambridge University Press; (2010). p. 225–51. [Google Scholar]
- 194.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry (2010) 68:1172–81. 10.1016/j.biopsych.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lipina TV, Zai C, Hlousek D, Roder JC, Wong AHC. Maternal immune activation during gestation interacts with DISC1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci (2013) 33:7654–66. 10.1523/JNEUROSCI.0091-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science (2013) 339:335–9. 10.1126/science.1226931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sakae N, Yamasaki N, Kitaichi K, Fukuda T, Yamada M, Yoshikawa H, et al. Mice lacking the schizophrenia-associated protein FEZ1 manifest hyperactivity and enhanced responsiveness to psychostimulants. Hum Mol Genet (2008) 17:3191–203. 10.1093/hmg/ddn215 [DOI] [PubMed] [Google Scholar]
- 198.Zhang H, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology (2008) 33:1611–23. 10.1038/sj.npp.1301537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) (2008) 197:115–26. 10.1007/s00213-007-1014-6 [DOI] [PubMed] [Google Scholar]
- 200.Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol Brain (2009) 2:35. 10.1186/1756-6606-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, et al. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet (2009) 18:3227–43. 10.1093/hmg/ddp261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cahill ME, Xie Z, Day M, Barbolina MV, Miller CA, Weiss C, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A (2009) 106:13058–63. 10.1073/pnas.0904636106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Flores R, III, Hirota Y, Armstrong B, Sawa A, Tomoda T. DISC1 regulates synaptic vesicle transport via a lithium-sensitive pathway. Neurosci Res (2011) 71:71–7. 10.1016/j.neures.2011.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci (2007) 27:9513–24. 10.1523/JNEUROSCI.1493-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry (2013) 18:557–67. 10.1038/mp.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, et al. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron (2009) 63:761–73. 10.1016/j.neuron.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beaulieu J. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci (2012) 37:7–16. 10.1503/jpn.110011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Beurel E. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci (2011) 4:18. 10.3389/fnmol.2011.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA, et al. A pivotal role of GSK-3 in synaptic plasticity. Front Mol Neurosci (2012) 5:13. 10.3389/fnmol.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dai D, Wang Y, Yuan J, Zhou X, Jiang D, Li J, et al. Meta-analyses of 10 polymorphisms associated with the risk of schizophrenia. Biomed Rep (2014) 2:729–36. 10.3892/br.2014.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sarras H, Semeralul MO, Fadel MP, Feldcamp LA, Labrie V, Wong AH. Elevated PICK1 mRNA in schizophrenia increased SRR mRNA in suicide. Schizophr Res (2010) 120:236–7 10.1016/j.schres.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 212.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry (2006) 11:557–66. 10.1038/sj.mp.4001792 [DOI] [PubMed] [Google Scholar]
- 213.Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res (2008) 1239:235–48. 10.1016/j.brainres.2008.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des (2009) 15:1688–98. 10.2174/138161209788168092 [DOI] [PubMed] [Google Scholar]
- 215.Labrie V, Wong AH, Roder JC. Contributions of the D-serine pathway to schizophrenia. Neuropharmacology (2012) 62:1484–503. 10.1016/j.neuropharm.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 216.Jia P, Sun J, Guo AY, Zhao Z. SZGR: a comprehensive schizophrenia gene resource. Mol Psychiatry (2010) 15:453–62. 10.1038/mp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet (2008) 4:e28. 10.1371/journal.pgen.0040028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li M, Luo XJ, Xiao X, Shi L, Liu XY, Yin LD, et al. Analysis of common genetic variants identifies RELN as a risk gene for schizophrenia in Chinese population. World J Biol Psychiatry (2013) 14:91–9. 10.3109/15622975.2011.587891 [DOI] [PubMed] [Google Scholar]
- 219.Kuang WJ, Sun RF, Zhu YS, Li SB. A new single-nucleotide mutation (rs362719) of the reelin (RELN) gene associated with schizophrenia in female Chinese Han. Genet Mol Res (2011) 10:1650–8. 10.4238/vol10-3gmr1343 [DOI] [PubMed] [Google Scholar]
- 220.Fatemi SH, Earle JA, McMenomy T. Reduction in reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry (2000) 5(654–63):571. 10.1038/sj.mp.4000783 [DOI] [PubMed] [Google Scholar]
- 221.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry (2005) 57:252–60. 10.1016/j.biopsych.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 222.Ovadia G, Shifman S. The genetic variation of RELN expression in schizophrenia and bipolar disorder. PLoS One (2011) 6:e19955. 10.1371/journal.pone.0019955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fatemi SH, Reutiman TJ, Folsom TD. Chronic psychotropic drug treatment causes differential expression of reelin signaling system in frontal cortex of rats. Schizophr Res (2009) 111:138–52. 10.1016/j.schres.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 224.Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol (1973) 148:141–51 10.1002/cne.901480202 [DOI] [PubMed] [Google Scholar]
- 225.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature (1995) 374:719–23 10.1038/374719a0 [DOI] [PubMed] [Google Scholar]
- 226.Kubo K, Honda T, Tomita K, Sekine K, Ishii K, Uto A, et al. Ectopic reelin induces neuronal aggregation with a normal birthdate-dependent “inside-out” alignment in the developing neocortex. J Neurosci (2010) 30:10953–66. 10.1523/JNEUROSCI.0486-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci (2006) 7:850–9. 10.1038/nrn2009 [DOI] [PubMed] [Google Scholar]
- 228.Chen Y, Beffert U, Ertunc M, Tang T, Kavalali ET, Bezprozvanny I, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci (2005) 25:8209–16. 10.1523/JNEUROSCI.1951-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, et al. Modulation of synaptic plasticity and memory by reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron (2005) 47:567–79. 10.1016/j.neuron.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 230.Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci (2010) 30:4636–49. 10.1523/JNEUROSCI.5284-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Folsom TD, Fatemi SH. The involvement of reelin in neurodevelopmental disorders. Neuropharmacology (2013) 68:122–35. 10.1016/j.neuropharm.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res (2004) 153:43–54. 10.1016/j.bbr.2003.10.033 [DOI] [PubMed] [Google Scholar]
- 233.Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in reelin haploinsufficient mice. Neurobiol Learn Mem (2006) 85:228–42. 10.1016/j.nlm.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 234.Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci (2006) 120:984–8. 10.1037/0735-7044.120.4.984 [DOI] [PubMed] [Google Scholar]
- 235.Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) (2006) 189:95–104. 10.1007/s00213-006-0530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci (2008) 27:2568–74. 10.1111/j.1460-9568.2008.06233.x [DOI] [PubMed] [Google Scholar]
- 237.Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, et al. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport (1999) 10:1329–34. 10.1097/00001756-199904260-00032 [DOI] [PubMed] [Google Scholar]
- 238.Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) (2009) 204:511–21. 10.1007/s00213-009-1483-x [DOI] [PubMed] [Google Scholar]
- 239.Van den Buuse M, Halley P, Hill R, Labots M, Martin S. Altered N-methyl-d-aspartate receptor function in reelin heterozygous mice: male-female differences and comparison with dopaminergic activity. Prog Neuropsychopharmacol Biol Psychiatry (2012) 37:237–46. 10.1016/j.pnpbp.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 240.Ognibene E, Adriani W, Granstrem O, Pieretti S, Laviola G. Impulsivity-anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res (2007) 1131:173–80. 10.1016/j.brainres.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 241.Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci (2003) 117:1257–75. 10.1037/0735-7044.117.6.1257 [DOI] [PubMed] [Google Scholar]
- 242.Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, et al. Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci (2006) 24:2061–70. 10.1111/j.1460-9568.2006.05083.x [DOI] [PubMed] [Google Scholar]
- 243.Teixeira CM, Martin ED, Sahun I, Masachs N, Pujadas L, Corvelo A, et al. Overexpression of reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology (2011) 36:2395–405. 10.1038/npp.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev (2002) 8:51–7 10.1002/mrdd.10004 [DOI] [PubMed] [Google Scholar]
- 245.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res (2009) 204:322–34. 10.1016/j.bbr.2008.12.022 [DOI] [PubMed] [Google Scholar]
- 246.Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry (2014) 4:e434. 10.1038/tp.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pang D, Syed S, Fine P, Jones PB. No association between prenatal viral infection and depression in later life – a long-term cohort study of 6152 subjects. Can J Psychiatry (2009) 54:565–70. [DOI] [PubMed] [Google Scholar]
- 248.Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl) (2011) 214:89–106. 10.1007/s00213-010-2035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One (2009) 4:e6354. 10.1371/journal.pone.0006354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev (2005) 29:913–47. 10.1016/j.neubiorev.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 251.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun (2006) 20:378–88. 10.1016/j.bbi.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 252.Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res (2006) 173:243–57. 10.1007/s00221-006-0419-5 [DOI] [PubMed] [Google Scholar]
- 253.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology (2008) 33:441–56. 10.1038/sj.npp.1301413 [DOI] [PubMed] [Google Scholar]
- 254.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun (2008) 22:469–86. 10.1016/j.bbi.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 255.Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull (2010) 36:607–23. 10.1093/schbul/sbn131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vuillermot S, Weber L, Feldon J, Meyer UA. Longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci (2010) 30:1270–87. 10.1523/JNEUROSCI.5408-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry (2006) 59:546–54. 10.1016/j.biopsych.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 258.Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res (2008) 190:156–9. 10.1016/j.bbr.2008.02.021 [DOI] [PubMed] [Google Scholar]
- 259.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology (2003) 28:1778–89. 10.1038/sj.npp.1300248 [DOI] [PubMed] [Google Scholar]
- 260.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res (2005) 39:311–23. 10.1016/j.jpsychires.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 261.Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, et al. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol (2009) 12:513–24. 10.1017/S1461145708009206 [DOI] [PubMed] [Google Scholar]
- 262.Dickerson DD, Overeem KA, Wolff AR, Williams JM, Abraham WC, Bilkey DK. Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl Psychiatry (2014) 4:e418. 10.1038/tp.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci (2003) 23:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci (2006) 26:4752–62. 10.1523/JNEUROSCI.0099-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bitanihirwe BKY, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol (2010) 13:981–96. 10.1017/S1461145710000192 [DOI] [PubMed] [Google Scholar]
- 266.Missault S, Van den Eynde K, Vanden Berghe W, Fransen E, Weeren A, Timmermans JP, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun (2014) 42:138–46. 10.1016/j.bbi.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 267.Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry (2014) 5:47. 10.3389/fpsyt.2014.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron (2006) 52:139–53. 10.1016/j.neuron.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 269.Pješčić KD, Nenadović MM, Jašović-Gašić M, Trajković G, Kostić M, Ristić-Dimitrijević R. Influence of psycho-social factors on the emergence of depression and suicidal risk in patients with schizophrenia. Psychiatr Danub (2014) 26:226–30. [PubMed] [Google Scholar]
- 270.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci (2006) 7:583–90. 10.1038/nrn1925 [DOI] [PubMed] [Google Scholar]
- 271.Belmaker RH. The future of depression psychopharmacology. CNS Spectr (2008) 13:682–7. [DOI] [PubMed] [Google Scholar]
- 272.Pryce CR, Klaus F. Translating the evidence for gene association with depression into mouse models of depression-relevant behaviour: current limitations and future potential. Neurosci Biobehav Rev (2013) 37:1380–402. 10.1016/j.neubiorev.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 273.Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents – relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev (2008) 32:1087–102. 10.1016/j.neubiorev.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 274.Sahakian BJ, Robbins TW. Isolation-rearing enhances tail pinch-induced oral behavior in rats. Physiol Behav (1977) 18:53–8 10.1016/0031-9384(77)90093-2 [DOI] [PubMed] [Google Scholar]
- 275.Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry (2000) 47:864–73. 10.1016/S0006-3223(99)00269-3 [DOI] [PubMed] [Google Scholar]
- 276.Gentsch C, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialization. Physiol Behav (1988) 43:13–6. 10.1016/0031-9384(88)90091-1 [DOI] [PubMed] [Google Scholar]
- 277.Bakshi VP, Swerdlow NR, Braff DL, Geyer MA. Reversal of isolation rearing-induced deficits in prepulse inhibition by seroquel and olanzapine. Biol Psychiatry (1998) 43:436–45. 10.1016/S0006-3223(97)00246-1 [DOI] [PubMed] [Google Scholar]
- 278.Cilia J, Reavill C, Hagan J, Jones D. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology (Berl) (2001) 156:327–37 10.1007/s002130100786 [DOI] [PubMed] [Google Scholar]
- 279.Day-Wilson K, Jones DNC, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience (2006) 141:1113–21. 10.1016/j.neuroscience.2006.04.048 [DOI] [PubMed] [Google Scholar]
- 280.Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology (1994) 10:61–72. 10.1038/npp.1994.8 [DOI] [PubMed] [Google Scholar]
- 281.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res (1996) 75:27–32. 10.1016/0166-4328(96)00181-7 [DOI] [PubMed] [Google Scholar]
- 282.Vale AL, Montgomery AM. Social interaction: responses to chlordiazepoxide and the loss of isolation-reared effects with paired-housing. Psychopharmacology (Berl) (1997) 133:127–32. 10.1007/s002130050382 [DOI] [PubMed] [Google Scholar]
- 283.Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology (1986) 25:663–4 10.1016/0028-3908(86)90224-8 [DOI] [Google Scholar]
- 284.Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr N, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res (2004) 152:279–95. 10.1016/j.bbr.2003.10.015 [DOI] [PubMed] [Google Scholar]
- 285.Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology (Berl) (1990) 102:364–72. 10.1007/BF02244105 [DOI] [PubMed] [Google Scholar]
- 286.Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol Behav (1997) 62:281–90. 10.1016/S0031-9384(97)00117-0 [DOI] [PubMed] [Google Scholar]
- 287.Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on preference by rats for a novel environment. Physiol Behav (1997) 62:299–303. 10.1016/S0031-9384(97)00116-9 [DOI] [PubMed] [Google Scholar]
- 288.Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on sucrose consumption in rats. Physiol Behav (1997) 62:291–7. 10.1016/S0031-9384(97)00116-9 [DOI] [PubMed] [Google Scholar]
- 289.Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol (2007) 10:595–607. 10.1017/S1461145706007401 [DOI] [PubMed] [Google Scholar]
- 290.Deehan J, Gerald A, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res (2007) 31:1692–8. 10.1111/j.1530-0277.2007.00466.x [DOI] [PubMed] [Google Scholar]
- 291.Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev (2003) 27:457–91. 10.1016/S0149-7634(03)00073-3 [DOI] [PubMed] [Google Scholar]
- 292.Schrijver NCA, Würbel H. Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci (2001) 115:437–42. 10.1037/0735-7044.115.2.437 [DOI] [PubMed] [Google Scholar]
- 293.Schrijver NCA, Pallier PN, Brown VJ, Würbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res (2004) 152:307–14. 10.1016/j.bbr.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 294.Li N, Wu X, Li L. Chronic administration of clozapine alleviates reversal-learning impairment in isolation-reared rats. Behav Pharmacol (2007) 18:135–45. 10.1097/FBP.0b013e3280d3ee83 [DOI] [PubMed] [Google Scholar]
- 295.Hellemans KGC, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res (2004) 150:103–15. 10.1016/j.devbrainres.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 296.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, et al. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol (2003) 183:600–9. 10.1016/S0014-4886(03)00248-6 [DOI] [PubMed] [Google Scholar]
- 297.Wongwitdecha N, Marsden CA. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacol Biochem Behav (1996) 53:531–4. 10.1016/0091-3057(95)02046-2 [DOI] [PubMed] [Google Scholar]
- 298.Lapiz MDS, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol (2003) 33:13–29. 10.1023/A:1021171129766 [DOI] [PubMed] [Google Scholar]
- 299.Gan JO, Bowline E, Lourenco FS, Pickel VM. Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience (2014) 258:174–83. 10.1016/j.neuroscience.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology (2004) 29:1980–7. 10.1038/sj.npp.1300507 [DOI] [PubMed] [Google Scholar]
- 301.Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm (2007) 114:893–8. 10.1007/s00702-007-0627-6 [DOI] [PubMed] [Google Scholar]
- 302.Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AME, Nencini P, et al. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res (2006) 168:323–5. 10.1016/j.bbr.2005.04.024 [DOI] [PubMed] [Google Scholar]
- 303.Silva-Gómez AB, Rojas D, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res (2003) 983:128–36. 10.1016/S0006-8993(03)03042-7 [DOI] [PubMed] [Google Scholar]
- 304.Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem (1995) 63:217–9. 10.1006/nlme.1995.1025 [DOI] [PubMed] [Google Scholar]
- 305.Comery TA, Stamoudis CX, Irwin SA, Greenough WT. Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol Learn Mem (1996) 66:93–6. 10.1006/nlme.1996.0049 [DOI] [PubMed] [Google Scholar]
- 306.Pascual R, Zamora-León SP, Valero-Cabré A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (2006) 66:7–14. [DOI] [PubMed] [Google Scholar]
- 307.Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet (2006) 15:1995–2002. 10.1093/hmg/ddl122 [DOI] [PubMed] [Google Scholar]
- 308.Munafò MR, Thiselton DL, Clark TG, Flint J. Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry (2006) 11:539–46. 10.1038/sj.mp.4001817 [DOI] [PubMed] [Google Scholar]
- 309.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet (2006) 141B:96–101. 10.1002/ajmg.b.30236 [DOI] [PubMed] [Google Scholar]
- 310.Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, et al. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry (2005) 10:366–74. 10.1038/sj.mp.4001661 [DOI] [PubMed] [Google Scholar]
- 311.Agim ZS, Esendal M, Briollais L, Uyan O, Meschian M, Martinez LAM, et al. Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. PLoS One (2013) 8:e53042. 10.1371/journal.pone.0053042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Athanasiu L, Mattingsdal M, Kähler AK, Brown A, Gustafsson O, Agartz I, et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res (2010) 44:748–53. 10.1016/j.jpsychires.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mei L, Xiong W. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci (2008) 9:437–52. 10.1038/nrn2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, et al. Family-based association study of neuregulin 1 with psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet (2009) 150B:693–702. 10.1002/ajmg.b.30895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Green EK, Raybould R, Macgregor S, Gordon-Smith K, Heron J, Hyde S, et al. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry (2005) 62:642–8. 10.1001/archpsyc.62.6.642 [DOI] [PubMed] [Google Scholar]
- 316.Prata DP, Breen G, Osborne S, Munro J, St Clair D, Collier DA. An association study of the neuregulin 1 gene, bipolar affective disorder and psychosis. Psychiatr Genet (2009) 19:113–6. 10.1097/YPG.0b013e32832a4f69 [DOI] [PubMed] [Google Scholar]
- 317.Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ, et al. Association of neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry (2007) 12:94–104. 10.1038/sj.mp.4001889 [DOI] [PubMed] [Google Scholar]
- 318.Walker RM, Christoforou A, Thomson PA, McGhee KA, Maclean A, Mühleisen TW, et al. Association analysis of neuregulin 1 candidate regions in schizophrenia and bipolar disorder. Neurosci Lett (2010) 478:9–13. 10.1016/j.neulet.2010.04.056 [DOI] [PubMed] [Google Scholar]
- 319.Buxbaum JD, Corfas G, Roy K. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci (2004) 7:575–80. 10.1038/nn1258 [DOI] [PubMed] [Google Scholar]
- 320.Mei L, Nave K. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron (2014) 83:27–49. 10.1016/j.neuron.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci (2008) 28:6872–83. 10.1523/JNEUROSCI.1815-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci (2008) 122:748–59. 10.1037/0735-7044.122.4.748 [DOI] [PubMed] [Google Scholar]
- 323.Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, et al. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res (2009) 1294:116–27. 10.1016/j.brainres.2009.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav (2007) 6:677–87. 10.1111/j.1601-183X.2006.00298.x [DOI] [PubMed] [Google Scholar]
- 325.Moy SS, Ghashghaei HT, Nonneman RJ, Weimer JM, Yokota Y, Lee D, et al. Deficient NRG1-ERBB signaling alters social approach: relevance to genetic mouse models of schizophrenia. J Neurodev Disord (2009) 1:302–12. 10.1007/s11689-009-9017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.O’Tuathaigh CM, O’Sullivan GJ, Kinsella A, Harvey RP, Tighe O, Croke DT, et al. Sexually dimorphic changes in the exploratory and habituation profiles of heterozygous neuregulin-1 knockout mice. Neuroreport (2006) 17:79–83. 10.1097/01.wnr.0000192738.31029.0a [DOI] [PubMed] [Google Scholar]
- 327.O’Tuathaigh CM, Babovic D, O’Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience (2007) 147:18–27. 10.1016/j.neuroscience.2007.03.051 [DOI] [PubMed] [Google Scholar]
- 328.O’Tuathaigh CMP, Harte M, O’Leary C, O’Sullivan GJ, Blau C, Lai D, et al. Schizophrenia-related endophenotypes in heterozygous neuregulin-1 ‘knockout’ mice. Eur J Neurosci (2010) 31:349–58. 10.1111/j.1460-9568.2009.07069.x [DOI] [PubMed] [Google Scholar]
- 329.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport (2005) 16:271–5. 10.1097/00001756-200502280-00014 [DOI] [PubMed] [Google Scholar]
- 330.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet (2002) 71:877–92 10.1086/342734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, et al. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport (2009) 20:1523–8. 10.1097/WNR.0b013e328330f6e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Deakin IH, Nissen W, Law AJ, Lane T, Kanso R, Schwab MH, et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb Cortex (2012) 22:1520–9. 10.1093/cercor/bhr223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, et al. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS One (2010) 5:e14185. 10.1371/journal.pone.0014185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Luo X, He W, Hu X, Yan R. Reversible overexpression of bace1-cleaved neuregulin-1 N-terminal fragment induces schizophrenia-like phenotypes in mice. Biol Psychiatry (2014) 76:120–7. 10.1016/j.biopsych.2013.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yin D, Chen Y, Lu Y, Bean JC, Sathyamurthy A, Shen C, et al. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron (2013) 78:644–57. 10.1016/j.neuron.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) (2007) 192:325–36. 10.1007/s00213-007-0721-3 [DOI] [PubMed] [Google Scholar]
- 337.Desbonnet L, O’Tuathaigh C, Clarke G, O’Leary C, Petit E, Clarke N, et al. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene x environment interaction. Brain Behav Immun (2012) 26:660–71. 10.1016/j.bbi.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 338.Hong J, Shu-Leong H, Tao X, Lap-Ping Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport (1998) 9:2861–4. 10.1097/00001756-199808240-00033 [DOI] [PubMed] [Google Scholar]
- 339.Lachman H, Papolos D, Saito T, Yu Y, Szumlanski C, Weinshilboum R. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics (1996) 6:243–50. 10.1097/00008571-199606000-00007 [DOI] [PubMed] [Google Scholar]
- 340.Okochi T, Ikeda M, Kishi T, Kawashima K, Kinoshita Y, Kitajima T, et al. Meta-analysis of association between genetic variants in COMT and schizophrenia: an update. Schizophr Res (2009) 110:140–8. 10.1016/j.schres.2009.02.019 [DOI] [PubMed] [Google Scholar]
- 341.Khoury MJ, Ioannidis JPA, Allen NC, Tanzi RE, Kavvoura FK, Bagade S, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet (2008) 40:827–34. 10.1038/ng.171 [DOI] [PubMed] [Google Scholar]
- 342.Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev (2013) 37:1597–610. 10.1016/j.neubiorev.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 343.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry (2013) 45:183–94. 10.1016/j.pnpbp.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 344.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A (2001) 98:6917–22. 10.1073/pnas.111134598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry (2003) 60:889–96. 10.1001/archpsyc.60.9.889 [DOI] [PubMed] [Google Scholar]
- 346.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci (Regul Ed) (2004) 8:325–35. 10.1016/j.tics.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 347.Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry (2002) 52:701–7. 10.1016/S0006-3223(02)01416-6 [DOI] [PubMed] [Google Scholar]
- 348.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A (2003) 100:6186–91. 10.1073/pnas.0931309100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry (2004) 161:359–61. 10.1176/appi.ajp.161.2.359 [DOI] [PubMed] [Google Scholar]
- 350.Ira E, Zanoni M, Ruggeri M, Dazzan P, Tosato S. COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J Psychiatry Neurosci (2013) 38:366–80. 10.1503/jpn.120178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bhakta SG, Zhang JP, Malhotra AK. The COMT Met158 allele and violence in schizophrenia: a meta-analysis. Schizophr Res (2012) 140:192–7. 10.1016/j.schres.2012.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lee LO, Prescott CA. Association of the catechol-O-methyltransferase val158met polymorphism and anxiety-related traits: a meta-analysis. Psychiatr Genet (2014) 24:52–69. 10.1097/YPG.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A (1998) 95:9991–6. 10.1073/pnas.95.17.9991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Haasio K, Huotari M, Nissinen E, Männistö PT. Tissue histopathology, clinical chemistry and behaviour of adult COMT-gene-disrupted mice. J Appl Toxicol (2003) 23:213–9. 10.1002/jat.909 [DOI] [PubMed] [Google Scholar]
- 355.Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Männistö PT. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-o-methyltransferase-disrupted mice. J Pharmacol Exp Ther (2002) 303:1309–16. 10.1124/jpet.102.043042 [DOI] [PubMed] [Google Scholar]
- 356.Huotari M, García-Horsman JA, Karayiorgou M, Gogos JA, Männistö PT. D-amphetamine responses in catechol-O-methyltransferase (COMT) disrupted mice. Psychopharmacology (Berl) (2004) 172:1–10. 10.1007/s00213-003-1627-3 [DOI] [PubMed] [Google Scholar]
- 357.Babovic D, O’Tuathaigh CM, O’Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Exploratory and habituation phenotype of heterozygous and homozygous COMT knockout mice. Behav Brain Res (2007) 183:236–9. 10.1016/j.bbr.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 358.O’Tuathaigh CMP, Hryniewiecka M, Behan A, Tighe O, Coughlan C, Desbonnet L, et al. Chronic adolescent exposure to Δ-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology (2010) 35:2262–73. 10.1038/npp.2010.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Babovic D, O’Tuathaigh CM, O’Connor AM, O’Sullivan GJ, Tighe O, Croke DT, et al. Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience (2008) 155:1021–9. 10.1016/j.neuroscience.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 360.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci (2008) 28:8709–23. 10.1523/JNEUROSCI.2077-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci (2004) 24:5331–5. 10.1523/JNEUROSCI.1124-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev (2012) 64:238–58. 10.1124/pr.111.005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World J Biol Chem (2014) 5:409–28. 10.4331/wjbc.v5.i4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Favalli G, Li J, Belmonte-de-Abreu P, Wong AHC, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res (2012) 46:1–11 10.1016/j.jpsychires.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 365.Lee B, Kim Y. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig (2010) 7:231–5. 10.4306/pi.2010.7.4.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry (2000) 5:293–300. 10.1038/sj.mp.4000718 [DOI] [PubMed] [Google Scholar]
- 367.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry (2003) 8:592–610. 10.1038/sj.mp.4001308 [DOI] [PubMed] [Google Scholar]
- 368.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci (2005) 25:372–83. 10.1523/JNEUROSCI.4035-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Iritani S, Niizato K, Nawa H, Ikeda K, Emson PC. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog Neuropsychopharmacol Biol Psychiatry (2003) 27:801–7. 10.1016/S0278-5846(03)00112-X [DOI] [PubMed] [Google Scholar]
- 370.Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res (2001) 52:79–86. 10.1016/S0920-9964(00)00084-0 [DOI] [PubMed] [Google Scholar]
- 371.Durany N, Thome J. Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry (2004) 19:326–37. 10.1016/j.eurpsy.2004.06.020 [DOI] [PubMed] [Google Scholar]
- 372.Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals (2008) 16:183–93. 10.1159/000111562 [DOI] [PubMed] [Google Scholar]
- 373.Gratacòs M, González JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry (2007) 61:911–22. 10.1016/j.biopsych.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 374.Aas M, Haukvik UK, Djurovic S, Tesli M, Athanasiu L, Bjella T, et al. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. J Psychiatr Res (2014) 59:14–21. 10.1016/j.jpsychires.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 375.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry (2003) 60:804–15. 10.1001/archpsyc.60.8.804 [DOI] [PubMed] [Google Scholar]
- 376.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res (2005) 136:29–37. 10.1016/j.molbrainres.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 377.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry J. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res (2002) 109:143–8. 10.1016/S0165-1781(02)00005-7 [DOI] [PubMed] [Google Scholar]
- 378.Guilloux J, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry (2012) 17:1130–42. 10.1038/mp.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tripp A, Oh H, Guilloux J, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry (2012) 169:1194–202. 10.1176/appi.ajp.2012.12020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kim D, Kim Y, Lee S, Lee H, Lee H, Lee B, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31:78–85 10.1016/j.pnpbp.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 381.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry (2003) 54:70–5. 10.1016/S0006-3223(03)00181-1 [DOI] [PubMed] [Google Scholar]
- 382.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry (2008) 64:527–32. 10.1016/j.biopsych.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J (2000) 19:1290–300. 10.1093/emboj/19.6.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A (1999) 96:15239–44. 10.1073/pnas.96.26.15239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav (2008) 7:906–14. 10.1111/j.1601-183X.2008.00430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Manning EE, Van den Buuse M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front Cell Neurosci (2013) 7:92. 10.3389/fncel.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci (2004) 24:7958–63. 10.1523/JNEUROSCI.1948-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and ± BDNF mutant mice. Exp Neurol (2001) 170:121–8. 10.1006/exnr.2001.7698 [DOI] [PubMed] [Google Scholar]
- 389.Koizumi H, Hashimoto K, Iyo M. Dietary restriction changes behaviours in brain-derived neurotrophic factor heterozygous mice: role of serotonergic system. Eur J Neurosci (2006) 24:2335–44. 10.1111/j.1460-9568.2006.05094.x [DOI] [PubMed] [Google Scholar]
- 390.Psotta L, Lessmann V, Endres T. Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiol Learn Mem (2013) 103:34–8. 10.1016/j.nlm.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 391.Klug M, Hill RA, Choy K, Kyrios M, Hannan AJ, Van den Buuse M. Long-term behavioral and NMDA receptor effects of young-adult corticosterone treatment in BDNF heterozygous mice. Neurobiol Dis (2012) 46:722–31. 10.1016/j.nbd.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 392.Klug M, Van den Buuse M. An investigation into “two hit” effects of BDNF deficiency and young-adult cannabinoid receptor stimulation on prepulse inhibition regulation and memory in mice. Front Behav Neurosci (2013) 7:149. 10.3389/fnbeh.2013.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci (2001) 115:1145–53. 10.1037/0735-7044.115.5.1145 [DOI] [PubMed] [Google Scholar]
- 394.Chourbaji S, Hellweg R, Brandis D, Zörner B, Zacher C, Lang UE, et al. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res (2004) 121:28–36. 10.1016/j.molbrainres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 395.Ibarguen-Vargas Y, Surget A, Vourc’h P, Leman S, Andres CR, Gardier AM, et al. Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res (2009) 202:245–51. 10.1016/j.bbr.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 396.Lindholm JSO, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf+/- heterozygous null mice. Neuropharmacology (2012) 62:391–7. 10.1016/j.neuropharm.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 397.Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience (2006) 142:49–58. 10.1016/j.neuroscience.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 398.Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry (2009) 66:84–90. 10.1016/j.biopsych.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry (2007) 61:187–97. 10.1016/j.biopsych.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 400.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience (2003) 121:341–54. 10.1016/S0306-4522(03)00426-3 [DOI] [PubMed] [Google Scholar]
- 401.Chen Z, Jing D, Bath KG, Ieraci A, Khan T, Siao C, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science (2006) 314:140–3. 10.1126/science.1129663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Nakajo Y, Miyamoto S, Nakano Y, Xue JH, Hori T, Yanamoto H. Genetic increase in brain-derived neurotrophic factor levels enhances learning and memory. Brain Res (2008) 1241:103–9. 10.1016/j.brainres.2008.08.080 [DOI] [PubMed] [Google Scholar]
- 403.Govindarajan A, Rao BSS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A (2006) 103:13208–13. 10.1073/pnas.0605180103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Zörner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, et al. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than “depressive”. Biol Psychiatry (2003) 54:972–82. 10.1016/S0006-3223(03)00418-9 [DOI] [PubMed] [Google Scholar]
- 405.Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCγ pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci (2004) 26:166–81. 10.1016/j.mcn.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 406.Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and Signaling. Hippocampus (2014) 24(10):1197–211. 10.1002/hipo.22302 [DOI] [PubMed] [Google Scholar]
- 407.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet (2012) 13:537–51. 10.1038/nrg3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry (2000) 157:1552–62. 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 409.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry (2013) 18:497–511. 10.1038/mp.2012.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Lipina TV, Niwa M, Jaaro-Peled H, Fletcher PJ, Seeman P, Sawa A, et al. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav (2010) 9:777–89. 10.1111/j.1601-183X.2010.00615.x [DOI] [PubMed] [Google Scholar]
- 411.Jaaro-Peled H, Niwa M, Foss CA, Murai R, de LR, Kamiya A, et al. Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum Mol Genet (2013) 22:1574–80. 10.1093/hmg/ddt007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S. Regulation of psychostimulant-induced signaling and gene expression in the striatum. J Neurochem (2008) 104:1440–9. 10.1111/j.1471-4159.2008.05240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Maher BJ, LoTurco JJ. Disrupted-in-schizophrenia (DISC1) functions presynaptically at glutamatergic synapses. PLoS One (2012) 7:e34053. 10.1371/journal.pone.0034053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Qiu S, Weeber EJ. Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol (2007) 97:2312–21. 10.1152/jn.00869.2006 [DOI] [PubMed] [Google Scholar]
- 415.Wang P, Si T. Use of antipsychotics in the treatment of depressive disorders. Shanghai Arch Psychiatry (2013) 25:134–40. 10.3969/j.issn.1002-0829.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Coplan JD, Gopinath S, Abdallah CG, Berry BR. A neurobiological hypothesis of treatment-resistant depression – mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci (2014) 8:189. 10.3389/fnbeh.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) (2003) 169:215–33. 10.1007/s00213-003-1582-z [DOI] [PubMed] [Google Scholar]
- 418.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology (1996) 14:301–7. 10.1016/0893-133X(95)00137-3 [DOI] [PubMed] [Google Scholar]
- 419.Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci (2010) 47:4–16. [PubMed] [Google Scholar]
- 420.Merritt K, McGuire P, Egerton A. Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Front Psychiatry (2013) 4:151. 10.3389/fpsyt.2013.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Umemori J, Takao K, Koshimizu H, Hattori S, Furuse T, Wakana S, et al. ENU-mutagenesis mice with a non-synonymous mutation in Grin1 exhibit abnormal anxiety-like behaviors, impaired fear memory, and decreased acoustic startle response. BMC Res Notes (2013) 6:203. 10.1186/1756-0500-6-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology (2012) 62:35–41 10.1016/j.neuropharm.2011.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Cioffi CL. Modulation of NMDA receptor function as a treatment for schizophrenia. Bioorg Med Chem Lett (2013) 23:5034–44. 10.1016/j.bmcl.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 424.Graziane N, Ishizuka K, Hayashi-Takagi A, Tomoda T, Yan Z, Kamiya A, et al. Disrupted-in-schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci (2010) 13:327–32. 10.1038/nn.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Wang Y, Ho U, Ko M, Liao C, Lee L. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct (2012) 217:337–51 10.1007/s00429-011-0355-4 [DOI] [PubMed] [Google Scholar]
- 426.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci (2009) 10:647–58. 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- 427.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci (2007) 30:79–97 10.1146/annurev.neuro.30.051606.094222 [DOI] [PubMed] [Google Scholar]
- 428.Zuo Y, Lin A, Chang P, Gan W. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron (2005) 46:181–9. 10.1016/j.neuron.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 429.Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology (2008) 33:141–65. 10.1038/sj.npp.1301563 [DOI] [PubMed] [Google Scholar]
- 430.Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry (2014) 77:52–8. 10.1016/j.biopsych.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 431.Penzes P, Buonanno A, Passafaro M, Sala C, Sweet RA. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem (2013) 126:165–82. 10.1111/jnc.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology (1990) 24:115–20. 10.1159/000119472 [DOI] [PubMed] [Google Scholar]
- 433.Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses (1995) 45:135–41. 10.1016/0306-9877(95)90062-4 [DOI] [PubMed] [Google Scholar]
- 434.Anderson G, Maes M, Berk M. Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry (2013) 42:101–14. 10.1016/j.pnpbp.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 435.McAllister AK. Major histocompatibility complex I in brain development and schizophrenia. Biol Psychiatry (2014) 75:262–8. 10.1016/j.biopsych.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology (2014) 155:2635–46. 10.1210/en.2014-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, et al. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav Immun (2014) 41:173–81. 10.1016/j.bbi.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 438.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry (2003) 60:1187–92. 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- 439.Insel TR. Rethinking schizophrenia. Nature (2010) 468:187–93 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- 440.Oliver PL. Challenges of analysing gene-environment interactions in mouse models of schizophrenia. ScientificWorldJournal (2011) 11:1411–20. 10.1100/tsw.2011.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry (2014) 5:48. 10.3389/fpsyt.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Abazyan B, Dziedzic J, Hua K, Abazyan S, Yang C, Mori S, et al. Chronic exposure of mutant DISC1 mice to lead produces sex-dependent abnormalities consistent with schizophrenia and related mental disorders: a gene-environment interaction study. Schizophr Bull (2014) 40:575–84. 10.1093/schbul/sbt071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Haque FN, Lipina TV, Roder JC, Wong AHC. Social defeat interacts with DISC1 mutations in the mouse to affect behavior. Behav Brain Res (2012) 233:337. 10.1016/j.bbr.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 444.Hida H, Mouri A, Noda Y. Behavioral phenotypes in schizophrenic animal models with multiple combinations of genetic and environmental factors. J Pharmacol Sci (2013) 121:185–91. 10.1254/jphs.12R15CP [DOI] [PubMed] [Google Scholar]
- 445.Lasić D, Bevanda M, Bošnjak N, Uglešić B, Glavina T, Franić T. Metabolic syndrome and inflammation markers in patients with schizophrenia and recurrent depressive disorder. Psychiatr Danub (2014) 26:214–9. [PubMed] [Google Scholar]
- 446.Malhotra N, Grover S, Chakrabarti S, Kulhara P. Metabolic syndrome in schizophrenia. Indian J Psychol Med (2013) 35:227–40 10.4103/0253-7176.119471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Martinac M, Pehar D, Karlović D, Babić D, Marcinko D, Jakovljević M. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clin Croat (2014) 53:55–71. [PubMed] [Google Scholar]
- 448.Daumit GL, Goff DC, Meyer JM, Davis VG, Davis SM, Nasrallah HA, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res (2008) 105:175–87. 10.1016/j.schres.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Jakovljevic M, Crncevic Z, Ljubicic D, Babic D, Topic R, Saric M. Mental disorders and metabolic syndrome: a fatamorgana or warning reality? Psychiatr Danub (2007) 19:76–86. [PubMed] [Google Scholar]
- 450.Benes FM. Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry (2009) 65:1003–5. 10.1016/j.biopsych.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 451.Spiga S, Mulas G, Piras F, Diana M. The “addicted” spine. Front Neuroanat (2014) 8:110. 10.3389/fnana.2014.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Banks PJ, Warburton EC, Brown MW, Bashir ZI. Mechanisms of synaptic plasticity and recognition memory in the perirhinal cortex. Prog Mol Biol Transl Sci (2014) 122:193–209. 10.1016/B978-0-12-420170-5.00007-6 [DOI] [PubMed] [Google Scholar]
- 453.Xavier AL, Menezes JR, Goldman SA, Nedergaard M. Fine-tuning the central nervous system: microglial modelling of cells and synapses. Philos Trans R Soc Lond B Biol Sci (2014) 369:20130593. 10.1098/rstb.2013.0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci (2013) 7:260. 10.3389/fncel.2013.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Hayashi-Takagi A, Sawa A. Disturbed synaptic connectivity in schizophrenia: convergence of genetic risk factors during neurodevelopment. Brain Res Bull (2010) 83:140–6. 10.1016/j.brainresbull.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 456.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods (2013) 10:413–20. 10.1038/nmeth.2434 [DOI] [PubMed] [Google Scholar]
- 457.Sasakura H, Tsukada Y, Takagi S, Mori I. Japanese studies on neural circuits and behavior of Caenorhabditis elegans. Front Neural Circuits (2013) 7:187. 10.3389/fncir.2013.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Kazama H. Systems neuroscience in Drosophila: conceptual and technical advantages. Neuroscience (2014). 10.1016/j.neuroscience.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 459.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry (2008) 13:36–64. 10.1038/sj.mp.4002106 [DOI] [PubMed] [Google Scholar]
- 460.Thomson PA, Malavasi EL, Grunewald E, Soares DC, Borkowska M, Millar JK. DISC1 genetics, biology and psychiatric illness. Front Biol (Beijing) (2013) 8:1–31 10.1007/s11515-012-1254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Maser JD, Norman SB, Zisook S, Everall IP, Stein MB, Schettler PJ, et al. Psychiatric nosology is ready for a paradigm shift in DSM-V. Clin Psychol (New York) (2009) 16:24–40 10.1111/j.1468-2850.2009.01140.x [DOI] [Google Scholar]
- 462.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell (2013) 153:910–8. 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]