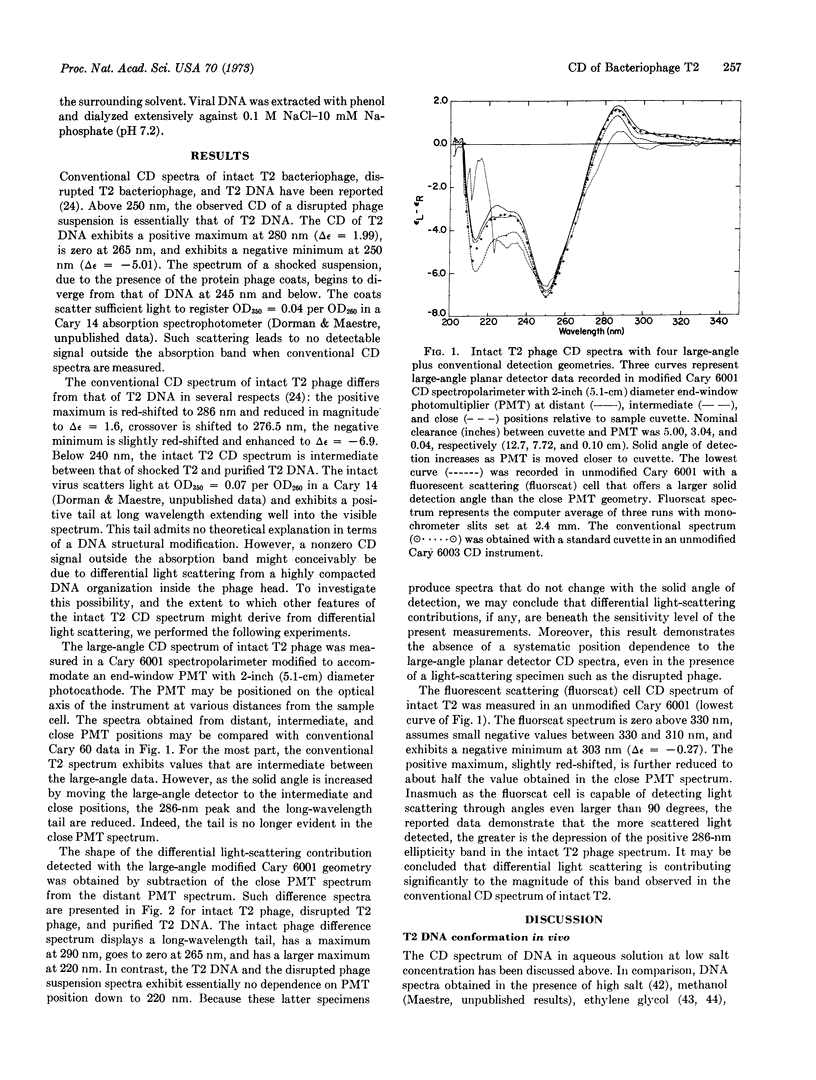
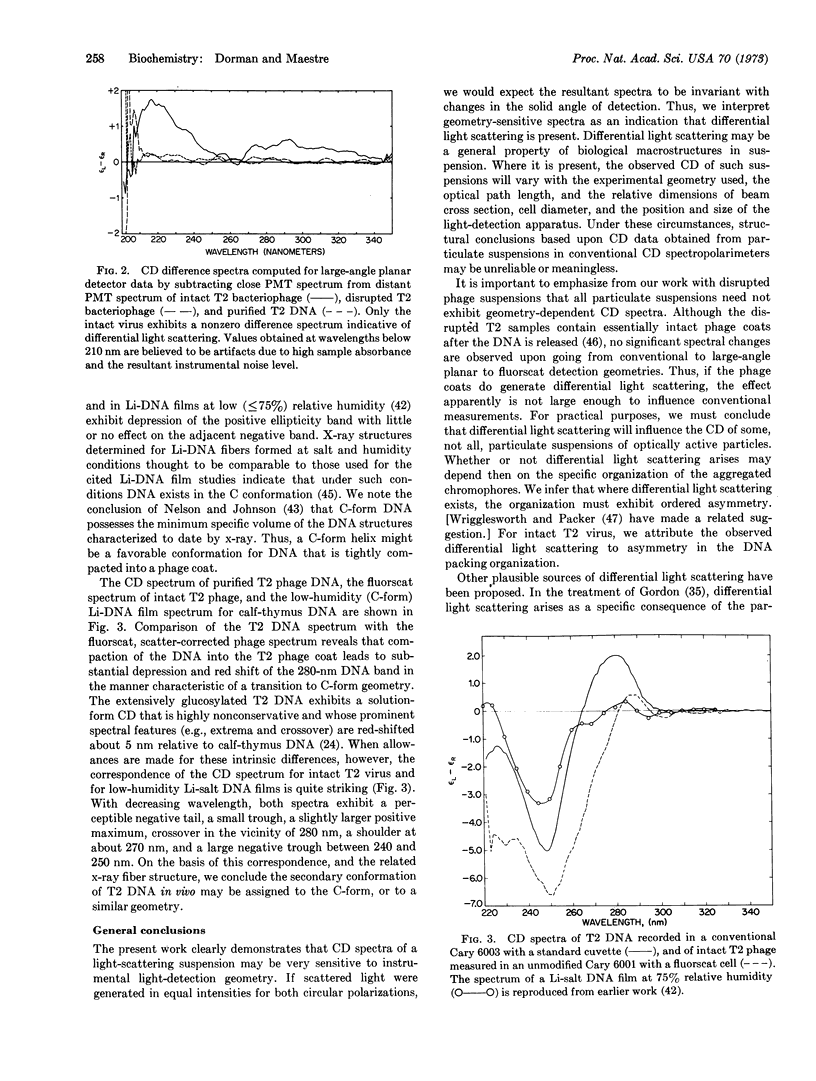
Abstract
Experimental techniques are presented that can be used to assay and correct for differential light scattering effects in circular dichroism spectra of biological macrostructures. The assay is based upon use of variable detector geometries that collect light over large solid angles. Disrupted T2 virus suspensions and purified T2 phage DNA exhibit geometry-independent spectra; the spectrum of intact T2 virus is highly sensitive to detection geometry. On the basis of spectra obtained after light-scattering correction, the structure of T2 DNA in the phage particle is assigned to the C form. We conclude that: (i) The measured circular dichroism of a light-scattering specimen may be highly sensitive to light-detection geometry of the instrument. This effect is indicative of differential scattering intensity for left and right circularly polarized light. (ii) Some optically active particles, although they scatter light intensely, exhibit circular dichroism that is independent of detection geometry and, therefore, apparently uninfluenced by differential light scattering. We infer that whether differential light scattering arises may depend upon the presence or absence of ordered asymmetry in the organization of the scattering particle. (iii) The circular dichroism of any light-scattering specimen should be measured again in apparatus designed for differential light-scattering correction as a prerequisite to meaningful structural conclusions. (iv) Differential scattering effects in circular dichroism may be potentially useful as a probe for large-order organization of the scattering particle.
Keywords: light-detection geometry, C-form DNA, ordered asymmetry
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Schaffhausen B., Langan T. A., Fasman G. D. Altered conformational effects of phosphorylated lysine-rich histone (f-1) in f-1--deoxyribonucleic acid complexes. Circular dichroism and immunological studies. Biochemistry. 1971 Mar 2;10(5):909–913. doi: 10.1021/bi00781a028. [DOI] [PubMed] [Google Scholar]
- Cantor K. P., Hearst J. E. The structure of metaphase chromosomes. I. Electrometric titration, magnesium ion binding and circular dichroism. J Mol Biol. 1970 Apr 14;49(1):213–229. doi: 10.1016/0022-2836(70)90387-6. [DOI] [PubMed] [Google Scholar]
- Carroll D. Optical properties of deoxyribonucleic acid--polylysine complexes. Biochemistry. 1972 Feb 1;11(3):421–426. doi: 10.1021/bi00753a019. [DOI] [PubMed] [Google Scholar]
- Davidson B., Fasman G. D. The double-stranded polyadenylic acid: poly-L-lysine complex. A conformational study and characterization. Arch Biochem Biophys. 1971 Jun;144(2):650–656. doi: 10.1016/0003-9861(71)90371-7. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Schaffhausen B., Goldsmith L., Adler A. Conformational changes associated with f-1 histone-deoxyribonucleic acid complexes. Circular dichroism studies. Biochemistry. 1970 Jul 7;9(14):2814–2822. doi: 10.1021/bi00816a010. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Valenzuela M. S., Adler A. J. Complexes of deoxyribonucleic acid with fragments of lysine-rich histone (f-1). Circular dichroism studies. Biochemistry. 1971 Sep 28;10(20):3795–3801. doi: 10.1021/bi00796a024. [DOI] [PubMed] [Google Scholar]
- Fric I., Sponar J. Circular dichroism of native and reconstituted nucleohistones. Biopolymers. 1971;10(9):1525–1531. doi: 10.1002/bip.360100908. [DOI] [PubMed] [Google Scholar]
- Glaser M., Simpkins H., Singer S. J., Sheetz M., Chan S. I. On the interactions of lipids and proteins in the red blood cell membrane. Proc Natl Acad Sci U S A. 1970 Mar;65(3):721–728. doi: 10.1073/pnas.65.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Singer S. J. Circular dichroism and the conformations of membrane proteins. Studies with red blood cell membranes. Biochemistry. 1971 May 11;10(10):1780–1787. doi: 10.1021/bi00786a008. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Artifacts in the measured optic activity of membrane suspensions. Arch Biochem Biophys. 1971 Feb;142(2):481–488. doi: 10.1016/0003-9861(71)90511-x. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Optical activity of membrane suspensions: calculation of artifacts by Mie scattering theory. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2365–2369. doi: 10.1073/pnas.68.10.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J. Mie scattering by optically active particles. Biochemistry. 1972 Feb 1;11(3):413–420. doi: 10.1021/bi00753a018. [DOI] [PubMed] [Google Scholar]
- Green G., Hahler H. R. Conformation changes of deoxyribonucleic acid and polydeoxynucleotides in water and ethylene glycol. Biochemistry. 1971 Jun 8;10(12):2200–2216. [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes M., Garrett R. A., Gratzer W. B. Structure of nucleic acid-poly base complexes. Biochemistry. 1970 Oct 27;9(22):4410–4416. doi: 10.1021/bi00824a600. [DOI] [PubMed] [Google Scholar]
- Henson P., Walker I. O. The partial dissociation of nucleohistone by salts. Circular dichroism and denaturation studies. Eur J Biochem. 1970 Nov;16(3):524–531. doi: 10.1111/j.1432-1033.1970.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Urry D. W. Correlation of light scattering and absorption flattening effects with distortions in the circular dichroism patterns of mitochondrial membrane fragments. Biochem Biophys Res Commun. 1969 Feb 21;34(4):404–411. doi: 10.1016/0006-291x(69)90396-9. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARVIN D. A., SPENCER M., WILKINS M. H., HAMILTON L. D. The molecular configuration of deoxyribonucleic acid. III. X-ray diffraction study of the C form of the lithium salt. J Mol Biol. 1961 Oct;3:547–565. doi: 10.1016/s0022-2836(61)80021-1. [DOI] [PubMed] [Google Scholar]
- Maestre M. F., Gray D. M., Cook R. B. Magnetic circular dichroism study on synthetic polynucleotides, bacteriophage structure, and DNA's. Biopolymers. 1971;10(12):2537–2553. doi: 10.1002/bip.360101214. [DOI] [PubMed] [Google Scholar]
- Maestre M. F., Tinoco I., Jr Optical rotatory dispersion of viruses. J Mol Biol. 1967 Feb 14;23(3):323–335. doi: 10.1016/s0022-2836(67)80108-6. [DOI] [PubMed] [Google Scholar]
- Matsuyama A., Tagashira Y., Nagata C. A circular dichroism study on the conformation of DNA in rat liver chromatin. Biochim Biophys Acta. 1971 Jun 30;240(2):184–190. doi: 10.1016/0005-2787(71)90656-3. [DOI] [PubMed] [Google Scholar]
- Nelson R. G., Johnson W. C., Jr Conformation of DNA in ethylene glycol. Biochem Biophys Res Commun. 1970 Oct 9;41(1):211–216. doi: 10.1016/0006-291x(70)90490-0. [DOI] [PubMed] [Google Scholar]
- Olins D. E. Interaction of lysine-rich histones and DNA. J Mol Biol. 1969 Aug 14;43(3):439–460. doi: 10.1016/0022-2836(69)90351-9. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Model nucleohistones: the interaction of F1 and F2al histones with native T7 DNA. J Mol Biol. 1971 May 14;57(3):437–455. doi: 10.1016/0022-2836(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Wetlaufer D. B. Light-scattering contributions to the circular dichroism of particulate systems. Arch Biochem Biophys. 1970 Aug;139(2):257–264. doi: 10.1016/0003-9861(70)90476-5. [DOI] [PubMed] [Google Scholar]
- Permogorov V. I., Debabov V. G., Sladkova I. A., Rebentish B. A. Structure of DNA and histones in the nucleohistone. Biochim Biophys Acta. 1970 Feb 18;199(2):556–558. doi: 10.1016/0005-2787(70)90107-3. [DOI] [PubMed] [Google Scholar]
- Ramm E. I., Vorob'ev V. I., Birshtein T. M., Bolotina I. A., Volkenshtein M. V. Circular dichroism of DNA and histones in the free state and in deoxyribonucleoprotein. Eur J Biochem. 1972 Feb 15;25(2):245–253. doi: 10.1111/j.1432-1033.1972.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Schneider M. J., Rosenheck K. Optical activity of biological membranes: scattering effects and protein conformation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):793–798. doi: 10.1073/pnas.66.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. T., Leng M., Felsenfeld G. Deoxyribonucleic acid-polylysine complexes. Structure and nucleotide specificity. Biochemistry. 1969 Aug;8(8):3219–3232. doi: 10.1021/bi00836a014. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of deoxyribonucleic acid complexes with arginine-rich histone IV (f2al). Biochemistry. 1971 Apr 27;10(9):1675–1683. doi: 10.1021/bi00785a027. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Conformation of deoxyribonucleic acid in chromatin: a circular dichroism study. J Mol Biol. 1970 Aug 28;52(1):125–129. doi: 10.1016/0022-2836(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Sober H. A. Circular dichroism of calf liver nucleohistone. Biochemistry. 1970 Aug 4;9(16):3103–3109. doi: 10.1021/bi00818a001. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Shih T. Y., Adler A. J., Fasman G. D. Electron microscopy and circular dichroism studies on chromatin. Biochemistry. 1972 Aug 1;11(16):3044–3054. doi: 10.1021/bi00766a016. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Hinners T. A., Masotti L. Calculation of distorted circular dichroism curves for poly-L-glutamic acid suspensions. Arch Biochem Biophys. 1970 Mar;137(1):214–221. doi: 10.1016/0003-9861(70)90428-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Ji T. H. Distortions in circular dichroism patterns of particulate (or membranous) systems. Arch Biochem Biophys. 1968 Dec;128(3):802–807. doi: 10.1016/0003-9861(68)90088-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Krivacic J. Differential scatter of left and right circularly polarized light by optically active particulate systems. Proc Natl Acad Sci U S A. 1970 Apr;65(4):845–852. doi: 10.1073/pnas.65.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Spelsberg T. C. Aspects of chromosomal structure. I. Circular dichroism studies. Biochemistry. 1971 Jun 22;10(13):2599–2605. doi: 10.1021/bi00789a029. [DOI] [PubMed] [Google Scholar]
- Wilhelm F. X., Champagne M. H., Daune M. P. Conformation du DNA dans la nucléoprotéine. Eur J Biochem. 1970 Aug;15(2):321–330. doi: 10.1111/j.1432-1033.1970.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Packer L. Optical rotary dispersion and circular dichroism studies on mitochondria: correlation of ultrastructure and metabolic state with molecular conformational changes. Arch Biochem Biophys. 1968 Dec;128(3):790–801. doi: 10.1016/0003-9861(68)90087-8. [DOI] [PubMed] [Google Scholar]



