Abstract
Background
The aim of this study was to evaluate the ability of dual acridine orange/ethidium bromide (AO/EB) staining to detect tumor cell apoptosis. According to apoptosis-associated changes of cell membranes during the process of apoptosis, a clear distinction is made between normal cells, early and late apoptotic cells, and necrotic cells.
Material/Method
We cultured human osteosarcoma cells with 30, 60, and 120 μg/ml kappa-selenocarrageenan. To assess the rates of cell proliferation and apoptosis, cells were fluorescently stained with acridine orange/ethidium bromide (AO/EB) or stained with propidium iodide (PI) and analyzed by flow cytometry. All experiments were repeated at least 3 times.
Result
Normal tumor cells, early and late apoptotic cells, and necrotic cells were examined using fluorescent microscopy. Early-stage apoptotic cells were marked by crescent-shaped or granular yellow-green acridine orange nuclear staining. Late-stage apoptotic cells were marked with concentrated and asymmetrically localized orange nuclear ethidium bromide staining. Necrotic cells increased in volume and showed uneven orange-red fluorescence at their periphery. Cells appeared to be in the process of disintegrating. The percentage of apoptotic osteosarcoma cells detected by dual acridine orange/ethidium bromide (AO/EB) staining was not significantly different from that detected using flow cytometry (P>0.05).
Conclusions
Our results suggest that dual acridine orange/ethidium bromide staining is an economic and convenient method to detect apoptosis in tumor cells and to test tumor chemosensitivity compared with flow cytometry.
MeSH Keywords: Apoptosis, Flow Cytometry, Osteosarcoma, Staining and Labeling
Background
Apoptosis is a type of genetically regulated programmed cell death that controls the development of multicellular organisms and tissues by eliminating physiologically redundant, physical damaged, and abnormal cells [1]. Studies focusing on the genes and signals regulating apoptosis have played an important role in basic oncology research. Radiotherapy and chemotherapy are essential for the treatment of malignant tumors in pre- and post-operative cancer patients. Chemotherapeutics destroy tumor cells and restrain their proliferation primarily by promoting tumor cell apoptosis [2]. The efficacy of anticancer drugs is measured by their ability to detect cancer cells and selectively promote their apoptosis.
The tumor drug sensitivity test (DST) is a method to identify the most effective drug to treat tumors based on their sensitivity. The genotype and pathogenesis of tumors vary, and malignant tumors are made up of a polymorphous, heterogeneous, and multi-differentiated cell population. Previous chemotherapies increased tumor drug resistance, thus having an unsatisfactory effect on patients [3]. Tumors can be resistant to one or more drugs, or exhibit sensitivity towards many drugs [4,5]. By reducing drug resistance and increasing the efficiency of DSTs, more effective individualized treatments can be established [6,7]. Detection of drug-induced tumor cell apoptosis in DSTs is of great importance. Multiple methods to detect apoptosis have been developed that detect changes in cell morphology and surface markers associated with apoptosis. However, these defects may present themselves concurrently [8]. This can obscure the result of DSTs, affecting the accuracy and validity of the drugs they select.
Dual acridine orange/ethidium bromide (AO/EB) fluorescent staining, visualized under a fluorescent microscope, can be used to identify apoptosis-associated changes of cell membranes during the process of apoptosis [9]. This method can also accurately distinguish cells in different stages of apoptosis [10,11]. Thus, AO/EB staining can feasibly be used in DSTs. Here, we introduce a simple method to identify cell apoptosis. Dual AO/EB staining was used to examine apoptosis in a human osteosarcoma cell line treated with the tumor inhibitor kappa-selenocarrageenan. We qualitatively and quantitatively analyzed the effects of kappa-selenocarrageenan using the AO/EB staining method. Flow cytometry is currently the most common method used in cell research. Flow cytometry can be used to measure the fluorescence intensity of intracellular Ca(2+) from bone marrow mesenchymal stem cells (MSCs) [12]. Cell-cycle distribution can be determined by flow cytometric analysis using propidium iodide (PI) staining and cellular apoptosis can be evaluated by flow cytometry and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay [13]. By comparing our technique with flow cytometry analysis technology, we concluded that dual AO/EB staining is a simple and accurate method that can be used in tumor DSTs.
Material and Methods
Dual AO/EB fluorescent staining
After obtaining IRB approval, a human osteosarcoma cell line (OS-732 cells, purchased from Beijing JiShuiTan Hospital, Beijing, China) in the logarithmic growth phase were digested with 0.25% trypsin (Hyclone, Logan, UT). RPMI1604 culture medium (Hyclone, Logan, UT) containing 10% fetal calf serum (FCS, Hyclone, Logan, UT) was deposited in each well of a 96-well plate (100 μl/well). Cells were added to a final concentration of 2×104/ml, and the plates were incubated. Cells were left untreated or treated with 30, 60, or 120 μg/ml of kappa-selenocarrageenan (Shanghai Tiancifu Biological engineering Co. Ltd., Shanghai, China). The samples in a 96-well plate were divided into 4 groups, with 24 well samples in each group corresponding to different reagent concentrations. After being cultured for 24 h, 48 h, and 4 d, 20 μl of trypsin was added into each well. When cells had sloughed off, suspensions (25 μl) were transferred to glass slides. Dual fluorescent staining solution (1 μl) containing 100 μg/ml AO and 100 μg/ml EB (AO/EB, Sigma, St. Louis, MO) was added to each suspension and then covered with a coverslip. The morphology of apoptotic cells was examined and 500 cells were counted within 20 min using a fluorescent microscope (OLYMPUS, Japan). Dual acridine orange/ethidium bromide (AO/EB) staining method was repeated 3 times at least.
Flow cytometry analysis of cell cycle and apoptosis
Cells were trypsinized (0.25% trypsin) 24 h after kappa-selenocarrageenan was applied to the sample. Single-cell suspensions (2×106 cells) were extracted and washed using phosphate-buffered saline. Samples were fixed using 70% alcohol at −20°C overnight. Lysis buffer (0.2 M Na2HPO4, 0.1 M citric acid, 0.1% Triton X-100 pH 7.8) was added to the samples and was incubated at room temperature for 45 min. Next, the cells were digested with 50 μg/ml RNase for 10 min. Cells were stained with PI (50 μg/ml) for 30 min (PI, Sigma, St. Louis, MO). The samples were analyzed using a flow cytometer (BD, Franklin Lakes, NJ). The cells with DNA content less than that of cells at the G1 phase were identified as apoptotic cells. Also, flow cytometry analysis was repeated at least 3 times.
Statistical analysis
All statistical analyses were performed using the SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) for Windows. P value of <0.05 was considered statistically significant.
Results
AO/EB double-staining and fluorescent microscopy
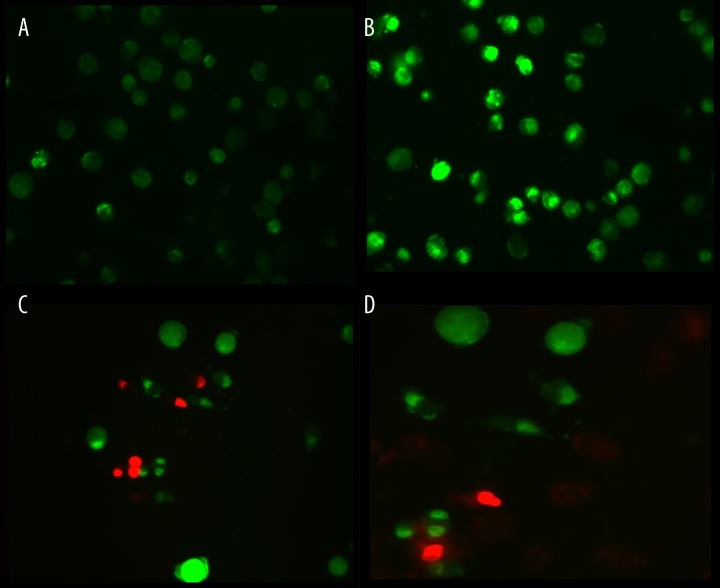
Osteosarcoma cells were labeled by AO/EB 24 h after kappa-selenocarrageenan was applied. Dual staining was examined under a fluorescent microscope. No significant apoptosis was detected in the negative control group (Figure 1A). Early-stage apoptotic cells, marked by crescent-shaped or granular yellow-green AO nuclear staining, were detected in the experimental group (Figure 1B). Staining was localized asymmetrically within the cells. With increasing concentrations and treatment lengths, the number of early-stage apoptotic cells increased. Late-stage apoptotic cells, with concentrated and asymmetrically localized orange nuclear EB staining, were also detected (Figure 1C). Necrotic cells increased in volume and showed uneven orange-red fluorescence at their periphery. The cells appeared to be in the process of disintegrating (Figure 1D).
Figure 1.
(A) Negative control group (normal cells): the circular nucleus uniformly distributed in the center of the cell. (B) Experimental group (early apoptotic cells): nucleus showed yellow-green fluorescence by acridine orange (AO) staining and concentrated into a crescent or granular that located in 1 side of cells. (C) Experimental group (late apoptotic cells): the nucleus of cell showed orange fluorescence by EB staining and gathered in concentration and located in bias. (D) Necrotic cells: The necrosis cells’ volume was increased, showing uneven orange-red fluorescence and an unapparent outline. It is becoming dissolved or near disintegration.
Flow cytometry analysis
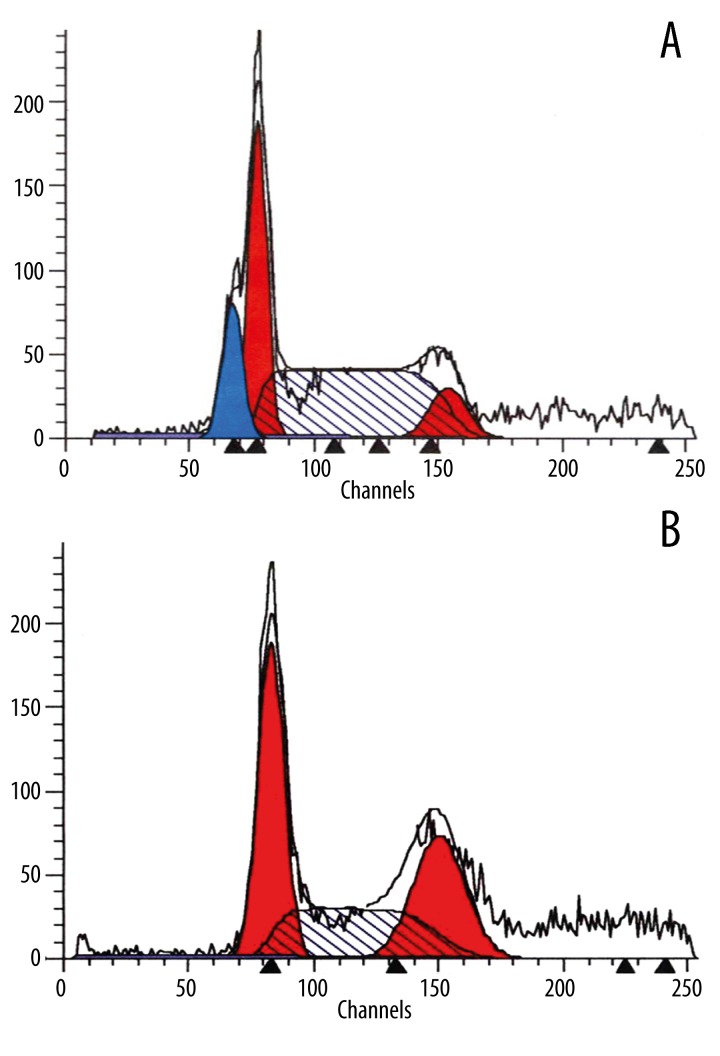
The hypodiploid peak appearing to the left of the G1 peak in the cell cycle histogram formed as a result of apoptosis. This peak is characteristic of karyopyknosis and DNA cleavage in apoptotic cells. No distinctive apoptotic peak was observed for the negative control group cultured for 24 h (Figure 2A). The apoptotic peak to the left of the G1 peak immediately appeared 24 h after 30 μg/ml of kappa-selenocarrageenan was applied to the osteosarcoma cells (Figure 2B). The number of apoptotic cells increased with increasing drug concentration and exposure time.
Figure 2.
(A) Negative control group: There was no distinct apoptotic peak. (B) Experimental group: The hypodiploid peak before the G1 peak showed in the cell cycle histogram was the apoptotic peak (Ap peak) formed because of apoptosis cells.
We compared the results attained using flow cytometry analysis and quantitative fluorescent staining. The results of our apoptotic cell count analyses are listed in Table 1. A Student’s t-test showed no significant difference between the 2 methods (Table 1).
Table 1.
Percentage of apoptotic cells identified using flow cytometry and AO/BR methods.
| Concentrations (%) | Flow cytometry (PI) | AO/BR | p value |
|---|---|---|---|
| 30 μg/ml | 6.25±0.9 | 6.68±1.2 | 0.69 |
| 60 μg/ml | 9.97±1.5 | 10.33±1.7 | 0.75 |
| 120 μg/ml | 20.14±1.8 | 20.46±2.0 | 0.84 |
PI – propidium iodide. Concentrations mean of different does of kappa-selenocarrageenan. The Two methods did not differ significantly (P>0.05, Student’s t-test). All experiments were repeated three times at least.
Discussion and Conclusions
Osteosarcoma is a systemic carcinoma that occurs primarily in children and adolescents. Greater than 80% of patients have systemic metastatic cancer when they enter the hospital, with the lungs being the most common site of osteosarcoma metastasis [14,15]. The long-term survival rate of the patients receiving surgical interventions is only 20–30%. Over the past 30 years, Rosen has been a strong proponent and has advocated for high-dose systemic chemotherapy, namely neoadjuvant chemotherapy, prior to surgical intervention, which has been a milestone in the history of osteosarcoma treatment [16]. This approach has greatly improved the 5-year survival rate of patients with osteosarcoma; however, nearly half of patients die from pulmonary metastasis because cancers become resistant to chemotherapy drugs within 2–3 years of treatment [17]. Because tumors are composed of a polymorphous, heterogeneous, and multi-differentiated cell population, their sensitivity to various chemotherapeutic drugs differs depending on the individual. The action and metabolism of drugs in vivo is a complex process, and chemotherapy is time-consuming and cost-ineffective. Therefore, the efficacy of chemotherapy is unsatisfactory [18–20]. Individualized treatment strategies have been proposed. In individualized treatment, DSTs are carried out in vitro to identify and select the most effective chemotherapeutics, and to determine its effective dose. Therefore, individualized medication could improve the targeting and efficacy of chemotherapeutic agents and reduce adverse reactions and drug resistance [18].
The primary mechanism by which chemotherapeutics destroy tumor cells is by inducing apoptosis. High levels of apoptosis in cancer cells are strongly associated with chemotherapeutic sensitivity [2]. Therefore, the main purpose of DSTs is to detect apoptosis. Multiple methods, such as PI, in situ nick translation, terminal deoxynucleotidyl, acidic denaturation, and thermal denaturation assays, have been developed to detect apoptosis by monitoring changes in cell morphology and surface markers [8]. These methods identify cell death; however, they also have drawbacks. These methods involve multi-step procedures, including diversion, washing, and transfer of samples, using both time and materials [21].
MTT assays have been used as DSTs in vitro, but this method has some weaknesses [22]. For example, this assay cannot distinguish between apoptotic and necrotic cells, and therefore cannot detect the toxic effects of drugs on the body [23,24]. While tumor cells undergo apoptosis in the presence of anticancer drugs, normal cells become necrotic if the drug is toxic. MTT assays cannot differentiate between these mechanisms of cell death. Therefore, the effects of drug may primarily be toxic, poisoning normal cells. These drugs have limited clinical application. Therefore, detection of tumor cell apoptosis is more valuable than generally assessing tumor cell viability. Dual AO/EB fluorescent staining can detect basic morphological changes in apoptotic cells. In addition, it allows for the distinction between normal cells, early and late apoptotic cells, and necrotic cells. Therefore, AO/EB staining is a qualitative and quantitative method to detect apoptosis [25].
We speculate that AO penetrated normal and early apoptotic cells with intact membranes, fluorescing green when bound to DNA. EB only entered cells with damaged membranes, such as late apoptotic and dead cells, emitting orange-red fluorescence when bound to concentrated DNA fragments or apoptotic bodies [21]. Furthermore, dual AO/EB staining is able to detect mild DNA injuries [9]. Therefore, to distinguish normal, early apoptotic, late apoptotic cells, and dead cells, nuclear morphology must be assessed. Fluorescent staining using AO alone has been used in the past; however, detection of cell apoptosis using AO/EB is a relatively new approach, and few papers have reported its use [8]. In comparison to AO staining, the AO/EB method improves the detection of apoptosis and can distinguish between late apoptotic and dead cells.
Flow cytometry is a classical method to detect cell apoptosis with high sensitivity and can be used for simultaneous cell-cycle analysis [26]. However, a general laboratory may not be equipped with a flow cytometer, and they are costly to run. No significant difference was observed between the ability of flow cytometry and AO/EB staining to detect apoptosis. Therefore, fluorescent staining can feasibly be applied to evaluate apoptosis in DSTs of osteosarcomas or other malignant tumors. OA/EB is a more economical and convenient method compared with flow cytometry.
Acknowledgements
We thank the members of our department for their helpful discussion and assistance.
Footnotes
Conflict of interest
All authors certify that this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. The authors have no conflicts of interest to declare.
Source of support: Departmental sources
References
- 1.Taraphdar AK, Roy M, Bhattacharya R. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr Sci. 2001;80:1387–96. [Google Scholar]
- 2.Yamamoto M, Maehara Y, Oda S, et al. The p53 tumor suppressor gene in anticancer agent-induced apoptosis and chemosensitivity of human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 1999;43:43–49. doi: 10.1007/s002800050861. [DOI] [PubMed] [Google Scholar]
- 3.Bown N, Reid M, Malcolm A, et al. Cytogenetic abnormalities of small round cell tumours. Med Pediatr Oncol. 1994;23:124–29. doi: 10.1002/mpo.2950230210. [DOI] [PubMed] [Google Scholar]
- 4.Efferth T, Konkimalla VB, Wang YF, et al. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin Cancer Res. 2008;14:2405–12. doi: 10.1158/1078-0432.CCR-07-4525. [DOI] [PubMed] [Google Scholar]
- 5.Samson DJ, Seidenfeld J, Ziegler K, et al. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–30. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman RM. In vitro sensitivity assays in cancer: a review, analysis, and prognosis. J Clin Lab Anal. 1991;5:133–43. doi: 10.1002/jcla.1860050211. [DOI] [PubMed] [Google Scholar]
- 7.Hatok J, Babusikova E, Matakova T, et al. In vitro assays for the evaluation of drug resistance in tumor cells. Clin Exp Med. 2009;9:1–7. doi: 10.1007/s10238-008-0011-3. [DOI] [PubMed] [Google Scholar]
- 8.Lecoeur H. Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp Cell Res. 2002;277:1–14. doi: 10.1006/excr.2002.5537. [DOI] [PubMed] [Google Scholar]
- 9.Gherghi IC, Girousi ST, Voulgaropoulos A, et al. Study of interactions between DNA-ethidium bromide (EB) and DNA-acridine orange (AO), in solution, using hanging mercury drop electrode (HMDE) Talanta. 2003;61:103–12. doi: 10.1016/S0039-9140(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 10.Leite M, Quinta-Costa M, Leite PS, et al. Critical evaluation of techniques to detect and measure cell death – study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol. 1999;19:139–51. doi: 10.1155/1999/176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baskić D, Popović S, Ristić P, et al. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Wei M, Shao J. Effects of verapamil on the immediate-early gene expression of bone marrow mesenchymal stem cells stimulated by mechanical strain in vitro. Med Sci Monit Basic Res. 2013;19:68–75. doi: 10.12659/MSMBR.883790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu JX, He YQ, Wang Y, et al. Plumbagin induces the apoptosis of human tongue carcinoma cells through the mitochondria-mediated pathway. Med Sci Monit Basic Res. 2013;19:228–36. doi: 10.12659/MSMBR.884004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picci P, Ferrari S, Bacci G, et al. Treatment recommendations for osteosarcoma and adult soft tissue sarcomas. Drugs. 1994;47:82–92. doi: 10.2165/00003495-199447010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106:1154–61. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 16.Rosen G, Tan C, Sanmaneechai A, et al. The rationale for multiple drug chemotherapy in the treatment of osteogenic sarcoma. Cancer. 1975;35:936–45. doi: 10.1002/1097-0142(197503)35:3+<936::aid-cncr2820350714>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Bacci G, Picci P, Ruggieri P, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities the istituto rizzoli experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990;65:2539–53. doi: 10.1002/1097-0142(19900601)65:11<2539::aid-cncr2820651125>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Efferth T, Konkimalla VB, Wang Y-F, et al. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin Cancer Res. 2008;14:2405–12. doi: 10.1158/1078-0432.CCR-07-4525. [DOI] [PubMed] [Google Scholar]
- 19.Nishio K, Nakamura T. [Conquering drug resistance in lung cancer]. Nihon Rinsho. 2000;58(5):1041–47. [in Japanese] [PubMed] [Google Scholar]
- 20.Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999;17:1625–31. doi: 10.1200/JCO.1999.17.5.1625. [DOI] [PubMed] [Google Scholar]
- 21.Ribble D, Goldstein NB, Norris DA, et al. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent JM. The use of the MTT assay to study drug resistance in fresh tumour samples. Recent Results Cancer Res. 2003;161:13–25. doi: 10.1007/978-3-642-19022-3_2. [DOI] [PubMed] [Google Scholar]
- 23.Carmichael J, Mitchell J, DeGraff W, et al. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br J Cancer. 1988;57:540–47. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips R, Bibby M, Double J. A critical appraisal of the predictive value of in vitro chemosensitivity assays. J Natl Cancer Inst. 1990;82:1457–68. doi: 10.1093/jnci/82.18.1457. [DOI] [PubMed] [Google Scholar]
- 25.Biffl WL, Moore EE, Moore FA, et al. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma. 1996;40:575–78. doi: 10.1097/00005373-199604000-00009. discussion 578–79. [DOI] [PubMed] [Google Scholar]
- 26.Meyer M, Essack M, Kanyanda S, et al. A low-cost flow cytometric assay for the detection and quantification of apoptosis using an anionic halogenated fluorescein dye. Biotechniques. 2008;45:317–20. doi: 10.2144/000112908. [DOI] [PubMed] [Google Scholar]