Abstract
Background
Persistent air leak is one of the most common complications of lung diseases and pulmonary resections. Prolonged hospitalization, increased morbidity, and increased overall treatment costs arise from persistent air leaks. The use of endobronchial valves (EBVs) in the management of air leaks is an important alternative, especially for patients who are not candidates for surgical treatment.
Material/Methods
We retrieved the included studies by performing a systematic search in PubMed and Scopus databases. The references of the included studies were also hand-searched.
Results
We retrieved 25 case reports and 3 case series from our literature search. The most common cause of persisting air leaks was spontaneous secondary pneumothorax (12/39, 31%). The left upper lobe (13/39, 33%) and right upper lobe (14/39, 36%) were the most frequent locations of air leaks. Most air leaks treated with EBVs ceased in less than 24 h. Three recurrences of air leak were reported and 2 cases of EBV migration were described. No deaths were reported in correlation with EBVs.
Conclusions
EBVs are a minimally invasive therapeutical option that may be suitable for the treatment of persistent air leaks regardless of the initial cause, especially in high-risk patients. Nevertheless, studies with better methodological quality are essential to standardize this technique and to provide more evidence on EBV safety issues.
MeSH Keywords: Bronchial Fistula, Bronchoscopy, Pulmonary Valve
Background
Persistent air leaks are defined as those lasting more than 5 days postoperatively, without forced expiration or expulsion maneuvers [1]. Increased morbidity, prolonged hospitalizations, and increased treatment costs are associated with persistent air leaks [2]. The most common treatment of persistent air leaks is the close medical observation of the patient (watchful waiting) under pleural drainage with or without suction [1]. Frequent causes of persistent air leak include previous surgical procedures, chest trauma, and spontaneous pneumothorax [3].
Various patient-associated and treatment-associated risk factors have been correlated with the formation of bronchopleural fistulas (BPFs). Patient-related factors are patient sex, smoking, nutrition (albumin <3.5 mg/dL), and reduced forced expiratory volume (FEV1) in 1 s [3]. Previous chest procedures such as chest tubes and thoracic surgical operations, history of preoperative chemotherapy or radiation treatment, acute respiratory distress syndrome (ARDS), mechanical ventilation, immunosuppressive factors (fever, chest infections, sepsis, treatment with steroids, and anemia), and other underlying lung-related diseases, were also correlated with BPF formation [4].
The principles of conservative management of air leaks include air drainage by the use of chest tubes and treatment of infection and nutrition deficits [5]. Currently, with the evolution of endoscopic methods [6,7], the endobronchial management of air leaks has become an alternative therapy to treat persistent air leaks. Interventional bronchoscopy may be a valuable alternative in the treatment of BPFs, especially at distal tracts of the bronchial tree, and could be an ideal therapeutic option for high-risk patients. In the past various methods have been used. In 1977, Hartmann et al. reported the first successful endobronchial management of BPF using tissue glue and lead shot [8]. Since then, many reports using different techniques have appeared. These include ethanol silver nitrate, cyanoacrylate compounds, coils, lead plugs, balloons, fibrin or tissue glue, antibiotics, gel foam, and autologous blood patches [4,9,10]. All have revealed inconsistent clinical results. Watanabe et al. developed the endobronchial Watanabe spigot, effective in reducing air leaks by endoscopic bronchial occlusion [11]. The advantage of the Watanabe spigots is that are easy to insert and remove with a simple bronchoscope.
The main technical limitation in endoscopic treatment is the size of the endobronchial fistula orifice. Openings with diameter over than 8 mm are not candidates for bronchoscopic treatments [4]. One-way endobronchial valves (EBVs) were initially used in cases of emphysema [12] and may also be an alternative for the treatment of persistent air leaks. The removability and simplicity of valve positioning, as well as the ability to locate the position through simple radiography are some of the advantages of EBV placement.
The objective of this article was to present the existing clinical evidence of the endobronchial valves in the treatment of persisting air leaks, based on the currently available literature.
Material and Methods
Data sources
We systematically reviewed the included results after performing an electronic search in PubMed (11 October 2013) and Scopus (11 October 2013). The search strategy used in both PubMed and Scopus databases included the combination of the key words: endobronchial AND valve. We also did a hand-search in the bibliographies of relevant articles for additional studies.
Study selection criteria
Studies reporting data on endobronchial valves in the treatment of persisting air leaks were included in this review. Abstracts, conference papers, animal studies, editorials, and studies published in languages other than English, German, French, Italian, and Spanish were excluded from this review.
Definitions
Persistent air leak was defined based on the presence of an air leak lasting more than 5 days postoperatively in case of previous surgical treatment. In studies where a different definition was used, we adopted the definition specified by the authors of the selected papers.
Results
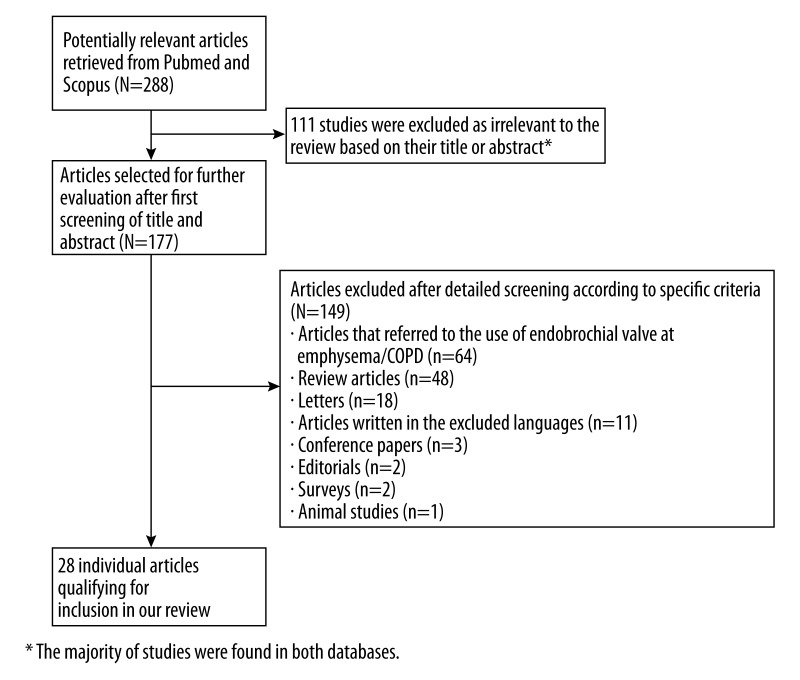
The database search performed in PubMed and Scopus revealed a total of 84 and 204 articles, respectively. The process of study selection is illustrated in detail in Figure 1. We included a total of 28 studies [2,13–39].
Figure 1.
Flow diagram of the detailed process of selection of articles for inclusion in the review.
Case reports of persisting air leaks treated with EBVs
Table 1 summarizes the available data from patients included in case reports of persisting air leaks treated with EBVs; data for 39 patients from 25 reports were available [13–24,26,28–39]. Patient ages were 18–88 years and most were males (24/39, 61%) while the main known risk factors present in patient medical histories were immunosuppressive treatment/disease (36/39, 92%), lung-related diseases (20/39, 51%), history of cancer (10/39, 26%), smoking (10/39, 26%), and cardiovascular disease (4/39, 10%). The median duration of air leak prior to EBV treatment was 12.5 days. The most common cause of persisting air leaks was spontaneous secondary pneumothorax (12/39, 31%). The left upper lobe (13/39, 33%) and the right upper lobe (14/39, 36%) were the most frequent location of air leaks. Three types of EBVs were used: Emphasys® (25/81, 31%), IBV Valve® (23/81, 28%), and Zephyr® (23/81, 28%). The median number of EBVs utilized per patient was 2 valves. Most air leaks treated with EBVs ceased in less than 24 hours (26/39, 66%).
Table 1.
Main characteristics and outcomes of the patients after the application of endobrochial valves.
| n/N (%) | |
|---|---|
| Demographics | |
| Age in years, median (range) | 60 (18–88) |
| Male sex | 24/39 (61) |
| Medical history | |
| Immunosuppresive treatment or disease | 36/39 (92) |
| Lung related diseases (COPD/emphysema) | 20/39 (51) |
| Cancer | 10/39 (26) |
| Smoking | 10/39 (26) |
| Cardiovascular disease | 4/39 (10) |
| Clinical features of air leak and EBVs characteristics | |
| Duration of air leak before EBV placement (in days), median (range) | 12.5 (7–2,190) |
| Causes of air leak | |
| Spontaneous secondary pneumothorax | 12/39 (31) |
| Postoperative pulmonary air leak | 8/39 (20) |
| Bronchopleural fistula | 8/39 (20) |
| Iatrogenic pneumothorax | 6/39 (15) |
| Alveolar-pleural/transdiaphragmatic fistula | 2/36 (5) |
| Parapneumonic pleural empyema | 2/36 (5) |
| Bronchocutaneous fistula | 1/39 (2) |
| Lung tree perforation | 1/39 (2) |
| Location of air leak | |
| Right upper | 14/39 (36) |
| Right middle | 3/39 (8) |
| Right lower | 3/39 (8) |
| Left upper | 13/39 (33) |
| Left lower | 6/39 (15) |
| Right main bronchus | 1/39 (2) |
| Type of EBV used | |
| Emphasys® | 25/81 (31) |
| IBV Valve® | 23/81 (28) |
| Zephyr® | 23/81 (28) |
| Number of EBVs (per patient), median (range) | 2 (1–5) |
| Duration of air leak after EBV placement | |
| <24 hours | 26/39 (66) |
| 24 hours ≤ × <48 hours | 3/39 (8) |
| >2 days | 10/39 (25) |
| Outcomes | |
| Removal of EBV | 17/39 (46) |
| Recurrence of air leak | 3/39 (8) |
| Migration of EBV | 2/39 (5) |
| Complications | 2/39 (5) |
| Recurrent chest infections | 1/39 (2) |
| Exacerbations of COPD | 1/39 (2) |
| Deaths* | 4/39 (10) |
None of the deaths were correlated with the utilization of EBVs.
COPD – chronic obstructive pulmonary disease, EBV – endobrochial valve.
EBVs were removed after the cessation of air leak in 17/39 patients (46%). Following the EBV treatment, complications were reported in 2 cases; 1 developed recurrent chest infections and 1 presented exacerbations of chronic obstructive pulmonary disease (COPD). Two patients died after the EBV placement but none of the deaths were related to the use of EBVs. Three recurrences of air leak were reported and 2 cases of EBV migration were described.
Case series of persisting air leaks treated with EBVs
We identified 3 case series including 60 patients with persisting air leaks (Table 2) 2,25,27]. All of the patients included in the studies were adults and most were males (29/47, 62%). The most common underlying diseases were lung-related pathologies, such as COPD, emphysema, and pneumonia. Most EBVs used were placed at the upper right and left lobes. Air leak recurrence, valve expectoration combined with moderate oxygen desaturation, valve malposition, pneumonia, methicillin-resistant Staphylococcus aureus colonization, signs of minor local granulation tissue, and an unspecified event were present in 10/60 patients (16.7%) in total. The mean duration of EBV use was 9–66 days. Removal of EBVs was performed in 24/60 patients (40%). None of the included patients died due to EBV placement.
Table 2.
Data from clinical studies regarding the use of endobronchial valves for the treatment of persisted air leak.
| First author, year [ref] | Publication type | Nr of patients, sex (M/F) | Patients’ age (in years) | Underlying disease (n/N) | Previous interventions (n/N) | Duration of air leak before EBV placement | Location of EBVs (n/N,%) | Type of EBVs | Complications related to EBVs | Days to EBVs removal (in days) | Persistence of air leak after EBV placement, (%) | Removal of EBVs n/N, (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Firlinger, 2013 [25] | Prospective study | 13 | NR | Empyema (4/13), pulmonary metastasis (1/13), pleural mesothelioma (3/13), pneumothorax (2/13), lung cancer (1/13), bronchial carcinoma (1/13), bronchiectasis (1/13) | Chest tube (4/13), lobectomy (3/13), decortication (2/13), radical pleurectomy (3/13), muscle flap/pleurodesis (1/13) | Median (range): 17 days (8–30) | LUL (2/19, 10.5), LLL (5/19, 26.4), RUL (10/19, 52.6), RLL (2/19, 10.5) | IBV® (13/19), Zephyr® (6/19) | 0/19†† | Mean (range): 9 (1–21) | 1/19, (5.2) | 11/13, (84.6) |
| Gillespie, 2011 [27] | Retrospective study | 7, (4/3) | Median (range): 58 (17–60) | Emphysema (5/7), neoplasia (3/7), pulmonary embolism (2/7), radiation fibrosis (2/7), fungal infection (1/7), empyema (4/7) | Surgical interventions (6/7), pleural procedures* (4/7) | Median (range): 4 wks (2 wks – 5 mo) | LUL (14/31, 45), LLL (10/31, 32.3), RUL (7/31, 22.6) | IBV® | Recurrence§ (1/7) | Mean (range): 37 (14–55) | Mean (range): 4.5 (0–27) | 5/7, (71.4) |
| Travaline, 2009 [2] | Retrospective study | 40, (25/15) | Mean (SD): 69 (14) | Neoplasia (12/40), COPD (12/40), pneumonia (7/40), rheumatoid arthritis (2/40), tuberculosis (1/40), trauma (1/40), aspergilloma (1/40), bronchiectasis (1/40), cor pulmonale (1/40), lung transplantation (1/40), multiple comorbidities (1/40) | Chest tube (39/40), Eloesser flap (1/40), blood patch (3/40), wedge resection (1/40), pleurodesis (1/40) | Median (IQR): 20 days (15–45) | NR** | Zephyr® | 6/40§§ | Mean (range): 66 (7–143)† | Decreased air leak: 18/40 (45), no change: 2/40 (5), no data: 1/40 (2.5) | 8/40, (20) |
Nr – number; wks – weeks; mo – months; NR – not referred; LLL – left lower lobe; LUL – left upper lobe; RUL – right upper lobe; RML – right middle lobe; RUL – right upper lobe; RLL – right lower lobe; RML – right middle lobe; IQR – interquartile range; SD – standard deviation; VATS – video-assisted thoracic surgery.
Pleurodesis or pleurectomy.
RUL (11/40), RML (3/40), RLL (3/40), LUL (11/40), LLL (5/40), RUL+RLL (1/40), RLL+RML (1/40), RUL+LUL (1/40). In 4 patients, the location was not referred.
Air leak returned after valve removal on the 15th day.
Valve expectoration, moderate oxygen desaturation, valve malposition, pneumonia, methicillin-resistant Staphylococcus aureus colonization and an unspecified event.
Only 8 patients removed the EBVs.
Signs of minor local granulation tissue in 3 patients.
Discussion
Prolonged pulmonary air leaks are present in 15% of thoracic procedures. Of the air leaks persisting at the 4th postoperative day, 83% will persist past the 7th postoperative day [1,40]. In addition, the incidence of prolonged air leaks after segmental pulmonary resections can rise up to 8%, 10% in cases of lobectomy, and lung volume reduction operations have an average incidence of 45% [3]. The treatment of persistent air leaks still remains a challenge due to the absence of a standard therapeutic approach. EBVs are an innovative treatment of prolonged pulmonary air leaks. Initially, EBVs were developed for the treatment of severe emphysema [3,41]. EBVs obstruct the bronchial airway, causing parenchymal atelectasis that follows the valve deployment while allowing distal secretions to be drained normally. The formed atelectasis possibly is a consequence of the reduction and eventually the cessation of the air leak.
The EBV system generally consists of a delivery catheter, a loader system, a guidewire, and the implantable valves [36]. The valves are composed of a self-expanding framework made of nitinol. Nitinol is a titanium/nickel-based alloy commonly used in implantable medical devices, which has biological tolerance and the capacity to maintain its preformed shape. There are currently 2 commercially available types of EBVs: the Zephyr EBV® (Pulmonx, Redwood City, CA) and the Spiration IBV® (Olympus Respiratory America, Redmond, WA). Among the first EBVs ever produced were also the Emphasys® (formerly Emphasys Medical Inc) which are not anymore commercially available. The placement of EBVs can be managed directly through the working channel of a flexible bronchoscope in segmental or subsegmental bronchi [3]. Although all commercial EBV systems are available in Europe, in the United States only the Spiration IBV® valve has been approved for humanitarian use by the FDA, in patients with the indication of prolonged or significant air leaks as a consequence of lung surgery or lung volume reduction operations [42]. Among the functional advantages of the EBVs is the ability of the valve system to be easily repositioned and even removed to maximize efficiency, and it can be easily removed in case of any valve-related complications.
The variability of EBV insertion methods is the result of the innovation of this particular technique. The larger lumen of the rigid bronchoscopes versus the narrow one of the flexible bronchoscopes offers more possibilities to identify the affected bronchus, and general anaesthesia is essential for the utilization of rigid bronchoscopes. In contrast, a flexible bronchoscope is longer and thinner than its rigid counterpart, permitting the practitioner to navigate and place the EBVs into an individual lobe or the more distal bronchi. In addition, flexible bronchoscopy can be performed under conscious sedation or through a tracheal tube in patients under mechanical ventilation. These characteristics are the apparent reasons why EBVs were implanted with flexible bronchoscopes in most of the included studies. Flexible bronchoscopy causes less discomfort for the patient than rigid bronchoscopy and the procedure can be performed easily and safely under moderate sedation. It is now the technique of choice for most bronchoscopy procedures.
BPF and alveolar pleural fistula (APF) are pathological communications between the tracheobronchial tree and the alveolar spaces with the pleural space, respectively. BPF and APF are frequent clinical problems after pulmonary resections, and associated with a significant morbidity and mortality [1]. The treatment of persistent air leaks due to BPF and APF necessitate the surgical intervention by either video-assisted thoracoscopic surgery (VATS) or thoracotomy. In our review, were retrieved 6 cases of BPF [14,22,23,29,34] and 2 cases of APF [17,30] With the exception of 1 patient, all the air leaks caused by fistulas ceased immediately after the valve placement. Additionally, Snell et al. have described the successful treatment with EBVs of a patient with bronchocutaneous fistula [35] in a patient with a 6-year history of persistent air leak due to a right upper lobectomy with right apical segmentectomy and combined thoracoplasty for Aspergillus sp. mycetoma. Nevertheless, the patient underwent several surgical attempts of closure and never presented any reduction at the air leak. Furthermore, 4 Zephyr® valves were deployed within the proper bronchial segments under flexible bronchoscopy, resulting in the complete fistula closure within 4 months. The gold standard for the treatment of BPF/APF and secondary pneumothorax is surgical management. Nevertheless, the treatment with EBVs should be considered as a first-line approach in high-risk patients or in cases where the surgical option has failed.
Collateral ventilation is one of the main drawbacks of the EBV technique in cases of intended lung volume reduction [43]. The presence of “passages” in the pulmonary parenchyma that bypass the normal airways is assumed to influence the effectiveness of bronchoscopic treatment of emphysema. None of the included studies stated that collateral ventilation was the cause of air leak recurrence [29,36]. Nevertheless, the identification of the affected bronchus seems to be essential to avoid episodes of recurrence. Among the selected studies, the most common method used for the recognition of the source of the air leak was use of a Fogarty catheter. The selection of the correct position for the location of EBVs is made after the insertion of an inflated Fogarty catheter at the proper bronchus. The immediate cessation of air flow at the chest drain is the principle indication for accurate valve insertion.
Another concern found in our review of the available literature is the safety of EBVs. According to the literature, none of the deaths registered after EBV implantation were correlated with the EBVs [16,18,32,36]. The information provided on the causes of deaths were not sufficient to completely exonerate the use of valves from any role in the deaths. Comparative studies are necessary to clarify all safety issues regarding the use of EBVs.
The utilization of EBVs has a substantial impact on total hospitalization costs. According to the size of the valves, the mean cost of each valve is 4500 Euros, ranging from 3500 to 5500 Euros per valve. To the EBV costs should also be added the costs of the catheter and the loader of the valve system. Wan et al. reported that the total costs per patient could be up to 7800 Euros excluding tax assessed value (TAV) or 8268 Euros including TAV [31,44]. Alternatively, Santini et al. suggests that the use of EBVs reduces the overall hospital cost by reducing the stay in the intensive care unit and consequently the duration of hospitalization [31].
Various limitations should be considered in the interpretation of the findings of this study. The small number of included studies and the limited number of patients reveals that the use EBVs for treatment of persistent air leaks is a technique that is still evolving. The limited available literature does not permit any comparison between the use of EBVs and alternative bronchoscopic interventions such as Watanabe spigots or fibrin glue. Lastly, although our literature search was extensive, it may have been selective by excluding abstracts, animal studies, conference papers, and editorials.
Conclusions
Prolonged and persistent air leaks are important clinical problems. The use of EBVs is proposed as an innovative and alternative treatment option. Studies with better methodological quality are important to standardize this technique, to evaluate the early and late complication rates between the different patient populations, to compare EBVs with other endoscopic methods, and to provide more evidence on safety. Although the high cost seems to be the main drawback, EBVs are a non-surgical, minimally invasive therapeutical option that may be appropriate for the treatment of persistent air leaks regardless of the initial cause, especially in high-risk patients.
Footnotes
Source of support: Self financing
References
- 1.Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66:1726–31. doi: 10.1016/s0003-4975(98)00958-8. [DOI] [PubMed] [Google Scholar]
- 2.Travaline JM, McKenna RJ, Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009;136:355–60. doi: 10.1378/chest.08-2389. [DOI] [PubMed] [Google Scholar]
- 3.Wood DE, Cerfolio RJ, Gonzalez X, Springmeyer SC. Bronchoscopic management of prolonged air leak. Clin Chest Med. 2010;31:127–33. doi: 10.1016/j.ccm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955–65. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 5.Shabalovskaya S, Anderegg J, Van Humbeeck J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008;4:447–67. doi: 10.1016/j.actbio.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Lozo Vukovac E, Lozo M, et al. Bronchoalveolar pH and inflammatory biomarkers in newly diagnosed IPF and GERD patients: a case-control study. Med Sci Monit. 2015;21:255–61. doi: 10.12659/MSM.889800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyman BM, Chung MM, Lark AL, et al. Endobronchial metastasis from primary anorectal melanoma. Am J Case Rep. 2013;14:253–57. doi: 10.12659/AJCR.889291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann W, Rausch V. New therapeutic application of the fiberoptic bronchoscope. Chest. 1977;71:237. doi: 10.1378/chest.71.2.237a. [DOI] [PubMed] [Google Scholar]
- 9.Sasada S, Tamura K, Chang YS, et al. Clinical evaluation of endoscopic bronchial occlusion with silicone spigots for the management of persistent pulmonary air leaks. Intern Med. 2011;50:1169–73. doi: 10.2169/internalmedicine.50.5016. [DOI] [PubMed] [Google Scholar]
- 10.Haga T, Kurihara M, Kataoka H, et al. Spontaneous pneumothorax with persistent air leakage and invasive procedures. Intern Med. 2013;52:2189–92. doi: 10.2169/internalmedicine.52.0732. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe Y, Matsuo K, Tamaoki A, et al. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchol. 2003;10:264–67. [Google Scholar]
- 12.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–44. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. 2010;188:253–57. doi: 10.1007/s00408-009-9204-0. [DOI] [PubMed] [Google Scholar]
- 14.Alexander ES, Healey TT, Martin DW, Dupuy DE. Use of endobronchial valves for the treatment of bronchopleural fistulas after thermal ablation of lung neoplasms. J Vasc Interv Radiol. 2012;23:1236–40. doi: 10.1016/j.jvir.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosino N, Ribechini A, Allidi F, Gabbrielli L. Use of endobronchial valves in persistent air leaks: a case report and review of the literature. Expert Rev Respir Med. 2013;7:85–90. doi: 10.1586/ers.12.76. [DOI] [PubMed] [Google Scholar]
- 16.Anile M, Venuta F, De Giacomo T, et al. Treatment of persistent air leakage with endobronchial one-way valves. J Thorac Cardiovasc Surg. 2006;132:711–12. doi: 10.1016/j.jtcvs.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Brichon PY, Poquet C, Arvieux C, Pison C. Successful treatment of a life-threatening air leakage, complicating severe abdominal sepsis, with a one-way endobronchial valve. Interact Cardiovasc Thorac Surg. 2012;15:779–80. doi: 10.1093/icvts/ivs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conforti S, Torre M, Fieschi S, et al. Successful treatment of persistent postoperative air leaks following the placement of an endobronchial one-way valve. Monaldi Arch Chest Dis. 2010;73:88–91. doi: 10.4081/monaldi.2010.304. [DOI] [PubMed] [Google Scholar]
- 19.De Giacomo T, Venuta F, Diso D, Coloni GF. Successful treatment with one-way endobronchial valve of large air-leakage complicating narrow-bore enteral feeding tube malposition. Eur J Cardiothorac Surg. 2006;30:811–12. doi: 10.1016/j.ejcts.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Dooms CA, De Leyn PR, Yserbyt J, et al. Endobronchial valves for persistent postoperative pulmonary air leak: accurate monitoring and functional implications. Respiration. 2012;84:329–33. doi: 10.1159/000339411. [DOI] [PubMed] [Google Scholar]
- 21.El-Sameed Y, Waness A, Al Shamsi I, Mehta AC. Endobronchial valves in the management of broncho-pleural and alveolo-pleural fistulae. Lung. 2012;190:347–51. doi: 10.1007/s00408-011-9369-1. [DOI] [PubMed] [Google Scholar]
- 22.Feller-Kopman D, Bechara R, Garland R, et al. Use of a removable endobronchial valve for the treatment of bronchopleural fistula. Chest. 2006;130:273–75. doi: 10.1378/chest.130.1.273. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson JS, Sprenger K, Van Natta T. Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest. 2006;129:479–81. doi: 10.1378/chest.129.2.479. [DOI] [PubMed] [Google Scholar]
- 24.Fielding DI, Bashirzadeh F, Deller D, et al. Life-saving closure of a pulmonary cavity by endobronchial valve placement. Am J Respir Crit Care Med. 2013;187:1145–46. doi: 10.1164/rccm.201210-1833LE. [DOI] [PubMed] [Google Scholar]
- 25.Firlinger I, Stubenberger E, Muller MR, et al. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann Thorac Surg. 2013;95:1243–49. doi: 10.1016/j.athoracsur.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Fischer W, Feller-Kopman D, Shah A, et al. Endobronchial valve therapy for pneumothorax as a bridge to lung transplantation. J Heart Lung Transplant. 2012;31:334–36. doi: 10.1016/j.healun.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg. 2011;91:270–73. doi: 10.1016/j.athoracsur.2010.07.093. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins M, Vaughan P, Place D, Kornaszewska M. Endobronchial valve migration. Eur J Cardiothorac Surg. 2011;40:1258–60. doi: 10.1016/j.ejcts.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell KM, Boley TM, Hazelrigg SR. Endobronchial valves for treatment of bronchopleural fistula. Ann Thorac Surg. 2006;81:1129–31. doi: 10.1016/j.athoracsur.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 30.Rosell A, Lopez-Lisbona R, Cubero N, et al. Endoscopic treatment of persistent alveolar-pleural air leaks with a unidirectional endobronchial valve. Arch Bronconeumol. 2011;47:371–73. doi: 10.1016/j.arbres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Santini M, Fiorelli A, Vicidomini G, et al. Iatrogenic air leak successfully treated by bronchoscopic placement of unidirectional endobronchial valves. Ann Thorac Surg. 2010;89:2007–10. doi: 10.1016/j.athoracsur.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Schiavon M, Marulli G, Zuin A, et al. Endobronchial valve for secondary pneumothorax in a severe emphysema patient. Thorac Cardiovasc Surg. 2011;59:509–10. doi: 10.1055/s-0030-1270758. [DOI] [PubMed] [Google Scholar]
- 33.Schweigert M, Kraus D, Ficker JH, Stein HJ. Closure of persisting air leaks in patients with severe pleural empyema – use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg. 2011;39:401–3. doi: 10.1016/j.ejcts.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Seyfried U, Firlinger I, Reiter M, et al. [A leak in the lung: endobronchial one-way valve placement as treatment for a persistent bronchopleural fistula]. Pneumologie. 2012;66:188–91. doi: 10.1055/s-0031-1291615. [in German] [DOI] [PubMed] [Google Scholar]
- 35.Snell GI, Holsworth L, Fowler S, et al. Occlusion of a broncho-cutaneous fistula with endobronchial one-way valves. Ann Thorac Surg. 2005;80:1930–32. doi: 10.1016/j.athoracsur.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Toma TP, Kon OM, Oldfield W, et al. Reduction of persistent air leak with endoscopic valve implants. Thorax. 2007;62:830–33. doi: 10.1136/thx.2005.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatappa N, Fadul R, Raymond D, et al. Endobronchial valves for treatment of bronchopleural fistula in granulomatous polyangitis: a longitudinal case report. J Bronchology Interv Pulmonol. 2013;20:186–88. doi: 10.1097/LBR.0b013e3182917513. [DOI] [PubMed] [Google Scholar]
- 38.Yu WC, Yeung YC, Chang Y, et al. Use of endobronchial one-way valves reveals questions on etiology of spontaneous pneumothorax: report of three cases. J Cardiothorac Surg. 2009;4:63. doi: 10.1186/1749-8090-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudbjartsson T, Helgadottir S, Ek L. One-way endobronchial valve for bronchopleural fistula after necrotizing pneumonia. Asian Cardiovasc Thorac Ann. 2013;21:498. doi: 10.1177/0218492313480052. [DOI] [PubMed] [Google Scholar]
- 40.Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113:1507–10. doi: 10.1378/chest.113.6.1507. [DOI] [PubMed] [Google Scholar]
- 41.Sterman DH, Mehta AC, Wood DE, et al. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration. 2010;79:222–33. doi: 10.1159/000259318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.U.S. Food and Drug Administration. News and events. FDA approves lung valve to control some air leaks after surgery. Oct 24, 2008. [accessed on 11th October, 2013]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116970.htm.
- 43.Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax. 2006;61:371–73. doi: 10.1136/thx.2006.060509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006;129:518–26. doi: 10.1378/chest.129.3.518. [DOI] [PubMed] [Google Scholar]