Abstract
Squalene and sterol carrier protein of liver plays a general role as a vehicle for cholesterol and its water-insoluble precursors; the carrier protein is essential for enzymic cholesterol synthesis. Liver microsomal enzymes contain a small amount of endogenous carrier protein, which is readily removed by washing or purification of the enzyme. Enzymic conversion to products of a cholesterol precursor·carrier protein complex is markedly faster than that for initially unbound sterol. The protomer form of the carrier protein has a molecular weight of 16,000; during sodium dodecyl sulfate gel electrophoresis one band is observed. Phospholipid facilitates the aggregation of the protomer to the oligomer form (>150,000 daltons; purified 720-fold) accompanied by the binding of cholesterol precursors to the oligomer. The carrier protein binds fatty acids as well as cholesterol precursors, suggesting that it may more generally be a lipid carrier protein with “squalene and sterol carrier protein” describing the functional aspects of the lipid carrier in cholesterol biosynthesis. Studies with several steroids and related compounds revealed that the binding sites of lipid carrier protein must contain highly specific hydrophobic and polar regions.
Keywords: liver, microsomal enzymes, phospholipids, fatty acids
Full text
PDF
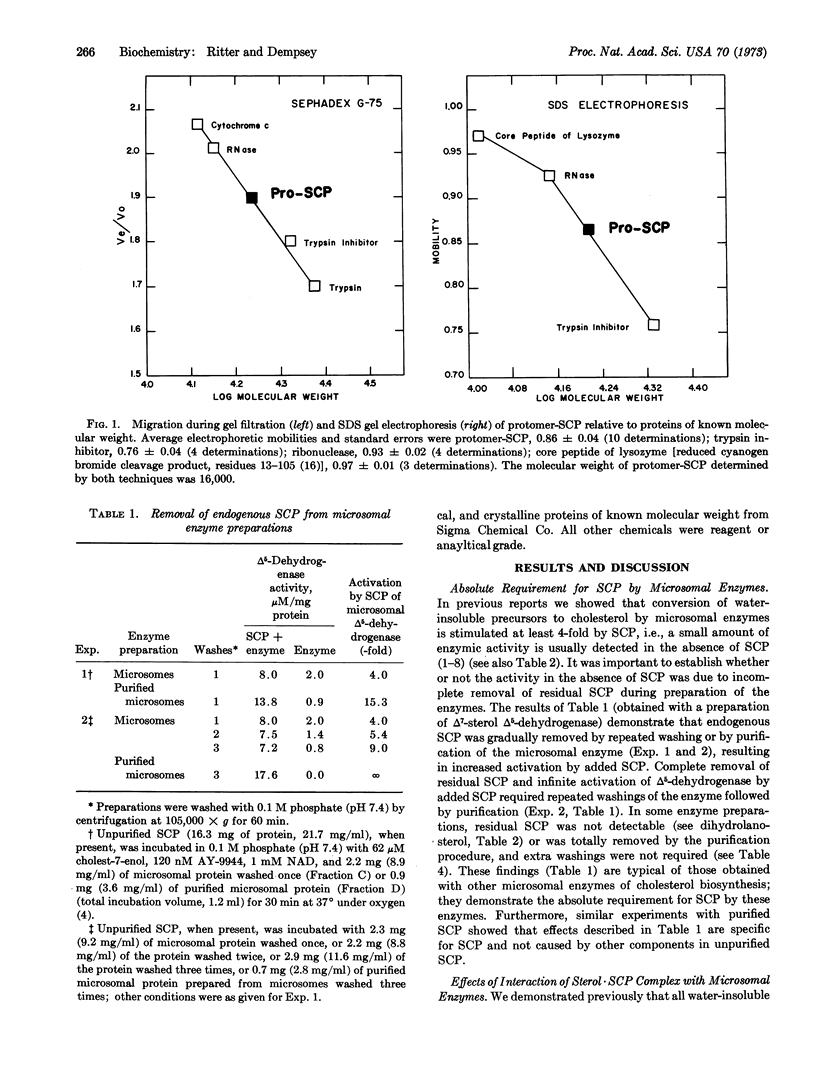



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Bonavida B., Miller A., Sercarz E. E. Structural basis for immune recognition of lysozymes. I. Effect of cyanogen bromide on hen egg-white lysozyme. Biochemistry. 1969 Mar;8(3):968–979. doi: 10.1021/bi00831a030. [DOI] [PubMed] [Google Scholar]
- DEMPSEY M. E., SEATON J. D., SCHROEPFER G. J., Jr, TROCKMAN R. W. THE INTERMEDIARY ROLE OF DELTA-5,7-CHOLESTADIEN-3-BETA-OL IN CHOLESTEROL BIOSYNTHESIS. J Biol Chem. 1964 May;239:1381–1387. [PubMed] [Google Scholar]
- Dempsey M. E. Inhibition of lipid biosynthesis. Ann N Y Acad Sci. 1968 Mar 26;148(3):631–646. doi: 10.1111/j.1749-6632.1968.tb27737.x. [DOI] [PubMed] [Google Scholar]
- Dempsey M. E. Pathways of enzymic synthesis and conversion to cholesterol of delta-5,7,24-cholestatrien-3 beta-ol and other naturally occurring sterols. J Biol Chem. 1965 Nov;240(11):4176–4188. [PubMed] [Google Scholar]
- FRANTZ I. D., Jr CHROMATOGRAPHY OF UNESTERIFIED STEROLS ON SILICIC ACID-SUPER-CEL. J Lipid Res. 1963 Apr;4:176–178. [PubMed] [Google Scholar]
- KENDREW J. C. Myoglobin and the structure of proteins. Science. 1963 Mar 29;139(3561):1259–1266. doi: 10.1126/science.139.3561.1259. [DOI] [PubMed] [Google Scholar]
- Kan K. W., Ritter M. C., Ungar F., Dempsey M. E. The role of a carrier protein in cholesterol and steroid hormone synthesis by adrenal enzymes, 1,2. Biochem Biophys Res Commun. 1972 Jul 25;48(2):423–429. doi: 10.1016/s0006-291x(72)80068-8. [DOI] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartha G., Bello J., Harker D. Tertiary structure of ribonuclease. Nature. 1967 Mar 4;213(5079):862–865. doi: 10.1038/213862a0. [DOI] [PubMed] [Google Scholar]
- Rilling H. C. The effect of sterol carrier protein on squalene synthesis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):470–475. doi: 10.1016/s0006-291x(72)80162-1. [DOI] [PubMed] [Google Scholar]
- Ritter M. C., Dempsey M. E. Purification and characterization of a naturally occurring activator of cholesterol biosynthesis from delta 5,7-cholestadienol and other precursors. Biochem Biophys Res Commun. 1970 Mar 12;38(5):921–929. doi: 10.1016/0006-291x(70)90809-0. [DOI] [PubMed] [Google Scholar]
- Scallen T. J., Schuster M. W., Dhar A. K. Evidence for a noncatalytic carrier protein in cholesterol biosynthesis. J Biol Chem. 1971 Jan 10;246(1):224–230. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Witiak D. T., Parker R. A., Brann D. R., Dempsey M. E., Ritter M. C., Connor W. E., Brahmankar D. M. Biological evaluation in vivo and in vitro of selected 5-alpha-cholestane-3-beta, 5-alpha, 6-beta-triol analogs as hypocholesterolemic agents. J Med Chem. 1971 Mar;14(3):216–222. doi: 10.1021/jm00285a010. [DOI] [PubMed] [Google Scholar]




