Abstract
Introduction
Tiotropium is prescribed for the treatment of chronic obstructive pulmonary disease (COPD) and delivered via HandiHaler® (18 μg once daily) or Respimat® Soft Mist™ inhaler (5 μg once daily). The recent TIOtropium Safety and Performance In Respimat® (TIOSPIR™) study demonstrated that both exhibit similar safety profiles. This analysis provides an updated comprehensive safety evaluation of tiotropium® using data from placebo-controlled HandiHaler® and Respimat® trials.
Methods
Pooled analysis of adverse event (AE) data from tiotropium HandiHaler® 18 μg and Respimat® 5 μg randomized, double-blind, parallel-group, placebo-controlled, clinical trials in patients with COPD (treatment duration ≥4 weeks). Incidence rates, rate ratios (RRs), and 95% confidence intervals (CIs) were determined for HandiHaler® and Respimat® trials, both together and separately.
Results
In the 28 HandiHaler® and 7 Respimat® trials included in this analysis, 11,626 patients were treated with placebo and 12,929 with tiotropium, totaling 14,909 (12,469 with HandiHaler®; 2,440 with Respimat®) patient-years of tiotropium exposure. Mean age was 65 years, and mean prebronchodilator forced expiratory volume in 1 second (FEV1) was 1.16 L (41% predicted). The risk (RR [95% CI]) of AEs (0.90 [0.87, 0.93]) and of serious AEs (SAEs) (0.94 [0.89, 0.99]) was significantly lower in the tiotropium than in the placebo group (HandiHaler® and Respimat® pooled results), and there was a numerically lower risk of fatal AEs (FAEs) (0.90 [0.79, 1.01]). The risk of cardiac AEs (0.93 [0.85, 1.02]) was numerically lower in the tiotropium group. Incidences of typical anticholinergic AEs, but not SAEs, were higher with tiotropium. Analyzed separately by inhaler, the risks of AE and SAE in the tiotropium groups remained lower than in placebo and similarly for FAEs.
Conclusion
This analysis indicates that tiotropium is associated with lower rates of AEs, SAEs, and similar rates of FAEs than placebo when delivered via HandiHaler® or Respimat® (overall and separately) in patients with COPD.
Keywords: tiotropium, HandiHaler®, Respimat®
Video abstract
Introduction
Chronic obstructive pulmonary disease (COPD) remains the fourth leading cause of death worldwide, although it is both preventable and treatable. It is a major cause of morbidity and mortality, and its economic and social burden is projected to increase in the coming decades owing to increased risk factors and aging of the population.1 Although characterized by the expiratory airflow (measured by forced expiratory volume in 1 second [FEV1]), COPD is associated with an increased incidence of comorbidities such as cardiovascular (CV) disease, musculoskeletal impairment, and diabetes mellitus, which can affect outcomes and may result in unanticipated adverse events (AEs). In addition, patients are often prescribed a number of medications for the management of the concomitant diseases, as well as their COPD, and therefore it is important to evaluate the long-term safety and efficacy of respiratory treatments.
Tiotropium bromide (SPIRIVA®, Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim am Rhein, Germany) is an inhaled, once-daily, long-acting anticholinergic bronchodilator indicated for maintenance therapy in patients with COPD,1 and has been shown to improve lung function, health-related quality of life (HRQoL), dyspnea, and exercise tolerance.2–6 Tiotropium has also been shown to reduce the number and risk of exacerbations (including exacerbations leading to hospitalization), and to delay the time to first exacerbation.5,7–9
Tiotropium has been available since 2002 as a single-dose dry-powder formulation delivered via the HandiHaler® (Boehringer Ingelheim Pharma GmbH & Co KG) device (18 μg),10 and since 2007 as an aqueous solution delivered via the multidose Respimat® Soft Mist™ inhaler (SMI) (Boehringer Ingelheim Pharma GmbH & Co KG) (5 μg once daily).11
Tiotropium HandiHaler® and Respimat® have similar efficacy, safety, and pharmacokinetic profiles,5,12–15 and are well established in most countries, with tiotropium HandiHaler® being the most prescribed COPD maintenance treatment worldwide (with more than 31 million patient-years of use).16
Results from the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) trial, which permitted the concomitant use of long-acting β2 agonists (LABAs) and theophylline, demonstrated fewer fatal AEs (FAEs) with tiotropium HandiHaler® versus placebo and no CV safety issues.5,17 In contrast, a post hoc pooled analysis of 6,096 patients in three 1-year trials and one 6-month trial using patient-level data and including vital status data of early discontinued patients showed numerically more deaths with tiotropium Respimat® 5 μg versus placebo, concentrated in patients with known cardiac rhythm disorders at study baseline.11,18
Subsequent meta-analyses based on aggregated data and reviews of the same Respimat® trial data have suggested there might be a significantly increased risk of death with tiotropium Respimat® versus placebo19–22; however, other meta-analyses, based on individual participant data, did not confirm this relationship between tiotropium HandiHaler®23–28 or Respimat®24 and CV or FAEs versus placebo in patients with COPD. Findings from the initial pooled safety analysis of tiotropium Respimat®11,18 drove the initiation of the large TIOtropium Safety and Performance In Respimat® (TIOSPIR™) trial (n=17,135), in which patients were treated over an average period of 2.3 years. The primary endpoint of TIOSPIR™ was noninferiority in all-cause mortality of Respimat® versus HandiHaler®. The TIOSPIR™ trial demonstrated similar safety (including fatal and CV events) and efficacy profiles for tiotropium Respimat® 2.5 and 5 μg, and HandiHaler® 18 μg, including patients with cardiac arrhythmias at baseline.29
The purpose of this report is to describe the findings of all placebo-controlled tiotropium HandiHaler® and tiotropium Respimat® trials available to date, as well as to assist healthcare professionals with their decisions regarding prescribing tiotropium delivered by either HandiHaler® or Respimat® SMI. The safety analysis was determined for HandiHaler® and Respimat® trials together as well as separately.
Methods
Study population
This pooled safety analysis followed a similar methodology to those described by Kesten et al26 and Celli et al24 and describes the risk of AEs by calculating a rate ratio (RR). Data from 35 Phase III and IV tiotropium clinical trials completed as of July 2012 (listed in Table 1) were included. Of these, 28 trials used tiotropium bromide dry powder (delivered via HandiHaler® 18 μg once daily), and 7 trials used tiotropium bromide solution (delivered via Respimat® 5 μg once daily). All placebo-controlled, double-blind, and parallel-group COPD trials of ≥4 weeks’ duration were included in the analysis.
Table 1.
Clinical trials included in the pooled analysis
| NCT trial number and/or publication | Boehringer Ingelheim trial number | Treatment duration (weeks) | Placebo (number of patients treated) | Tiotropium (number of patients treated) |
|---|---|---|---|---|
| HandiHaler® | 8,343 | 9,647 | ||
| Casaburi et al3,a | 205.114/117 | 49 | 191 | 279 |
| Casaburi et al3,a | 205.115/128 | 49 | 180 | 271 |
| Calverley et al43,a | 205.123 | 6 | 40 | 81 |
| McNicholas et al44,a | 205.124 | 4 | 30 | 65 |
| Brusasco et al2,a and Donohue et al45,a | 205.130 | 24 | 201 | 209 |
| O’Donnell46,a | 205.131 | 6 | 100 | 98 |
| Brusasco et al2,a | 205.137 | 24 | 199 | 193 |
| NCT002740147a | 205.214 | 48 | 510 | 500 |
| Verkindre et al47,a | 205.215 | 12 | 54 | 46 |
| Celli et al48,a | 205.218 | 4 | 41 | 40 |
| NCT0027450849,a | 205.223 | 6 | 130 | 131 |
| NCT0027452150,a | 205.230 | 25 | 53 | 55 |
| NCT001443395,a | 205.235 | 206 | 3,006 | 2,986 |
| NCT0015723551,a | 205.247 | 25 | 117 | 117 |
| NCT0027405352,a | 205.256 | 38 | 288 | 266 |
| NCT0027457353,a | 205.257 | 12 | 403 | 1,236 |
| NCT0027726454,a | 205.259 | 48 | 305 | 608 |
| NCT002745479,a | 205.266 | 26 | 915 | 914 |
| NCT0014432655,a | 205.269 | 12 | 127 | 123 |
| NCT0040523655,a | 205.270 | 52 | 73 | 69 |
| NCT0027407956,a | 205.276 | 12 | 195 | 200 |
| NCT0014419657,a | 205.281 | 12 | 117 | 107 |
| NCT0023940858,a | 205.282 | 12 | 164 | 147 |
| NCT0023946059,a | 205.284 | 12 | 96 | 100 |
| NCT0010682160,a | 205.294 | 8 | 86 | 80 |
| NCT0015298461,a | 205.301 | 12 | 244 | 228 |
| NCT0052399162 | 205.365 | 24 | 219 | 238 |
| NCT0052551263 | 205.368 | 96 | 259 | 260 |
| Respimat® | 3,283 | 3,282 | ||
| NCT0023947335 | 205.251 | 12 | 91 | 88 |
| NCT0024043535 | 205.252 | 12 | 90 | 92 |
| NCT0016884436 | 205.254 | 48 | 319 | 332 |
| NCT0016883136 | 205.255 | 48 | 334 | 338 |
| NCT0038708812 | 205.372 | 48 | 1,965 | 1,952 |
| NCT0012243464 | 1,205.4 | 4 | 55 | 53 |
| NCT0052899665 | 1,205.14 | 24 | 429 | 427 |
| Total | 11,626 | 12,929 |
Note:
Studies included in Kesten et al26 meta-analysis.
Abbreviations: BI, Boehringer Ingelheim; NCT, National Clinical Trials (clinicaltrials.gov) identifier number.
The trials used similar inclusion and exclusion criteria. Patients who had a diagnosis of COPD with FEV1≤70% of forced vital capacity, who were aged ≥40 years and had ≥10 pack-years of smoking history were eligible for inclusion. Exclusion criteria included a diagnosis of asthma, symptomatic prostatic hypertrophy or bladder neck obstruction, narrow-angle glaucoma, and known hypersensitivity to trial medication or components. Patients with significant disease other than COPD that could significantly confound the trial results or preclude trial completion were also excluded. Other exclusion criteria in earlier trial protocols were heart failure resulting in hospitalization in the previous 3 years, cardiac arrhythmia requiring drug treatment, or myocardial infarction (MI) within the past year. Other than these specific criteria, heart failure and ischemic heart disease were not excluded. More recent trials used less stringent exclusion criteria, such as life-threatening cardiac arrhythmia or arrhythmia requiring a change in medication within the last year, heart failure resulting in hospitalization in the past year, and/or MI within the preceding 6 months. In study 205.235 (UPLIFT®), moderate to severe renally impaired patients were excluded, while in all other studies, only severe renally impaired patients were excluded. Written informed consent was obtained from all patients, and all protocols were approved by an ethics committee.
All trials permitted the concomitant use of theophyllines, inhaled corticosteroids (ICS), modest daily doses of oral corticosteroids (provided the dosing was stable), and short-acting β2 agonists. Nine out of 35 trials, including the long-term safety studies 205.235 (UPLIFT®), 205.266, and 205.372, also permitted the use of LABAs as prescribed.
Adverse event reporting
AEs occurring during the period at risk (defined as the period during which the patient received the study drug and up to 30 days thereafter) were reported by the investigator. Definitions of AEs and serious AEs (SAEs) followed the International Conference on Harmonisation guidelines.30 The cause of death was adjudicated by a Clinical Endpoints Committee in studies 205.235 and 205.372 only. Therefore, the investigator-reported preferred terms (PTs) were used for the pooled analysis rather than the adjudicated terms.
Categorization of AEs
All AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0. The dictionary provides individual PTs, and assigns the PTs to so-called system organ classes (SOCs). In some cases, the PTs are assigned to more than one SOC, in which case the “primary” SOC that was most relevant to the AE has been selected. To capture clinical endpoints of interest more comprehensively, and to improve the precision of rate estimates, PTs from different SOCs were combined and referred to as pharmacovigilance (PV) endpoints. Where feasible and appropriate, in order to standardize and facilitate comparability, standardized MedDRA queries (SMQs) have been used, rather than PV endpoints. A similar approach has been used and is described in previous publications.24,26
A composite endpoint of major adverse CV events (MACE) was included in the analysis. The composite endpoint represented FAEs in the SOC cardiac disorders and SOC vascular disorders combined with MI (fatal and nonfatal), stroke (fatal and nonfatal), and the sudden death, sudden cardiac death, and cardiac death PTs. MACE with fatal outcomes are referred to as fatal MACE. The clinical endpoint MI was defined by the sub-SMQ MI (broad), while for the clinical endpoint of “stroke,” a PV endpoint was used in the analysis.
An individual patient may be represented in several PTs but was represented only once when the data were displayed according to a pooled term, ie, SOC, PV endpoint/SMQ, or MACE.
Statistical analysis
In order to adjust for treatment durations in the different clinical trials, incidence rates (IRs) were computed as follows: the number of patients experiencing an event/patient-years at risk. Time at risk was the time from start of treatment to onset of a predetermined event. For patients who did not experience a specific event, the shortest of either the time to death or the time to end of treatment +30 days was used (on-treatment analysis).
To measure the strength of the effect, incidence RRs of tiotropium versus placebo were calculated on the basis of a Cochran–Mantel–Haenszel test stratified by trial. To indicate the stability or precision of the effect estimate, the width of the 95% confidence intervals (CI) was utilized. An RR>1 indicates an increased risk with tiotropium, and an RR<1 indicates a decreased risk with tiotropium.
Results
Study population
Of the 28 HandiHaler® and 7 Respimat® trials included in this analysis (Table 1), 11,626 patients were treated with placebo (8,343 with HandiHaler® and 3,283 with Respimat®) and 12,929 with tiotropium (9,647 with HandiHaler® and 3,282 with Respimat®), totaling 14,909 (12,469 with HandiHaler®; 2,440 with Respimat®) patient-years of tiotropium exposure.
Baseline demographics were similar between treatment groups (Table 2). The cohort was predominantly male (75.4%) with a mean age of 65 years. The prebronchodilator mean FEV1 was 1.16 L (41% predicted). Baseline concomitant medication use was also similar between treatment groups, with 84.0% of patients receiving respiratory medication of any type, and 48.4% receiving CV medication of any type; 40.7% of patients were receiving LABAs and 54.3% ICS. More patients enrolled into the Respimat® trials were receiving long-acting inhaled anticholinergics at baseline (9.7%) than those enrolled into the HandiHaler® trials (1.7%), probably because these were not available or not commonly used at the time when most HandiHaler® trials were conducted.
Table 2.
Patient baseline characteristics
| Characteristics | HandiHaler®
|
Respimat®
|
Total
|
|||
|---|---|---|---|---|---|---|
| Placebo n=8,343 |
Tiotropium n=9,647 |
Placebo n=3,283 |
Tiotropium n=3,282 |
Placebo n=11,626 |
Tiotropium n=12,929 |
|
| Age, mean (SD), years | 64.63 (8.90) | 64.38 (8.76) | 64.73 (8.85) | 64.64 (8.94) | 64.66 (8.89) | 64.45 (8.80) |
| Male, n (%) | 6,327 (75.8) | 7,315 (75.8) | 2,429 (74.0) | 2,451 (74.7) | 8,756 (75.3) | 9,766 (75.5) |
| Current smoker, n (%) | 2,809 (33.7) | 3,329 (34.5) | 1,238 (37.7) | 1,233 (37.6) | 4,047 (34.8) | 4,562 (35.3) |
| Baseline spirometry, mean prebronchodilator (SD) | ||||||
| FEV1, L | 1.17 (0.46) | 1.18 (0.47) | 1.11 (0.41) | 1.11 (0.41) | 1.15 (0.45) | 1.16 (0.46) |
| FEV1, % predicted | 41.22 (14.20) | 41.66 (14.43) | 40.30 (12.45) | 40.11 (12.32) | 40.96 (13.73) | 41.27 (13.94) |
| FVC, L | 2.51 (0.82) | 2.49 (0.82) | 2.45 (0.79) | 2.45 (0.78) | 2.49 (0.81) | 2.48 (0.81) |
| FEV1/FVC | 0.47 (0.12) | 0.48 (0.13) | 0.46 (0.11) | 0.46 (0.11) | 0.47 (0.12) | 0.48 (0.12) |
| Baseline concomitant medicationa, n (%) | ||||||
| Any respiratory medication | 7,057 (84.6) | 8,021 (83.1) | 2,767 (84.3) | 2,784 (84.8) | 9,824 (84.5) | 10,805 (83.6) |
| SAMA | 3,295 (39.5) | 3,709 (38.4) | 982 (29.9) | 1,040 (31.7) | 4,277 (36.8) | 4,749 (36.7) |
| LAMA | 150 (1.8) | 150 (1.6) | 325 (9.9) | 313 (9.5) | 475 (4.1) | 463 (3.6) |
| β2 agonists | 6,199 (74.3) | 6,789 (70.4) | 2,440 (74.3) | 2,469 (75.2) | 8,639 (74.3) | 9,258 (71.6) |
| Short acting | 5,044 (60.5) | 5,630 (58.4) | 1,816 (55.3) | 1,815 (55.3) | 6,860 (59.0) | 7,445 (57.6) |
| Long acting | 3,340 (40.0) | 3,520 (36.5) | 1,538 (46.8) | 1,597 (48.7) | 4,878 (42.0) | 5,117 (39.6) |
| Steroids | 4,731 (56.7) | 5,379 (55.8) | 1,844 (56.2) | 1,821 (55.5) | 6,575 (56.6) | 7,200 (55.7) |
| ICS | 4,564 (54.7) | 5,184 (53.7) | 1,807 (55.0) | 1,785 (54.4) | 6,371 (54.8) | 6,969 (53.9) |
| OCS | 547 (6.6) | 635 (6.6) | 100 (3.0) | 100 (3.0) | 647 (5.6) | 735 (5.7) |
| ICS+LABA | 2,687 (32.2) | 2,904 (30.1) | 1,358 (41.4) | 1,395 (42.5) | 4,045 (34.8) | 4,299 (33.3) |
| Xanthines | 1,705 (20.4) | 2,103 (21.8) | 638 (19.4) | 667 (20.3) | 2,343 (20.2) | 2,770 (21.4) |
| Oxygen | 399 (4.8) | 414 (4.3) | 63 (1.9) | 54 (1.6) | 462 (4.0) | 468 (3.6) |
| Mucolytics | 411 (4.9) | 473 (4.9) | 228 (6.9) | 237 (7.2) | 639 (5.5) | 710 (5.5) |
| Other respiratory medication | 178 (2.1) | 196 (2.0) | 35 (1.1) | 46 (1.4) | 213 (1.8) | 242 (1.9) |
| Any CV medication | 4,599 (55.1) | 5,283 (54.8) | 1,690 (51.5) | 1,723 (52.5) | 6,289 (54.1) | 7,006 (54.2) |
| Cardiac disorder presentb, n (%) | 2,048 (25.8) | 2,257 (26.8) | 852 (26.0) | 891 (27.1) | 2,900 (25.8) | 3,148 (26.9) |
| Cardiac arrhythmia presentb, n (%) | 611 (7.7) | 719 (8.5) | 350 (10.7) | 428 (13.0) | 961 (8.6) | 1,147 (9.8) |
| Renal disorder presentb,c, n (%) | 127 (1.6) | 151 (1.8) | 54 (1.6) | 68 (2.1) | 181 (1.6) | 219 (1.9) |
Notes:
Includes patients with combination therapy.
Excluded study 205.257, for which diagnoses could not be mapped.
Listed as concomitant disease (any comorbidity at baseline coded to renal and/or urinary disorders).
Abbreviations: CV, cardiovascular; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity, ICS, inhaled corticosteroid; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroid; SAMA, short-acting muscarinic antagonist; SD, standard deviation.
Overall AEs
Overall, 62.6% and 65.5% of patients treated with tiotropium and placebo, respectively, had at least one AE during the trial, and the risk of an AE was significantly reduced with tiotropium (RR [95% CI]: 0.90 [0.87, 0.93]). Tiotropium also significantly reduced the risk of SAEs: 21.7% of tiotropium- and 22.8% of placebo-treated patients had events (RR [95% CI]: 0.94 [0.89, 0.99]). There was a numerically lower risk of death in the tiotropium (4.0%) than in the placebo (4.5%) group (RR [95% CI]: 0.90 [0.79, 1.01]). No increased risk of AEs, SAEs, and FAEs for the tiotropium group was observed in patients with cardiac disorders present at baseline, with all RR<1 (Table 3). Similar results were obtained when the analysis was performed by inhaler type (HandiHaler® or Respimat®) (Table 3). No significantly increased risk of AEs, SAEs, and FAEs for the tiotropium HandiHaler® and Respimat® groups of all patients or patients with cardiac disorders, cardiac arrhythmia, or renal disorders present at baseline was observed, with the exception of the previously described increase in FAEs in patients with cardiac arrhythmia at baseline in the tiotropium Respimat® group (RR [95% CI]: 3.25 [1.23, 8.60]).11,18 (For some of the subgroups, there was at least one trial with patients in only one treatment group, and thus no statistics could be derived [no RR shown for the subgroups]).
Table 3.
IRs (per 100 patient-years) and RRs for all AEs, by subgroup
| HandiHaler®
|
Respimat®
|
Total
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo
|
Tiotropium
|
RR (95% CI) | Placebo
|
Tiotropium
|
RR (95% CI) | Placebo
|
Tiotropium
|
RR (95% CI) | |||||||
| n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | ||||
| Total | n=8,343 | n=9,647 | n=3,283 | n=3,282 | n=11,626 | n=12,929 | |||||||||
| AEs | 5,362 (64.3) | 143.76 | 5,850 (60.6) | 131.53 | 0.88 (0.85, 0.91)a | 2,257 (68.7) | 179.86 | 2,243 (68.3) | 170.10 | 0.94 (0.89, 0.99)a | 7,619 (65.5) | 152.85 | 8,093 (62.6) | 140.35 | 0.90 (0.87, 0.93)a |
| SAEs | 2,158 (25.9) | 23.67 | 2,311 (24.0) | 22.28 | 0.94 (0.89, 1.00)a | 496 (15.1) | 20.81 | 491 (15.0) | 19.48 | 0.94 (0.83, 1.06) | 2,654 (22.8) | 23.08 | 2,802 (21.7) | 21.73 | 0.94 (0.89, 0.99)a |
| FAEs | 478 (5.7) | 4.15 | 451 (4.7) | 3.46 | 0.85 (0.75, 0.97)a | 45 (1.4) | 1.75 | 64 (2.0) | 2.37 | 1.37 (0.93, 2.00) | 523 (4.5) | 3.71 | 515 (4.0) | 3.27 | 0.90 (0.79, 1.01) |
| Cardiac disorder presentb | n=2,048 | n=2,257 | n=852 | n=891 | n=2,900 | n=3,148 | |||||||||
| AEs | 1,390 (67.9) | 161.25 | 1,547 (68.5) | 152.15 | 0.88 (0.81, 0.94)a | 607 (71.2) | 196.11 | 649 (72.8) | 202.38 | 1.05 (0.94, 1.17) | 1,997 (68.9) | 170.46 | 2,196 (69.8) | 164.20 | 0.93 (0.87, 0.98)a |
| SAEs | 715 (34.9) | 35.09 | 755 (33.5) | 31.29 | 0.90 (0.81, 1.00)a | 164 (19.2) | 27.37 | 184 (20.7) | 27.82 | 1.01 (0.82, 1.25) | 879 (30.3) | 33.34 | 939 (29.8) | 30.54 | 0.92 (0.84, 1.01) |
| FAEs | 165 (8.1) | 5.87 | 149 (6.6) | 4.46 | 0.77 (0.62, 0.96)a | 17 (2.0) | 2.57 | 29 (3.3) | 4.01 | 1.56 (0.86, 2.84) | 182 (6.3) | 5.24 | 178 (5.7) | 4.38 | 0.84 (0.69, 1.04) |
| Cardiac arrhythmia presentb,c | n=611 | n=719 | n=350 | n=428 | n=961 | n=1,147 | |||||||||
| AEs | 406 (66.4) | 182.17 | 497 (69.1) | 177.44 | 256 (73.1) | 212.44 | 320 (74.8) | 211.69 | 1.01 (0.86, 1.19) | 662 (68.9) | 192.80 | 817 (71.2) | 189.44 | ||
| SAEs | 199 (32.6) | 38.74 | 219 (30.5) | 31.75 | 69 (19.7) | 28.00 | 94 (22.0) | 29.72 | 1.05 (0.77, 1.44) | 268 (27.9) | 35.26 | 313 (27.3) | 31.11 | ||
| FAEs | 49 (8.0) | 6.80 | 49 (6.8) | 5.24 | 5 (1.4) | 1.83 | 21 (4.9) | 6.08 | 3.25 (1.23, 8.60)a | 54 (5.6) | 5.44 | 70 (6.1) | 5.47 | ||
| Renal disorder presentb,c | n=127 | n=151 | n=54 | n=68 | n=181 | n=219 | |||||||||
| AEs | 92 (72.4) | 198.94 | 111 (73.5) | 191.13 | 41 (75.9) | 229.72 | 51 (75.0) | 284.70 | 133 (73.5) | 207.51 | 162 (74.0) | 213.19 | |||
| SAEs | 52 (40.9) | 41.36 | 63 (41.7) | 40.79 | 12 (22.2) | 34.19 | 18 (26.5) | 41.23 | 64 (35.4) | 39.80 | 81 (37.0) | 40.89 | |||
| FAEs | 7 (5.5) | 3.35 | 13 (8.6) | 5.79 | 0 (0.0) | 0.00 | 3 (4.4) | 6.10 | 7 (3.9) | 2.81 | 16 (7.3) | 5.85 | |||
Notes:
Significantly different from 1.
Excluded study 205.257, for which diagnoses could not be mapped.
For the subgroups, there is at least one trial with patients in only one treatment group, thus no statistics are derived (no RR shown for the subgroups).
Abbreviations: AE, adverse event; CI, confidence interval; FAE, fatal adverse event; IR, incidence rate; RR, rate ratio; SAE, serious adverse event.
Cardiovascular and respiratory AEs
Pooled CV and respiratory terms based on clinical categories of public health interest, or on possible pathophysiologic associations suggested by either previous published reports or potential biologic mechanisms, are reported in the present analysis, with AEs and SAEs shown in Tables 4 and 5, respectively.
Table 4.
IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory AEs
| HandiHaler®
|
Respimat®
|
Total
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo n=8,343 |
Tiotropium n=9,647 |
RR (95% CI) | Placebo n=3,283 |
Tiotropium n=3,282 |
RR (95% CI) | Placebo n=11,626 |
Tiotropium n=12,929 |
RR (95% CI) | |||||||
| n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | ||||
| Cardiac disordersa | 778 (9.3) | 7.22 | 785 (8.1) | 6.40 | 0.89 (0.80, 0.98)b | 183 (5.6) | 7.31 | 217 (6.6) | 8.25 | 1.13 (0.93, 1.38) | 961 (8.3) | 7.24 | 1,002 (7.8) | 6.73 | 0.93 (0.85, 1.02) |
| AF/flutterc | 145 (1.7) | 1.26 | 147 (1.5) | 1.13 | 0.91 (0.72, 1.14) | 24 (0.7) | 0.93 | 22 (0.7) | 0.82 | 0.86 (0.48, 1.54) | 169 (1.5) | 1.20 | 169 (1.3) | 1.07 | 0.90 (0.73, 1.12) |
| Cardiac arrestc | 31 (0.4) | 0.27 | 23 (0.2) | 0.17 | 0.68 (0.40, 1.17) | 3 (0.1) | 0.12 | 7 (0.2) | 0.26 | 2.28 (0.59, 8.81) | 34 (0.3) | 0.24 | 30 (0.2) | 0.19 | 0.82 (0.50, 1.34) |
| Cardiac failured | 256 (3.1) | 2.24 | 231 (2.4) | 1.77 | 0.80 (0.67, 0.96)b | 40 (1.2) | 1.56 | 38 (1.2) | 1.41 | 0.90 (0.58, 1.41) | 296 (2.5) | 2.12 | 269 (2.1) | 1.71 | 0.81 (0.69, 0.96)b |
| Ischemic heart diseasee | 215 (2.6) | 1.89 | 225 (2.3) | 1.75 | 0.92 (0.76, 1.11) | 32 (1.0) | 1.25 | 54 (1.6) | 2.01 | 1.61 (1.04, 2.49)b | 247 (2.1) | 1.77 | 279 (2.2) | 1.79 | 1.01 (0.85, 1.19) |
| MIf | 114 (1.4) | 0.99 | 112 (1.2) | 0.85 | 0.87 (0.67, 1.14) | 21 (0.6) | 0.82 | 16 (0.5) | 0.59 | 0.73 (0.38, 1.39) | 135 (1.2) | 0.95 | 128 (1.0) | 0.81 | 0.85 (0.67, 1.09) |
| Palpitationsc | 59 (0.7) | 0.51 | 74 (0.8) | 0.56 | 1.09 (0.77, 1.55) | 29 (0.9) | 1.13 | 40 (1.2) | 1.49 | 1.33 (0.82, 2.14) | 88 (0.8) | 0.62 | 114 (0.9) | 0.72 | 1.17 (0.88, 1.55) |
| Supraventricular tachycardiac | 27 (0.3) | 0.23 | 32 (0.3) | 0.24 | 1.06 (0.64, 1.78) | 21 (0.6) | 0.82 | 16 (0.5) | 0.59 | 0.73 (0.38, 1.39) | 48 (0.4) | 0.34 | 48 (0.4) | 0.30 | 0.92 (0.61, 1.37) |
| Tachycardiac | 52 (0.6) | 0.45 | 64 (0.7) | 0.49 | 1.05 (0.73, 1.53) | 27 (0.8) | 1.05 | 18 (0.5) | 0.67 | 0.64 (0.35, 1.17) | 79 (0.7) | 0.56 | 82 (0.6) | 0.52 | 0.92 (0.67, 1.25) |
| Ventricular tachycardia/fibrillationc | 27 (0.3) | 0.23 | 19 (0.2) | 0.14 | 0.64 (0.35, 1.16) | 2 (0.1) | 0.08 | 3 (0.1) | 0.11 | 1.39 (0.24, 8.26) | 29 (0.2) | 0.20 | 22 (0.2) | 0.14 | 0.69 (0.40, 1.21) |
| Vascular disordersa | 610 (7.3) | 5.65 | 670 (6.9) | 5.48 | 0.98 (0.88, 1.09) | 128 (3.9) | 5.09 | 122 (3.7) | 4.60 | 0.90 (0.70, 1.15) | 738 (6.3) | 5.54 | 792 (6.1) | 5.32 | 0.96 (0.87, 1.07) |
| Aneurysmc | 41 (0.5) | 0.35 | 53 (0.5) | 0.40 | 1.16 (0.77, 1.75) | 5 (0.2) | 0.19 | 7 (0.2) | 0.26 | 1.32 (0.42, 4.13) | 46 (0.4) | 0.32 | 60 (0.5) | 0.38 | 1.18 (0.80, 1.74) |
| Hypertensionc | 386 (4.6) | 3.49 | 393 (4.1) | 3.12 | 0.89 (0.77, 1.03) | 81 (2.5) | 3.20 | 97 (3.0) | 3.65 | 1.14 (0.85, 1.53) | 467 (4.0) | 3.43 | 490 (3.8) | 3.21 | 0.93 (0.82, 1.06) |
| Strokec | 96 (1.2) | 0.83 | 114 (1.2) | 0.87 | 1.07 (0.81, 1.41) | 17 (0.5) | 0.66 | 13 (0.4) | 0.48 | 0.73 (0.35, 1.49) | 113 (1.0) | 0.80 | 127 (1.0) | 0.80 | 1.02 (0.79, 1.32) |
| Respiratory, thoracic, and mediastinal disordersa | 3,676 (44.1) | 60.44 | 3,786 (39.2) | 51.15 | 0.81 (0.78, 0.85)b | 1,490 (45.4) | 83.64 | 1,346 (41.0) | 68.38 | 0.82 (0.76, 0.88)b | 5,166 (44.4) | 65.69 | 5,132 (39.7) | 54.77 | 0.81 (0.78, 0.85)b |
| COPD exacerbationc | 2,951 (35.4) | 42.70 | 2,907 (30.1) | 34.48 | 0.80 (0.76, 0.84)b | 1,147 (34.9) | 57.82 | 949 (28.9) | 42.84 | 0.74 (0.68, 0.80)b | 4,098 (35.2) | 46.07 | 3,856 (29.8) | 36.22 | 0.78 (0.75, 0.81)b |
| Pneumoniac | 543 (6.5) | 4.92 | 591 (6.1) | 4.74 | 0.95 (0.84, 1.07) | 100 (3.0) | 3.94 | 99 (3.0) | 3.71 | 0.95 (0.72, 1.25) | 643 (5.5) | 4.74 | 690 (5.3) | 4.56 | 0.95 (0.85, 1.06) |
| Respiratory failurec | 215 (2.6) | 1.87 | 189 (2.0) | 1.45 | 0.80 (0.66, 0.97)b | 28 (0.9) | 1.09 | 26 (0.8) | 0.96 | 0.89 (0.52, 1.52) | 243 (2.1) | 1.73 | 215 (1.7) | 1.36 | 0.81 (0.67, 0.97)b |
Notes:
Primary SOC.
Significantly different from 1.
PV endpoint (see Table S1 for details).
SMQ cardiac failure (narrow).
SMQ ischemic heart disease sub-SMQ other ischemic heart disease (broad).
SMQ ischemic heart disease sub-SMQ MI (broad).
Abbreviations: AE, adverse event; AF, atrial fibrillation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; IR, incidence rate; MI, myocardial infarction; PV, pharmacovigilance; RR, rate ratio; SMQ, standardized Medical Dictionary for Regulatory Activities queries; SOC, system organ class.
Table 5.
IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory SAEs
| HandiHaler®
|
Respimat®
|
Total
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo n=8,343 |
Tiotropium n=9,647 |
RR (95% CI) | Placebo n=3,283 |
Tiotropium n=3,282 |
RR (95% CI) | Placebo n=11,626 |
Tiotropium n=12,929 |
RR (95% CI) | |||||||
| n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | ||||
| Cardiac disordersa | 477 (5.7) | 4.25 | 441 (4.6) | 3.45 | 0.81 (0.71, 0.93)b | 69 (2.1) | 2.70 | 84 (2.6) | 3.13 | 1.16 (0.84, 1.59) | 546 (4.7) | 3.96 | 525 (4.1) | 3.39 | 0.86 (0.76, 0.97)b |
| AF/flutterc | 85 (1.0) | 0.73 | 82 (0.9) | 0.62 | 0.86 (0.64, 1.17) | 6 (0.2) | 0.23 | 8 (0.2) | 0.30 | 1.23 (0.43, 3.57) | 91 (0.8) | 0.64 | 90 (0.7) | 0.57 | 0.89 (0.66, 1.19) |
| Cardiac arrestc | 30 (0.4) | 0.26 | 23 (0.2) | 0.17 | 0.71 (0.41, 1.22) | 3 (0.1) | 0.12 | 7 (0.2) | 0.26 | 2.28 (0.59, 8.81) | 33 (0.3) | 0.23 | 30 (0.2) | 0.19 | 0.85 (0.52, 1.39) |
| Cardiac failured | 170 (2.0) | 1.47 | 157 (1.6) | 1.20 | 0.82 (0.66, 1.02) | 28 (0.9) | 1.09 | 28 (0.9) | 1.04 | 0.95 (0.56, 1.61) | 198 (1.7) | 1.40 | 185 (1.4) | 1.17 | 0.84 (0.68, 1.02) |
| Ischemic heart diseasee | 119 (1.4) | 1.03 | 108 (1.1) | 0.83 | 0.80 (0.61, 1.03) | 15 (0.5) | 0.58 | 20 (0.6) | 0.74 | 1.27 (0.65, 2.49) | 134 (1.2) | 0.95 | 128 (1.0) | 0.81 | 0.85 (0.67, 1.08) |
| MIf | 106 (1.3) | 0.92 | 105 (1.1) | 0.80 | 0.88 (0.67, 1.16) | 18 (0.5) | 0.70 | 16 (0.5) | 0.59 | 0.84 (0.43, 1.65) | 124 (1.1) | 0.88 | 121 (0.9) | 0.76 | 0.87 (0.68, 1.13) |
| Palpitationsc | 5 (0.1) | 0.04 | 7 (0.1) | 0.05 | 1.20 (0.37, 3.89) | 1 (0.0) | 0.04 | 1 (0.0) | 0.04 | 0.98 (0.06, 15.68) | 6 (0.1) | 0.04 | 8 (0.1) | 0.05 | 1.16 (0.39, 3.44) |
| Supraventricular tachycardiac | 13 (0.2) | 0.11 | 15 (0.2) | 0.11 | 1.05 (0.50, 2.23) | 2 (0.1) | 0.08 | 2 (0.1) | 0.07 | 0.95 (0.14, 6.53) | 15 (0.1) | 0.11 | 17 (0.1) | 0.11 | 1.04 (0.52, 2.09) |
| Tachycardiac | 6 (0.1) | 0.05 | 7 (0.1) | 0.05 | 0.89 (0.28, 2.88) | 1 (0.0) | 0.04 | 2 (0.1) | 0.07 | 1.96 (0.18, 21.62) | 7 (0.1) | 0.05 | 9 (0.1) | 0.06 | 1.04 (0.37, 2.95) |
| Ventricular tachycardia/fibrillationc | 21 (0.3) | 0.18 | 17 (0.2) | 0.13 | 0.73 (0.38, 1.39) | 0 (0.0) | 0.00 | 1 (0.0) | 0.04 | 21 (0.2) | 0.15 | 18 (0.1) | 0.11 | 0.78 (0.41, 1.46) | |
| Vascular disordersa | 126 (1.5) | 1.09 | 155 (1.6) | 1.19 | 1.12 (0.88, 1.41) | 17 (0.5) | 0.66 | 10 (0.3) | 0.37 | 0.56 (0.26, 1.23) | 143 (1.2) | 1.01 | 165 (1.3) | 1.05 | 1.05 (0.84, 1.32) |
| Aneurysmc | 28 (0.3) | 0.24 | 33 (0.3) | 0.25 | 1.07 (0.65, 1.77) | 3 (0.1) | 0.12 | 2 (0.1) | 0.07 | 0.64 (0.11, 3.75) | 31 (0.3) | 0.22 | 35 (0.3) | 0.22 | 1.03 (0.63, 1.67) |
| Hypertensionc | 23 (0.3) | 0.20 | 17 (0.2) | 0.13 | 0.67 (0.36, 1.25) | 0 (0.0) | 0.00 | 3 (0.1) | 0.11 | 23 (0.2) | 0.16 | 20 (0.2) | 0.13 | 0.79 (0.44, 1.44) | |
| Strokec | 76 (0.9) | 0.66 | 94 (1.0) | 0.72 | 1.12 (0.83, 1.52) | 13 (0.4) | 0.51 | 10 (0.3) | 0.37 | 0.74 (0.33, 1.68) | 89 (0.8) | 0.63 | 104 (0.8) | 0.66 | 1.07 (0.80, 1.42) |
| Respiratory, thoracic, and mediastinal disordersa | 1,161 (13.9) | 11.20 | 1,123 (11.6) | 9.40 | 0.84 (0.77, 0.91)b | 245 (7.5) | 9.85 | 224 (6.8) | 8.53 | 0.87 (0.73, 1.04) | 1,406 (12.1) | 10.94 | 1,347 (10.4) | 9.25 | 0.84 (0.78, 0.91)b |
| COPD exacerbationc | 971 (11.6) | 9.22 | 944 (9.8) | 7.81 | 0.84 (0.77, 0.92)b | 220 (6.7) | 8.82 | 180 (5.5) | 6.83 | 0.78 (0.64, 0.95)b | 1,191 (10.2) | 9.15 | 1,124 (8.7) | 7.63 | 0.83 (0.76, 0.90)b |
| Pneumoniac | 382 (4.6) | 3.38 | 402 (4.2) | 3.15 | 0.92 (0.80, 1.06) | 64 (1.9) | 2.50 | 54 (1.6) | 2.01 | 0.81 (0.56, 1.16) | 446 (3.8) | 3.22 | 456 (3.5) | 2.95 | 0.91 (0.80, 1.03) |
| Respiratory failurec | 192 (2.3) | 1.67 | 162 (1.7) | 1.24 | 0.77 (0.62, 0.95)b | 23 (0.7) | 0.89 | 19 (0.6) | 0.70 | 0.80 (0.43, 1.46) | 215 (1.8) | 1.52 | 181 (1.4) | 1.14 | 0.77 (0.63, 0.94)b |
Notes:
Primary SOC.
Significantly different from 1.
PV endpoint (see Table S1 for details).
SMQ cardiac failure (narrow).
SMQ ischemic heart disease sub-SMQ other ischemic heart disease (broad).
SMQ ischemic heart disease sub-SMQ myocardial infarction (broad).
Abbreviations: AF, atrial fibrillation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; IR, incidence rate; MI, myocardial infarction; PV, pharmacovigilance; RR, rate ratio; SAE, serious adverse event; SMQ, standardized Medical Dictionary for Regulatory Activities queries; SOC, system organ class.
In total, 8.0% of patients had at least one cardiac AE during the study, and 6.2% of patients had at least one vascular AE (Table 4). Tiotropium was not associated with an increased risk of cardiac AEs (RR [95% CI]: 0.93 [0.85, 1.02]) or vascular AEs (RR [95% CI]: 0.96 [0.87, 1.07]) (Table 4). There was no indication of an increased risk of MI or stroke (RR [95% CI]: 0.85 [0.67, 1.09] or RR [95% CI]: 1.02 [0.79, 1.32]). There was no increased risk associated with tiotropium for ischemic heart disease and hypertension (RR [95% CI]: 1.01 [0.85, 1.19] and RR [95% CI]: 0.93 [0.82, 1.06]). Respiratory, thoracic, and mediastinal disorders were common (41.9% of patients) and were associated with a significantly decreased risk with tiotropium (RR [95% CI]: 0.81 [0.78, 0.85]), including for COPD exacerbations and respiratory failure (RR [95% CI]: 0.78 [0.75, 0.81] and RR [95% CI]: 0.81 [0.67, 0.97]). Similar results were obtained when the HandiHaler® and Respimat® groups were analyzed separately, with no increased risk of cardiac, vascular, and respiratory, thoracic, and mediastinal disorders or stroke in the tiotropium groups except for an increased risk of ischemic heart disease in the tiotropium Respimat® group (RR [95% CI]: 1.61 [1.04, 2.49]) (Table 4).
Overall, 4.4% of patients had at least one cardiac SAE during the study, and 1.3% of patients had at least one vascular SAE (Table 5). Tiotropium was associated with a decreased risk for cardiac SAEs (RR [95% CI]: 0.86 [0.76, 0.97]) and a similar risk for vascular SAEs (RR [95% CI]: 1.05 [0.84, 1.32]) (Table 5) compared with placebo. Tiotropium was not associated with an increased risk for the SAEs of ischemic heart disease, MI, or stroke (RR [95% CI]: 0.85 [0.67, 1.08], RR [95% CI]: 0.87 [0.68, 1.13], or RR [95% CI]: 1.07 [0.80, 1.42], respectively). Respiratory, thoracic, and mediastinal disorder SAEs were common (occurring in 13% of patients), and were associated with a significantly decreased risk with tiotropium (RR [95% CI]: 0.84 [0.78, 0.91]), including for COPD exacerbations and respiratory failure (RR [95% CI]: 0.83 [0.76, 0.90] and RR [95% CI]: 0.77 [0.63, 0.94]). Numerical differences for tiotropium Respimat® and HandiHaler® versus placebo could be observed for single terms; however, overall similar results were obtained for SAEs occurring when the HandiHaler® and Respimat® groups were analyzed separately (Table 5).
Major adverse CV events
MACE and fatal MACE are shown in Table 6. There was no evidence of increased risk for MACE (RR [95% CI]: 0.87 [0.75, 1.01]) or fatal MACE (including death unknown, RR [95% CI]: 0.90 [0.74, 1.10]) for the tiotropium group. Similarly, no increased risk was observed with the tiotropium HandiHaler® and Respimat® groups separately (Table 6).
Table 6.
IRs (per 100 patient-years) and RRs for MACE, by inhaler type
| HandiHaler®
|
Respimat®
|
Total
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo n=8,343 |
Tiotropium n=9,647 |
RR (95% CI) | Placebo n=3,283 |
Tiotropium n=3,282 |
RR (95% CI) | Placebo n=11,626 |
Tiotropium n=12,929 |
RR (95% CI) | |||||||
| n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | ||||
| MACE | 309 (3.7) | 2.71 | 299 (3.1) | 2.31 | 0.86 (0.74, 1.01) | 49 (1.5) | 1.91 | 46 (1.4) | 1.71 | 0.90 (0.60, 1.34) | 358 (3.1) | 2.56 | 345 (2.7) | 2.20 | 0.87 (0.75, 1.01) |
| Fatal MACEa | 174 (2.1) | 1.50 | 159 (1.6) | 1.21 | 0.82 (0.66, 1.02) | 18 (0.5) | 0.70 | 31 (0.9) | 1.15 | 1.66 (0.93, 2.96) | 192 (1.7) | 1.35 | 190 (1.5) | 1.20 | 0.90 (0.74, 1.10) |
Notes:
Including death unknown. Fatal MACE includes the following terms: cardiac SOC (fatal) or vascular SOC (fatal) or SMQ ischemic heart disease sub-SMQ myocardial infarction (broad) (fatal) or stroke #PV (fatal) or sudden death PT or cardiac death PT or sudden cardiac death PT or death PT.
Abbreviations: CI, confidence interval; IR, incidence rate; MACE, major adverse cardiovascular event; PT, preferred term; PV, pharmacovigilance; RR, rate ratio; SMQ, standardized Medical Dictionary for Regulatory Activities queries; SOC, system organ class.
Anticholinergic events
Potential anticholinergic AEs and SAEs are depicted in Tables 7 and 8, respectively. The PV endpoints shown are a summary of AEs for presumed, potential, or hypothetical events that may be a consequence of anticholinergic pharmacology.
Table 7.
IRs (per 100 patient-years) and RRs for potential anticholinergic AEs
| Placebo n=11,626
|
Tiotropium n=12,929
|
RR (95% CI) | |||
|---|---|---|---|---|---|
| n (%) | IR | n (%) | IR | ||
| Eye disordersa | 418 (3.6) | 3.05 | 471 (3.6) | 3.09 | 1.01 (0.88, 1.15) |
| Glaucomab | 38 (0.3) | 0.27 | 41 (0.3) | 0.26 | 0.97 (0.62, 1.52) |
| Vision blurredc | 67 (0.6) | 0.47 | 62 (0.5) | 0.39 | 0.80 (0.57, 1.14) |
| Gastrointestinal disordersa | 1,569 (13.5) | 12.65 | 2,082 (16.1) | 15.58 | 1.20 (1.13, 1.29)d |
| Abdominal painc | 206 (1.8) | 1.47 | 246 (1.9) | 1.57 | 1.04 (0.86, 1.25) |
| Constipationc | 185 (1.6) | 1.32 | 264 (2.0) | 1.69 | 1.28 (1.06, 1.54)d |
| Dry mouthc | 196 (1.7) | 1.40 | 519 (4.0) | 3.39 | 2.35 (1.99, 2.77)d |
| Dyspepsiac | 376 (3.2) | 2.73 | 515 (4.0) | 3.38 | 1.21 (1.06, 1.38)d |
| Dysphagiac | 24 (0.2) | 0.17 | 33 (0.3) | 0.21 | 1.21 (0.71, 2.08) |
| Gastroesophageal reflux diseasec | 261 (2.2) | 1.87 | 374 (2.9) | 2.42 | 1.25 (1.06, 1.46)d |
| Gastrointestinal obstructione | 33 (0.3) | 0.23 | 64 (0.5) | 0.40 | 1.76 (1.16, 2.69)d |
| Stomatitisc | 36 (0.3) | 0.25 | 49 (0.4) | 0.31 | 1.12 (0.73, 1.73) |
| Metabolism and nutrition disordersa | 639 (5.5) | 4.76 | 663 (5.1) | 4.41 | 0.92 (0.83, 1.03) |
| Dehydrationc | 32 (0.3) | 0.22 | 40 (0.3) | 0.25 | 1.14 (0.71, 1.82) |
| Hyperglycemia/new-onset diabetes mellitusf | 212 (1.8) | 1.52 | 225 (1.7) | 1.44 | 0.94 (0.78, 1.13) |
| Nervous system disordersa | 1,051 (9.0) | 8.02 | 1,233 (9.5) | 8.51 | 1.02 (0.94, 1.11) |
| Dizzinessc | 280 (2.4) | 2.01 | 333 (2.6) | 2.15 | 1.03 (0.88, 1.21) |
| Headachec | 398 (3.4) | 2.88 | 475 (3.7) | 3.09 | 1.00 (0.88, 1.14) |
| Sleep disturbancec | 204 (1.8) | 1.46 | 242 (1.9) | 1.55 | 1.05 (0.87, 1.26) |
| Syncopec | 72 (0.6) | 0.51 | 85 (0.7) | 0.54 | 1.06 (0.77, 1.45) |
| Psychiatric disordersa | 533 (4.6) | 3.90 | 615 (4.8) | 4.06 | 1.01 (0.90, 1.14) |
| Anxiety symptoms/fearsc | 194 (1.7) | 1.38 | 203 (1.6) | 1.29 | 0.89 (0.73, 1.09) |
| Cognitive impairmentc | 2 (0.0) | 0.01 | 1 (0.0) | 0.01 | 0.46 (0.04, 5.09) |
| Confusionc | 22 (0.2) | 0.15 | 18 (0.1) | 0.11 | 0.70 (0.37, 1.31) |
| Insomniac | 184 (1.6) | 1.31 | 227 (1.8) | 1.45 | 1.10 (0.90, 1.34) |
| Restlessnessc | 13 (0.1) | 0.09 | 12 (0.1) | 0.08 | 0.65 (0.29, 1.46) |
| Depression and self-injuryg | 173 (1.5) | 1.23 | 209 (1.6) | 1.33 | 1.08 (0.88, 1.32) |
| Renal and urinary disordersa | 341 (2.9) | 2.46 | 396 (3.1) | 2.56 | 1.04 (0.90, 1.20) |
| Dysuriac | 23 (0.2) | 0.16 | 52 (0.4) | 0.33 | 2.07 (1.26, 3.39)d |
| Renal failurec | 68 (0.6) | 0.48 | 73 (0.6) | 0.46 | 0.97 (0.70, 1.36) |
| Urinary retentionc | 36 (0.3) | 0.25 | 62 (0.5) | 0.39 | 1.56 (1.03, 2.36)d |
| Urinary tract infectionc | 287 (2.5) | 2.06 | 350 (2.7) | 2.26 | 1.08 (0.92, 1.26) |
Notes:
Primary SOC.
SMQ glaucoma (narrow).
PV endpoint (see Table S1 for details).
Significantly different from 1.
SMQ gastrointestinal perforation, ulceration, hemorrhage, or obstruction.
SMQ hyperglycemia/new-onset diabetes mellitus (narrow).
SMQ depression and self-injury (excluding suicide and self-injury) (narrow).
Abbreviations: AE, adverse event; CI, confidence interval; IR, incidence rate; PV, pharmacovigilance; RR, rate ratio; SMQ, standardized Medical Dictionary for Regulatory Activities queries; SOC, system organ class.
Table 8.
IRs (per 100 patient-years) and RRs for potential anticholinergic SAEs
| Placebo n=11,626
|
Tiotropium n=12,929
|
RR (95% CI) | |||
|---|---|---|---|---|---|
| n (%) | IR | n (%) | IR | ||
| Eye disordersa | 33 (0.3) | 0.23 | 38 (0.3) | 0.24 | 1.07 (0.67, 1.71) |
| Glaucomab | 1 (0.0) | 0.01 | 2 (0.0) | 0.01 | 1.85 (0.17, 20.37) |
| Vision blurredc | 1 (0.0) | 0.01 | 1 (0.0) | 0.01 | 0.92 (0.06, 14.77) |
| Gastrointestinal disordersa | 266 (2.3) | 1.90 | 284 (2.2) | 1.82 | 0.96 (0.81, 1.14) |
| Abdominal painc | 28 (0.2) | 0.20 | 30 (0.2) | 0.19 | 0.94 (0.56, 1.57) |
| Constipationc | 5 (0.0) | 0.04 | 7 (0.1) | 0.04 | 1.28 (0.40, 4.08) |
| Dyspepsiac | 28 (0.2) | 0.20 | 26 (0.2) | 0.16 | 0.86 (0.50, 1.47) |
| Dysphagiac | 4 (0.0) | 0.03 | 5 (0.0) | 0.03 | 1.12 (0.30, 4.24) |
| Gastroesophageal reflux diseasec | 14 (0.1) | 0.10 | 16 (0.1) | 0.10 | 1.06 (0.51, 2.19) |
| Gastrointestinal obstructiond | 27 (0.2) | 0.19 | 45 (0.3) | 0.28 | 1.51 (0.93, 2.44) |
| Stomatitisc | 0 (0.0) | 0.00 | 2 (0.0) | 0.01 | |
| Metabolism and nutrition disordersa | 77 (0.7) | 0.54 | 71 (0.5) | 0.45 | 0.82 (0.59, 1.13) |
| Dehydrationc | 12 (0.1) | 0.08 | 20 (0.2) | 0.13 | 1.47 (0.71, 3.07) |
| Hyperglycemia/new-onset diabetes mellituse | 22 (0.2) | 0.15 | 29 (0.2) | 0.18 | 1.14 (0.65, 2.00) |
| Nervous system disordersa | 205 (1.8) | 1.46 | 222 (1.7) | 1.41 | 0.98 (0.81, 1.19) |
| Dizzinessc | 26 (0.2) | 0.18 | 23 (0.2) | 0.14 | 0.82 (0.47, 1.43) |
| Headachec | 5 (0.0) | 0.04 | 5 (0.0) | 0.03 | 0.93 (0.27, 3.20) |
| Sleep disturbancec | 5 (0.0) | 0.04 | 0 (0.0) | 0.00 | 0.00 |
| Syncopec | 23 (0.2) | 0.16 | 40 (0.3) | 0.25 | 1.57 (0.94, 2.64) |
| Psychiatric disordersa | 58 (0.5) | 0.41 | 46 (0.4) | 0.29 | 0.71 (0.48, 1.04) |
| Anxiety symptoms/fearsc | 10 (0.1) | 0.07 | 7 (0.1) | 0.04 | 0.62 (0.23, 1.63) |
| Confusionc | 5 (0.0) | 0.04 | 5 (0.0) | 0.03 | 0.93 (0.27, 3.27) |
| Insomniac | 1 (0.0) | 0.01 | 0 (0.0) | 0.00 | 0.00 |
| Restlessnessc | 2 (0.0) | 0.01 | 0 (0.0) | 0.00 | 0.00 |
| Depression and self-injuryf | 20 (0.2) | 0.14 | 15 (0.1) | 0.09 | 0.68 (0.35, 1.31) |
| Renal and urinary disordersa | 84 (0.7) | 0.59 | 108 (0.8) | 0.68 | 1.16 (0.87, 1.55) |
| Dysuriac | 0 (0.0) | 0.00 | 3 (0.0) | 0.02 | |
| Renal failurec | 32 (0.3) | 0.22 | 46 (0.4) | 0.29 | 1.31 (0.83, 2.06) |
| Urinary retentionc | 12 (0.1) | 0.08 | 24 (0.2) | 0.15 | 1.87 (0.93, 3.73) |
| Urinary tract infectionc | 28 (0.2) | 0.20 | 37 (0.3) | 0.23 | 1.19 (0.73, 1.95) |
Notes:
Primary SOC.
SMQ glaucoma (narrow).
PV endpoint (see Table S1 for details).
SMQ gastrointestinal perforation, ulceration, hemorrhage, or obstruction.
SMQ hyperglycemia/new-onset diabetes mellitus (narrow).
SMQ depression and self-injury (excluding suicide and self-injury) (narrow).
Abbreviations: IR, incidence rate; PV, pharmacovigilance; RR, rate ratio; SAE, serious adverse event; SMQ, standardized Medical Dictionary for Regulatory Activities queries; SOC, system organ class.
Dry mouth (RR [95% CI]: 2.35 [1.99, 2.77]), constipation (RR [95% CI]: 1.28 [1.06, 1.54]), and urinary retention (RR [95% CI]: 1.56 [1.03, 2.36]) occurred more frequently with tiotropium versus placebo, with dry mouth being the most common AE, occurring in 4% of patients in the tiotropium group (Table 7). Gastrointestinal obstruction, dyspepsia, dysuria, and gastroesophageal reflux also increased with tiotropium use. The incidence of glaucoma or the worsening of an existing glaucoma at baseline was not increased with tiotropium, as shown by an RR close to 1 (RR [95% CI]: 0.97 [0.62, 1.52]). There was no increased risk for metabolism and nutrition, nervous system, and psychiatric AEs (Table 7). Potential anticholinergic SAEs were not associated with an increased risk with tiotropium (Table 8). Similar results were obtained for anticholinergic AEs and SAEs occurring when the HandiHaler® and Respimat® groups were analyzed separately (data not shown).
Fatal events
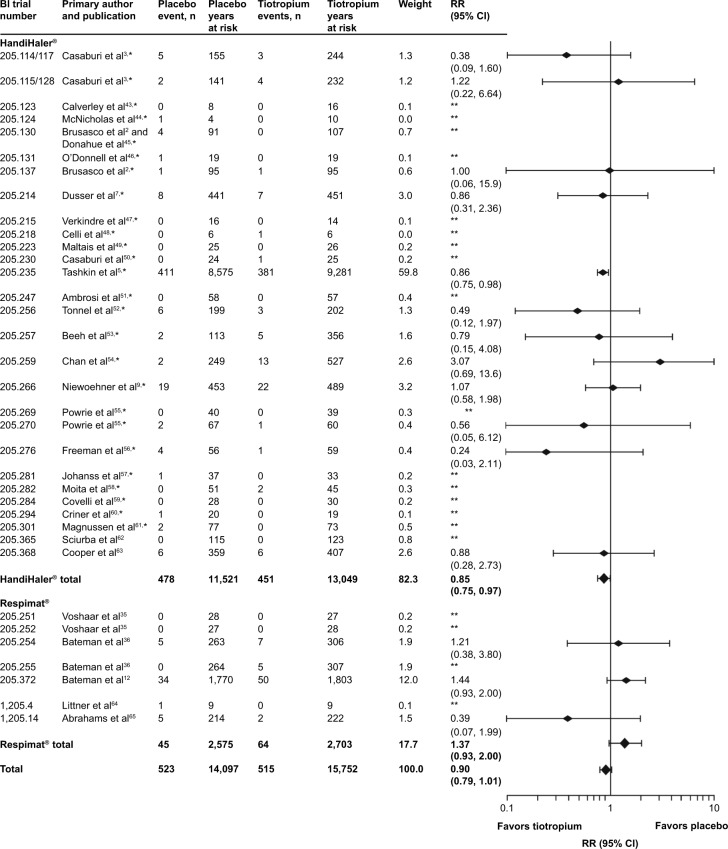
As noted earlier, there was a numerically lower risk of death in the tiotropium group (RR [95% CI]: 0.90 [0.79, 1.01]). Figure 1 depicts all-cause FAEs by trial. Most individual trials were associated with a wide CI, a few of which exhibited higher IRs for the tiotropium group.
Figure 1.
Number of events, time at risk, and RRs for all-cause FAEs.
Notes: *Studies included in Kesten et al26 meta-analysis; **RR could not be calculated for studies where 0 events occurred in one or both treatment groups.
Abbreviations: BI, Boehringer Ingelheim; CI, confidence interval; FAE, fatal adverse event; RR, rate ratio.
Table 9 depicts the most common causes of death according to SOC (with a frequency of ≥3% of total FAEs within any of the treatment arms in either study pool, ie, HandiHaler®, Respimat®, or both combined) and PT (with a frequency of ≥10 FAEs within the pooled analysis). Respiratory, thoracic, and mediastinal disorders were the most common cause of death (occurring in 1.2% of patients), and were associated with a reduced overall risk for tiotropium (RR [95% CI]: 0.76 [0.61, 0.96]). None of the SOCs were associated with a significantly increased risk for tiotropium. When the HandiHaler® and Respimat® groups were analyzed separately, neither tiotropium HandiHaler® nor tiotropium Respimat® was associated with significantly increased FAEs according to SOC, while the risk associated with tiotropium HandiHaler® was additionally decreased for respiratory, thoracic, and mediastinal disorders as well as for cardiac disorders (Table 9).
Table 9.
IRs (per 100 patient-years) and RRs for FAEs according to SOC and PTa
| HandiHaler®
|
Respimat®
|
Total
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo n=8,343 |
Tiotropium n=9,647 |
RR (95% CI) | Placebo n=3,283 |
Tiotropium n=3,282 |
RR (95% CI) | Placebo n=11,626 |
Tiotropium n=12,929 |
RR (95% CI) | |||||||
| n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | n (%) | IR | ||||
| Respiratory, thoracic, and mediastinal disorders | 143 (1.7) | 1.23 | 118 (1.2) | 0.90 | 0.74 (0.58, 0.95)b | 16 (0.5) | 0.62 | 16 (0.5) | 0.59 | 0.96 (0.48, 1.92) | 159 (1.4) | 1.12 | 134 (1.0) | 0.84 | 0.76 (0.61, 0.96)b |
| COPD | 67 (0.8) | 0.57 | 61 (0.6) | 0.46 | 0.82 (0.58, 1.16) | 4 (0.1) | 0.16 | 8 (0.2) | 0.30 | 1.88 (0.56, 6.28) | 71 (0.6) | 0.50 | 69 (0.5) | 0.43 | 0.88 (0.63, 1.22) |
| Respiratory failure | 51 (0.6) | 0.44 | 33 (0.3) | 0.25 | 0.59 (0.38, 0.91)b | 5 (0.2) | 0.19 | 2 (0.1) | 0.07 | 0.37 (0.07, 1.99) | 56 (0.5) | 0.39 | 35 (0.3) | 0.22 | 0.57 (0.37, 0.87)b |
| Acute respiratory failure | 8 (0.1) | 0.07 | 8 (0.1) | 0.06 | 0.92 (0.35, 2.46) | 2 (0.1) | 0.08 | 0 (0.0) | 0.00 | 0 | 10 (0.1) | 0.07 | 8 (0.1) | 0.05 | 0.74 (0.29, 1.89) |
| Neoplasms benign, malignant, and unspecified | 118 (1.4) | 1.01 | 117 (1.2) | 0.89 | 0.91 (0.70, 1.17) | 5 (0.2) | 0.19 | 8 (0.2) | 0.30 | 1.53 (0.50, 4.68) | 123 (1.1) | 0.87 | 125 (1.0) | 0.79 | 0.93 (0.73, 1.20) |
| Lung neoplasm malignant | 19 (0.2) | 0.16 | 21 (0.2) | 0.16 | 1.00 (0.54, 1.85) | 2 (0.1) | 0.08 | 4 (0.1) | 0.15 | 1.92 (0.35, 10.59) | 21 (0.2) | 0.15 | 25 (0.2) | 0.16 | 1.09 (0.61, 1.93) |
| Cardiac disorders | 106 (1.3) | 0.91 | 89 (0.9) | 0.67 | 0.75 (0.56, 0.99)b | 12 (0.4) | 0.47 | 23 (0.7) | 0.85 | 1.86 (0.92, 3.75) | 118 (1.0) | 0.83 | 112 (0.9) | 0.70 | 0.80 (0.66, 1.11) |
| Cardiac failure | 10 (0.1) | 0.09 | 14 (0.1) | 0.11 | 1.25 (0.55, 2.83) | 4 (0.1) | 0.16 | 2 (0.1) | 0.07 | 0.47 (0.08, 2.66) | 14 (0.1) | 0.10 | 16 (0.1) | 0.10 | 1.04 (0.50, 2.14) |
| MI and acute MI | 25 (0.3) | 0.21 | 20 (0.2) | 0.15 | 0.71 (0.39,1.30) | 3 (0.1) | 0.12 | 6 (0.2) | 0.22 | 1.94 (0.48, 7.81) | 28 (0.2) | 0.20 | 26 (0.2) | 0.16 | 0.84 (0.49, 1.45) |
| Cardiac arrest | 17 (0.2) | 0.15 | 10 (0.1) | 0.08 | 0.53 (0.24,1.17) | 2 (0.1) | 0.08 | 2 (0.1) | 0.07 | 0.98 (0.14, 6.90) | 19 (0.2) | 0.13 | 12 (0.1) | 0.08 | 0.58 (0.28, 1.20) |
| Cardiopulmonary failure | 8 (0.1) | 0.07 | 9 (0.1) | 0.07 | 1.00 (0.38, 2.63) | 2 (0.1) | 0.08 | 1 (0.0) | 0.04 | 0.49 (0.04, 5.36) | 10 (0.1) | 0.07 | 10 (0.1) | 0.06 | 0.90 (0.37, 2.19) |
| Cardiorespiratory arrest | 6 (0.1) | 0.05 | 7 (0.1) | 0.05 | 1.09 (0.37, 3.23) | 1 (0.0) | 0.04 | 4 (0.1) | 0.15 | 3.92 (0.44, 35.09) | 7 (0.1) | 0.05 | 11 (0.1) | 0.07 | 1.49 (0.58, 3.81) |
| Cardiac failure congestive | 10 (0.1) | 0.09 | 6 (0.1) | 0.05 | 0.51 (0.18, 1.45) | 0 (0.0) | 0.00 | 1 (0.0) | 0.04 | 10 (0.1) | 0.07 | 7 (0.1) | 0.04 | 0.60 (0.22, 1.63) | |
| Infections and infestations | 63 (0.8) | 0.54 | 61 (0.6) | 0.46 | 0.88 (0.62, 1.26) | 9 (0.3) | 0.35 | 7 (0.2) | 0.26 | 0.75 (0.28, 2.04) | 72 (0.6) | 0.51 | 68 (0.5) | 0.43 | 0.87 (0.62, 1.21) |
| Pneumonia | 26 (0.3) | 0.22 | 26 (0.3) | 0.20 | 0.91 (0.53, 1.57) | 3 (0.1) | 0.12 | 2 (0.1) | 0.07 | 0.63 (0.10, 3.90) | 29 (0.2) | 0.20 | 28 (0.2) | 0.18 | 0.89 (0.53, 1.49) |
| Sepsis | 17 (0.2) | 0.15 | 8 (0.1) | 0.06 | 0.44 (0.19, 1.01) | 1 (0.0) | 0.04 | 0 (0.0) | 0.00 | 0 | 18 (0.2) | 0.13 | 8 (0.1) | 0.05 | 0.41 (0.18, 0.95)b |
| Septic shock | 8 (0.1) | 0.07 | 6 (0.1) | 0.05 | 0.69 (0.24, 2.00) | 3 (0.1) | 0.12 | 1 (0.0) | 0.04 | 0.33 (0.03, 3.12) | 11 (0.1) | 0.08 | 7 (0.1) | 0.04 | 0.59 (0.23, 1.54) |
| General disorders and administration site conditions | 55 (0.7) | 0.47 | 60 (0.6) | 0.45 | 1.00 (0.69, 1.44) | 4 (0.1) | 0.16 | 10 (0.3) | 0.37 | 2.38 (0.76, 7.43) | 59 (0.5) | 0.41 | 70 (0.5) | 0.44 | 1.09 (0.77, 1.54) |
| Death | 28 (0.3) | 0.24 | 37 (0.4) | 0.28 | 1.20 (0.73, 1.97) | 3 (0.1) | 0.12 | 5 (0.2) | 0.18 | 1.57 (0.39, 6.36) | 31 (0.3) | 0.22 | 42 (0.3) | 0.26 | 1.24 (0.78, 1.97) |
| Sudden death | 11 (0.1) | 0.09 | 12 (0.1) | 0.09 | 1.00 (0.44, 2.27) | 1 (0.0) | 0.04 | 2 (0.1) | 0.07 | 1.96 (0.18, 21.63) | 12 (0.1) | 0.08 | 14 (0.1) | 0.09 | 1.08 (0.50, 2.33) |
| Nervous system disorders | 16 (0.2) | 0.14 | 18 (0.2) | 0.14 | 1.02 (0.52, 2.01) | 1 (0.0) | 0.04 | 1 (0.0) | 0.04 | 0.92 (0.06, 13.48) | 17 (0.1) | 0.12 | 19 (0.1) | 0.12 | 1.02 (0.53, 1.96) |
| Vascular disorders | 17 (0.2) | 0.15 | 8 (0.1) | 0.06 | 0.44 (0.19, 1.02) | 1 (0.0) | 0.04 | 0 (0.0) | 0.00 | 0 | 18 (0.2) | 0.13 | 8 (0.1) | 0.05 | 0.42 (0.18, 0.96)b |
| Renal and urinary disorders | 1 (0.0) | 0.01 | 6 (0.1) | 0.05 | 5.75 (0.67, 49.16) | 0 (0.0) | 0.00 | 2 (0.1) | 0.07 | 1 (0.0) | 0.01 | 8 (0.1) | 0.05 | 7.65 (0.93, 63.05) | |
Notes:
SOCs with a frequency of at least 3% of the total number of fatal events within any of the treatment arms in either study pool, ie, HandiHaler®, Respimat® or both combined are shown; PTs with an occurrence of at least 10 fatal events within the pooled analysis are shown.
Significantly different from 1.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; FAE, fatal adverse event; IR, incidence rate; MI, myocardial infarction; PT, preferred term; RR, rate ratio; SOC, system organ class.
Discussion
Long-term safety evaluation of treatment is essential for prescribing physicians. The present safety analysis reflects the large safety database of placebo-controlled studies with tiotropium, a long-established (over 10 years) treatment for COPD. This analysis of 24,555 patients, which adds to the last comprehensive safety review of 2009 (17,014 patients),26 includes data with Respimat® – in all, 28 tiotropium HandiHaler® and 7 tiotropium Respimat® trials (compared with 26 HandiHaler® trials from the previous safety review). The recent TIOSPIR™ trial did not show any relevant differences between tiotropium HandiHaler® and tiotropium Respimat®, in particular relating to safety. This led to the development of the present pooled safety analysis, and provides the justification for the pooling of both inhaler types. Additionally, the pooled studies had similar entry criteria, assessment procedures, and data collection methods; the patient baseline characteristics were also comparable between the HandiHaler® and Respimat® trials.
This pooled safety analysis shows that IRs for overall AEs and SAEs were significantly lower in the tiotropium group compared with those in the placebo group, while the incidence of FAEs was numerically lower in the tiotropium group. In the tiotropium HandiHaler® group, IRs were significantly lower for all AEs, while for the tiotropium Respimat® group, AEs were significantly lower and SAEs and FAEs were comparable to the placebo group. Overall, these results demonstrate that both tiotropium Respimat® and tiotropium HandiHaler® do not increase the risk of an AE. Furthermore, there was no evidence to show an increased risk of cardiac or vascular events, including MACE, with either tiotropium HandiHaler® or tiotropium Respimat®. A higher incidence of ischemic heart disease for tiotropium versus placebo was seen in the Respimat® group but not in the HandiHaler® group; however, the IRs were lower in the placebo Respimat® group than in the placebo HandiHaler® group (IR: 1.25 versus 1.89), and no significant difference in the risk of MI was seen between the Respimat® and HandiHaler® groups in the TIOSPIR™ trial.
FAEs by SOC and PT did not reveal an imbalance between the placebo and tiotropium groups, either pooled or by inhaler type. As expected, known anticholinergic AEs (such as dry mouth, constipation, intestinal obstruction, and urinary retention) were more frequent in the tiotropium versus placebo groups, although SAEs were not. The large patient database size and large tiotropium exposure in terms of patient years provide a robust estimate of the IRs for these effects and allow a sound assessment of risks and benefits with tiotropium HandiHaler® and Respimat® in patients with COPD.
We have carried out further analysis on patients with renal impairment. The results were presented at the European Respiratory Society Annual Congress 2014.31 The safety of tiotropium was analyzed in trials where creatinine clearance was reported at baseline. Incidence RRs of AEs, SAEs, and FAEs with tiotropium HandiHaler® or Respimat® versus placebo showed no association with mild to moderately impaired renal function (10,805 patients were evaluable: n=4,282 in HandiHaler® trials; n=6,523 in Respimat® trials). Results for severe renal impairment were, however, limited owing to low patient numbers (n=52).
The results reported here support the 2009 safety analysis,26 which showed a reduced risk of overall AEs, SAEs, and FAEs with tiotropium HandiHaler®. The safety results within the Kesten et al26 analysis were also consistent with those seen in the UPLIFT® trial (with the analyses for HandiHaler® trials being driven predominantly by the data from UPLIFT®, based on large patient numbers and 4-year trial length), and indicated a reduction in cardiac AEs and MACE associated with tiotropium, and no increased risk of vascular AEs. Our analysis presents a numerically reduced risk of cardiac AEs with tiotropium in both inhalers. The risk of respiratory disorders was significantly reduced in both the Kesten et al26 and our analyses, while the recognized anticholinergic effects were higher with tiotropium use. A limitation of comparing the results of the present analysis with those of the Kesten et al26 pooled analysis is that the latter included all but two of the HandiHaler® trials included in our pooled analysis, so that its results are driven mainly by the findings from the HandiHaler® trials (28 HandiHaler® trials versus 7 Respimat® trials).
The results from this safety review support the recent findings of the largest randomized, double-blind, parallel-group trial performed to date in COPD, involving 17,135 patients – the TIOSPIR™ trial.29 TIOSPIR™ showed that tiotropium Respimat® (2.5 or 5 μg once daily) had a similar safety profile and exacerbation efficacy as tiotropium HandiHaler® (18 μg once daily) (risk of death HR [95% CI]: 0.96 [0.84, 1.09] for Respimat® 5 μg and 1.00 [0.87, 1.14] for Respimat® 2.5 μg; risk of first exacerbation HR [95% CI]: 0.98 [0.93, 1.03] for Respimat® 5 μg). Our analysis, similar to earlier analyses, found an increased risk of FAEs in patients with cardiac arrhythmia at baseline for the tiotropium Respimat® group. This finding may be due to an unusually low IR in the placebo Respimat® group (1.83), compared with an IR of 6.08 in the active treatment group. The corresponding IRs in the HandiHaler® database were higher (placebo: 6.80, tiotropium: 5.24). Direct comparison of tiotropium HandiHaler® and Respimat® in the TIOSPIR™ trial, which was powered to assess the all-cause mortality noninferiority, did not indicate a mortality imbalance for patients with cardiac arrhythmias at baseline (RR [95% CI]: 0.81 [0.58, 1.12] for tiotropium Respimat® 5 μg versus HandiHaler®).29 Furthermore, the accompanying editorial by Jenkins32 concluded that both HandiHaler® and Respimat® have equal safety profiles.
The findings of our analysis contrast with retrospective analyses regarding CV events and inhaled anticholinergics, which suggested that CV deaths were increased with inhaled anticholinergic, in particular with ipratropium.33,34 Limitations to these meta-analyses have been described previously,24 and include improper design due to lack of data, errors in article identification and data extraction, as well as improper accounting for differential trial discontinuation. A mortality imbalance was seen with tiotropium Respimat® in a pooled analysis of three 1-year trials and one 6-month trial,11,18 and with subsequent meta-analyses.19–22 These meta-analyses were based on the same trials as the present review, but were carried out differently. In the Singh et al22 and Karner et al20 meta-analyses, which included five randomized, controlled trials (NCT00239473,35 NCT00240435,35 NCT00168844,36 NCT00168831,36 and NCT00387088), tiotropium Respimat® was associated with an increased risk of FAEs. These analyses were limited due to imprecise estimates owing to fairly low event rates and were performed on published aggregated on-treatment data, including the higher 10 μg dose, rather than using individual patient data, possibly leading to bias.21 The Dong et al19 meta-analysis, which also associated tiotropium Respimat® with a higher risk of FAEs, in particular for CV death, in patients with severe COPD and in those at a higher daily dose (10 μg), only used three Respimat® trials (NCT00168844,36 NCT00168831,36 and NCT0038708812). All of the trials included in these meta-analyses were also included in the current analysis, which totaled seven Respimat® trials (all placebo-controlled trials available to date). The high number of patients and patient-years of exposure to tiotropium included in our review, and especially the use of individual patient data, ensures the robustness of the analysis.
Although most prospective, randomized, double-blind, clinical trials with tiotropium HandiHaler® and Respimat® in COPD have been conducted by Boehringer Ingelheim and are included in this analysis, a few other studies have been carried out. The Investigating New Standards for Prophylaxis In Reduction of Exacerbations (INSPIRE) study observed an increase in mortality in the tiotropium (HandiHaler®) group compared with the salmeterol and fluticasone groups. However, the interpretation beyond the primary exacerbation endpoint should be done with caution as the withdrawal of the run-in medication, salmeterol and oral prednisolone (30 mg), at the time of randomization may also have affected the outcome and results of this trial.37,38 Additional studies of tiotropium HandiHaler® were performed in an open-label fashion, for which patient-level data were not available.39,40
Pooling clinical data into meta-analyses includes such limitations as differences in trial populations, trial design, and physician diagnostic reporting. However, the present safety review reduced these limitations by analyzing the data on a patient level and controlling for differences in exposure between treatment groups, therefore reducing bias and confounding. In addition, the analysis of patient-level data also allows grouping of AEs, such as SMQs, PV endpoints, and subgroup analyses defined by potential risk factors. Potential problems in combining studies have been minimized because the trials were conducted using consistent inclusion and exclusion criteria, the same diagnostic criteria, and nearly identical approaches to data collection. A potential limitation may arise from the accuracy of causes of death reported by investigators, as only a few trials had an adjudication committee (studies 205.235 and 205.372). However, for consistency with data capture for AEs, SAEs, and other trials, the investigator-reported causes were used for the pooled analysis rather than the adjudicated terms from the clinical endpoints committee.
In our analysis, some HandiHaler® trials (such as UPLIFT® and 205.266) as well as the Respimat® trials had relatively broad inclusion criteria (including patients with stable cardiac disorders), and allowed concomitant respiratory medication including ICS, LABAs, and theophylline. Therefore, the patient population analyzed here generally reflects real-world heterogeneous populations and phenotypes of patients with COPD, as far as is possible in randomized, controlled trials. The absolute rates of FAEs observed for the groups reported in this analysis (IR/100 patient-years: 3.71 and 3.27 for the placebo and tiotropium groups, respectively) are comparable with those of mortality in general in COPD for which observed mortality IRs are 1.7–2.2 for Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage 2 patients and 3.07–4.29 for GOLD Stage 3 and 4 patients.41,42 Furthermore, the increased size of the safety database, pooling 35 studies to include 12,929 tiotropium-treated patients, providing 14,909 patient-years’ exposure to tiotropium, provides a robust dataset.
In summary, the present analysis describes the pooled safety data for tiotropium HandiHaler® (28 trials) and Respimat® (7 trials), as well as by inhaler type, in 24,555 patients and 14,909 patient-years of exposure to tiotropium. The results indicate that tiotropium, given via either HandiHaler® or Respimat®, does not increase the overall risks of AEs, SAEs, FAEs, or CV events. Given the evidence, provided by the TIOSPIR™ trial, that both have an equal safety profile, and given their established efficacy in terms of bronchodilation, reduction in exacerbations, and improvement in HRQoL, physicians may consider tiotropium in either formulation depending on availability and their preference or that of the patient.
Supplementary material
Table S1.
PV endpoint definition
| PV endpoint/SMQ | MedDRA PT |
|---|---|
| Abdominal pain | Abdominal pain; abdominal pain lower; abdominal pain upper; abdominal rebound tenderness; abdominal tenderness; epigastric discomfort; gastrointestinal pain |
| Aneurysm | Aneurysm; aneurysm arteriovenous; aneurysm ruptured; aortic aneurysm; aortic aneurysm rupture; aortic aneurysm syphilitic; aortic dissection; aortic dissection rupture; aortic intramural hematoma; aortic stent insertion; artery dissection; cardiac aneurysm; carotid aneurysm rupture; cerebral aneurysm ruptured syphilitic; Charcot–Bouchard microaneurysms; coronary artery aneurysm; coronary artery dissection; dissecting coronary artery aneurysm; femoral artery aneurysm; femoral artery dissection; hemorrhage coronary artery; hepatic artery aneurysm; infective aneurysm; intracranial aneurysm; mycotic aneurysm; peripheral artery aneurysm; peripheral artery dissection; pulmonary artery aneurysm; renal aneurysm; renal artery dissection; retinal aneurysm; ruptured cerebral aneurysm; splenic artery aneurysm; subclavian artery aneurysm; superior vena cava dilatation; venous aneurysm |
| Anxiety symptoms/fears | Acrophobia; activation syndrome; acute stress disorder; agitation; agitation neonatal; agoraphobia; animal phobia; anticipatory anxiety; anxiety; anxiety disorder; anxiety disorder due to a general medical condition; arachnophobia; autophobia; Burnout syndrome; claustrophobia; compulsions; compulsive hand washing; dysmorphophobia; emetophobia; fear; fear of animals; fear of closed spaces; fear of crowded places; fear of death; fear of disease; fear of eating; fear of falling; fear of needles; fear of open spaces; fear of pregnancy; fear of weight gain; generalized anxiety disorder; haphephobia; hydrophobia; limited symptom panic attack; nervousness; neurosis; noctiphobia; nocturnal fear; nosophobia; obsessive thoughts; obsessive-compulsive disorder; ochlophobia; osmophobia; pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection; panic attack; panic disorder; panic disorder with agoraphobia; panic disorder without agoraphobia; panic reaction; paruresis; performance fear; phagophobia; pharmacophobia; phobia; phobia of driving; phobia of exams; phobia of flying; phobic avoidance; phonophobia; photaugiaphobia; post-traumatic stress disorder; postpartum neurosis; postpartum stress disorder; social fear; social phobia; stress; tension; thanatophobia |
| Cardiac arrest | Atrioventricular dissociation; cardiac arrest; cardiac death; cardiac massage; cardiorespiratory arrest; cardiogenic shock; pulseless electrical activity; resuscitation |
| Cardiac failure (SMQ cardiac failure [narrow]) | Acute left ventricular failure; acute pulmonary edema; acute right ventricular failure; cardiac asthma; cardiac failure; cardiac failure acute; cardiac failure chronic; cardiac failure congestive; cardiac failure high output; cardiogenic shock; cardiopulmonary failure; cardiorenal syndrome; chronic left ventricular failure; chronic right ventricular failure; cor pulmonale; cor pulmonale acute; cor pulmonale chronic; ejection fraction decreased; hepatic congestion; hepatojugular reflux; left ventricular failure; low cardiac output syndrome; neonatal cardiac failure; pulmonary edema; pulmonary edema neonatal; right ventricular failure; ventricular failure |
| Cognitive impairment | Cognitive disorder |
| Confusion | Confusion postoperative; confusional state; disorientation |
| Constipation | Colonic pseudo-obstruction; constipation; feces hard; infrequent bowel movements; postprocedural constipation |
| COPD exacerbation | Chronic obstructive pulmonary disease; infective exacerbation of chronic obstructive airways disease; obstructive airways disorder |
| Dehydration | Dehydration |
| Depression and self-injury (SMQ depression and self-injury (excluding suicide and self-injury) (narrow) | Activation syndrome; adjustment disorder with depressed mood; adjustment disorder with mixed anxiety and depressed mood; agitated depression; anhedonia; antidepressant therapy; childhood depression; decreased interest; depressed mood; depression; depression postoperative; depressive symptom; dysphoria; dysthymic disorder; electroconvulsive therapy; feeling guilty; feeling of despair; feelings of worthlessness; major depression; menopausal depression; postpartum depression |
| Dizziness | Dizziness; dizziness exertional; dizziness postural; vertigo; vertigo positional |
| Dry mouth | Aptyalism; dry mouth; dry throat; lip dry |
| Dyspepsia (including reflux) | Abdominal discomfort; dyspepsia; epigastric discomfort; esophageal pain; esophagitis; gastritis; gastroesophageal reflux disease; reflux gastritis; reflux laryngitis |
| Dysphagia | Dysphagia; esophageal hypomotility; malignant dysphagia |
| Dysuria | Dysuria; strangury |
| Gastroesophageal reflux disease | Dyspepsia; esophageal pain; esophagitis; reflux gastritis; gastroesophageal reflux; disease; reflux laryngitis |
| Gastrointestinal obstruction (SMQ gastrointestinal perforation, ulceration, hemorrhage, or obstruction) | Anal stenosis; anastomotic stenosis; anastomotic ulcer, obstructive; anorectal stenosis; barium impaction; colonic obstruction; colonic stenosis; distal ileal obstruction syndrome; distal intestinal obstruction syndrome; duodenal obstruction; duodenal scarring; duodenal stenosis; duodenal ulcer perforation, obstructive; duodenal ulcer, obstructive; esophageal obstruction; esophageal stenosis; fibrosing colonopathy; gastric stenosis; gastric ulcer hemorrhage, obstructive; gastric ulcer perforation, obstructive; gastric ulcer, obstructive; gastrointestinal anastomotic leak; gastrointestinal hypomotility; gastrointestinal motility disorder; gastrointestinal obstruction; gastrointestinal stenosis; ileal stenosis; impaired gastric emptying; intestinal obstruction; intestinal stenosis; jejuna tenosis; large intestinal obstruction; large intestinal obstruction reduction; large intestinal stricture; necrotizing colitis; necrotizing gastritis; necrotizing esophagitis; neonatal intestinal obstruction; obstruction gastric; peptic ulcer perforation, obstructive; peptic ulcer, obstructive; prepyloric stenosis; rectal obstruction; rectal stenosis; small intestinal bacterial overgrowth; small intestinal obstruction; small intestinal stenosis |
| Glaucoma (SMQ glaucoma) (narrow) | Angle closure glaucoma; borderline glaucoma; developmental glaucoma; diabetic glaucoma; fundoscopy abnormal; glaucoma; glaucoma drug therapy; glaucoma surgery; glaucoma traumatic; glaucomatocyclitic crises; glaucomatous optic disc atrophy; gonioscopy abnormal; halo vision; intraocular pressure; fluctuation; intraocular pressure increased; intraocular pressure test abnormal; iridotomy; loss of visual contrast sensitivity; normal tension glaucoma; ocular hypertension; open angle glaucoma; ophthalmic fluid drainage; optic discs blurred; optic nerve cup/disc ratio increased; optic nerve cupping; phacolytic glaucoma; phacotrabeculectomy; pigmentary glaucoma; pupillary light reflex tests abnormal; slit-lamp tests abnormal; trabeculectomy; trabeculoplasty; visual field tests abnormal |
| Headache | Basilar migraine; chronic paroxysmal hemicrania; cluster headache; complicated migraine; drug withdrawal headache; headache; migraine; migraine with aura; migraine without aura; ophthalmoplegic migraine; post-traumatic headache; postictal headache; retinal migraine; SUNCT syndrome; sinus headache; status migrainosus; temporomandibular joint syndrome; tension headache; typical aura without headache |
| Hyperglycemia/new-onset diabetes mellitus (SMQ Hyperglycemia/new-onset diabetes mellitus) (narrow) | Blood 1,5-anhydroglucitol decreased; blood glucose increased; diabetes complicating pregnancy; diabetes mellitus; diabetes mellitus inadequate control; diabetes with hyperosmolarity; diabetic coma; diabetic hyperglycemic coma; diabetic hyperosmolar coma; diabetic ketoacidosis; diabetic ketoacidotic hyperglycemic coma; fructosamine increased; gestational diabetes; glucose tolerance impaired; glucose tolerance impaired in pregnancy; glucose urine present; glycosuria; glycosuria during pregnancy; glycosylated hemoglobin increased; hyperglycemia; hyperglycemic hyperosmolar nonketotic syndrome; impaired fasting glucose; insulin resistance; insulin resistance syndrome; insulin-resistant diabetes; insulin-requiring Type 2 diabetes mellitus; ketoacidosis; ketonuria; ketosis; latent autoimmune diabetes in adults; metabolic syndrome; neonatal diabetes mellitus; pancreatogenous diabetes; Type 1 diabetes mellitus; Type 2 diabetes mellitus; urine ketone body present |
| Hypertension | Accelerated hypertension; blood pressure ambulatory increased; blood pressure diastolic increased; blood pressure inadequately controlled; blood pressure increased; blood pressure orthostatic increased; blood pressure systolic increased; diastolic hypertension; endocrine hypertension; essential hypertension; hypertension; hypertensive angiopathy; hypertensive cardiomegaly; hypertensive cardiomyopathy; hypertensive crisis; hypertensive emergency; hypertensive encephalopathy; hypertensive heart disease; hypertensive nephropathy; labile hypertension; Liddle’s syndrome; malignant hypertension; malignant hypertensive heart disease; malignant renal hypertension; mean arterial pressure increased; neurogenic hypertension; orthostatic hypertension; paradoxical pressor response; procedural hypertension; renal hypertension; renovascular hypertension; secondary hypertension; systolic hypertension |
| Insomnia | Initial insomnia; insomnia; middle insomnia; poor quality sleep; sleep disorder; sleep phase rhythm disturbance; somnambulism |
| Ischemic heart disease (SMQ ischemic heart disease sub-SMQ other ischemic heart disease) (broad) | Angina pectoris; angina unstable; arteriogram coronary abnormal; arteriosclerosis coronary artery; arteriospasm coronary; cardiac stress test abnormal; computerized tomogram; coronary artery abnormal; coronary angioplasty; coronary arterial stent insertion; coronary artery bypass; coronary artery disease; coronary artery dissection; coronary artery insufficiency; coronary artery restenosis; coronary artery stenosis; coronary endarterectomy; coronary no-reflow phenomenon; coronary ostial stenosis; coronary revascularization; dissecting coronary artery aneurysm; electrocardiogram signs of myocardial; electrocardiogram ST segment depression; electrocardiogram ST–T segment abnormal; electrocardiogram ST–T segment depression; electrocardiogram T-wave abnormal; electrocardiogram T-wave inversion; exercise electrocardiogram abnormal; exercise test abnormal; external counterpulsation; hemorrhage coronary artery; in-stent coronary artery restenosis; ischemic cardiomyopathy; microvascular angina; myocardial ischemia; other ischemic heart disease (broad) ischemia; percutaneous coronary intervention; prinzmetal angina; stress cardiomyopathy; stress echocardiogram abnormal; subclavian coronary steal; syndrome; subendocardial ischemia |
| MI (SMQ ischemic heart disease sub-SMQ myocardial infarction) (broad) | Acute coronary syndrome; acute myocardial infarction; blood creatine phosphokinase; MB abnormal; blood creatine phosphokinase; MB increased; blood creatine phosphokinase abnormal; blood creatine phosphokinase increased; cardiac enzymes increased; coronary artery embolism; coronary artery occlusion; coronary artery reocclusion; coronary artery thrombosis; coronary bypass thrombosis; electrocardiogram electrically inactive area; electrocardiogram Q-wave abnormal; electrocardiogram ST segment abnormal; electrocardiogram ST segment elevation; electrocardiogram ST–T segment elevation; infarction; Kounis syndrome; myocardial infarction; myocardial reperfusion injury; myocardial stunning; papillary muscle infarction; postprocedural myocardial infarction; postinfarction angina; scan myocardial perfusion abnormal; silent myocardial infarction; troponin I increased; troponin T increased; troponin increased; vascular graft occlusion |
| Palpitations | Extrasystoles; palpitations; supraventricular extrasystoles; ventricular extrasystoles |
| Pneumonia | Atypical mycobacterial pneumonia; bronchopneumonia; congenital pneumonia; embolic pneumonia; Enterobacter pneumonia; lobar pneumonia; miliary pneumonia; neonatal pneumonia; pneumonia; pneumonia adenoviral; pneumonia anthrax; pneumonia bacterial; pneumonia blastomyces; pneumonia Bordetella; pneumonia Chlamydial; pneumonia Cryptococcal; pneumonia Cytomegaloviral; pneumonia Escherichia; pneumonia fungal; pneumonia hemophilus; pneumonia helminthic; pneumonia herpes viral; pneumonia influenzal; pneumonia Klebsiella; pneumonia Legionella; pneumonia measles; pneumonia Moraxella; pneumonia mycoplasmal; pneumonia necrotizing; pneumonia parainfluenzae viral; pneumonia pneumococcal; pneumonia primary atypical; pneumonia respiratory syncytial viral; pneumonia Salmonella; pneumonia Staphylococcal; pneumonia Streptococcal; pneumonia toxoplasmal; pneumonia Tularemia; pneumonia viral; post procedural pneumonia |
| Renal failure | Acute prerenal failure; anuria; cardiorenal syndrome; diabetic end-stage renal disease; hepatorenal failure; postoperative renal failure; renal failure; renal failure acute; renal failure chronic |
| Respiratory failure | Acute respiratory failure; cardiopulmonary failure; chronic respiratory failure; hypoxia; respiratory acidosis; respiratory failure; respiratory paralysis |
| Restlessness | Agitation; psychomotor hyperactivity; restlessness |
| Sleep disturbance | Abnormal dreams; insomnia; nightmare; poor quality sleep; sedation; sleep disorder; sleep phase rhythm disturbance; sleep talking; somnambulism; somnolence |
| Stomatitis | Aphthous stomatitis; mouth ulceration; oral mucosal eruption; oropharyngeal blistering; stomatitis; stomatitis hemorrhagic; stomatitis necrotizing |
| Stroke | Amaurosis fugax; basal ganglia hemorrhage; basilar artery occlusion; basilar artery thrombosis; brachiocephalic artery occlusion; brain stem hemorrhage; brain stem infarction; brain stem ischemia; brain stem stroke; brain stem thrombosis; carotid aneurysm rupture; carotid arterial embolus; carotid artery occlusion; carotid artery thrombosis; central nervous system hemorrhage; cerebellar artery occlusion; cerebellar artery thrombosis; cerebellar embolism; cerebellar hematoma; cerebellar hemorrhage; cerebellar infarction; cerebellar ischemia; cerebral arteriovenous malformation hemorrhagic; cerebral artery embolism; cerebral artery occlusion; cerebral artery thrombosis; cerebral hematoma; cerebral hemorrhage; cerebral hemorrhage fetal; cerebral hemorrhage neonatal; cerebral infarction; cerebral infarction fetal; cerebral ischemia; cerebral thrombosis; cerebrovascular accident; embolic cerebral infarction; embolic stroke; hemorrhage intracranial; hemorrhagic cerebral infarction; hemorrhagic stroke; hemorrhagic transformation stroke; intracranial hematoma; intracranial tumor hemorrhage; intraoperative cerebral artery occlusion; intraventricular hemorrhage; intraventricular hemorrhage neonatal; ischemic cerebral infarction; ischemic stroke; lacunar infarction; lateral medullary syndrome; pituitary hemorrhage; pituitary infarction; postprocedural stroke; precerebral artery occlusion; putamen hemorrhage; reversible ischemic neurological deficit; ruptured cerebral aneurysm; stroke in evolution; subarachnoid hemorrhage; subarachnoid hemorrhage neonatal; subdural hemorrhage neonatal; thalamic infarction; thalamus hemorrhage; thrombotic cerebral infarction; thrombotic stroke; transient ischemic attack; vertebral artery dissection; vertebral artery occlusion; vertebral artery thrombosis |
| Supraventricular tachycardia | Atrial tachycardia; postural orthostatic tachycardia syndrome; sinus tachycardia; supraventricular tachycardia; tachycardia paroxysmal |
| Syncope | Loss of consciousness; presyncope; syncope |
| Tachycardia | Heart rate increased; sinus tachycardia; tachycardia |
| Urinary retention | Bladder neck obstruction; residual urine volume increased; urinary hesitation; urinary retention; urinary retention postoperative; urinary tract obstruction; urine flow decreased |
| Urinary tract infection | Adenoviral hemorrhagic; cystitis; asymptomatic bacteriuria; bacterial pyelonephritis; bacteriuria; bladder candidiasis; cystitis; cystitis Escherichia; cystitis glandularis; cystitis gonococcal; cystitis hemorrhagic; cystitis interstitial; cystitis Klebsiella; cystitis noninfective; cystitis pseudomonal; cystitis radiation; cystitis ulcerative; cytomegalovirus urinary tract infection; emphysematous cystitis; eosinophilic cystitis; Escherichia urinary tract infection; fungal cystitis; genitourinary tract infection; kidney infection; perinephric abscess; pyelocystitis; pyelonephritis; pyelonephritis acute; pyelonephritis chronic; pyelonephritis mycoplasmal; pyonephrosis; renal abscess; renal cyst infection; schistosomiasis bladder; Streptococcal urinary tract infection; trigonitis; tuberculosis bladder; ureter abscess; ureteritis; urethral abscess; urethral carbuncle; urethral stricture post infection; urethritis; urinary bladder abscess; urinary tract abscess; urinary tract infection; urinary tract infection bacterial; urinary tract infection enterococcal; urinary tract infection fungal; urinary tract infection neonatal; urinary tract infection pseudomonal; urinary tract infection staphylococcal; urosepsis; viral hemorrhagic cystitis |
| Ventricular tachycardia/fibrillation | Ventricular fibrillation; ventricular tachycardia |
| Vision blurred | Accommodation disorder; altered visual depth perception; diplopia; erythropsia; glare; halo vision; hypermetropia; loss of visual contrast sensitivity; malignant myopia; myopia; night blindness; oscillopsia; phosphenes; photopsia; presbyopia; refraction disorder; tunnel vision |
Abbreviations: MB, creatine kinase-MB; MedDRA, Medical Dictionary for Regulatory Activities; MI, myocardial infarction; PT, preferred term; PV, pharmacovigilance; SMQ, standardized MedDRA queries.
Acknowledgments
The authors would like to thank Dr Inge Leimer and Dr Katrin Kupas for their assistance with the statistical analysis. Writing assistance was provided by Sarah J Petit and Natalie Dennis of PAREXEL, funded jointly by Boehringer Ingelheim and Pfizer. The abstract of this paper was presented at the American Thoracic Society International Conference 2014 as a poster presentation with interim findings. The poster’s abstract was published within “Conference Abstracts” of ATS Journals: D44. COPD: Treatment from Bronchodilators to Other Pharmacological Interventions, May 1, 2014, A5973-A5973; http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A5973. The actual paper, however, has never been published.
Author contributions
DMGH, RD, DT, and CH participated in the study design; AM participated in data collection and data analysis. All authors contributed toward drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr David MG Halpin has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, GSK, Intermune, Novartis, and Pfizer, and nonfinancial support from Boehringer Ingelheim and Novartis. Dr Ronald Dahl has received consulting fees, lecture fees, and grant support from Boehringer Ingelheim and Novartis. Dr Chistoph Hallmann and Dr Achim Mueller are employees of Boehringer Ingelheim. Dr Donald Tashkin has received grants or personal fees for consulting, speaking, or acting on the advisory board from Astra Zeneca, Boehringer Ingelheim, Forest, GlaxoSmithKline, Novartis, Pfizer, Sunovion, and Theravance. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. GOLD; 2014. [Accessed December 15, 2014]. updated. Available from: http://www.goldcopd.com/uploads/users/files/GOLD_Report_2014_Oct30.pdf. [Google Scholar]
- 2.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125:249–259. doi: 10.1378/chest.125.1.249. [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 6.Vincken W, van Noord JA, Greefhorst AP, et al. Dutch/Belgian Tiotropium Study Group Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 7.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–555. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 8.Halpin D, Menjoge S, Viel K. Patient-level pooled analysis of the effect of tiotropium on COPD exacerbations and related hospitalisations. Prim Care Respir J. 2009;18:106–113. doi: 10.4104/pcrj.2009.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 10.Boehringer Ingelheim Spiriva 18 microgram inhalation powder, hard capsule. Summary of Product Characteristics (SPC), Electonic Medicines Compendium (EMC) [Accessed July 19, 2013]. Available from: http://www.medicines.org.uk/emc/medicine/10039/SPC/Spiriva+18+microgram+inhalation+powder%2c+hard+capsule/
- 11.Boehringer Ingelheim Spiriva Respimat 2.5 microgram solution for inhalation. Summary of Product Characteristics (SPC), Electonic Medicines Compendium (EMC) [Accessed July 19, 2013]. Available from: http://www.medicines.org.uk/emc/medicine/20134/SPC.
- 12.Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104:1460–1472. doi: 10.1016/j.rmed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose M, Fujimoto T, Fukuchi Y. Tiotropium 5microg via Respimat and 18microg via HandiHaler; efficacy and safety in Japanese COPD patients. Respir Med. 2010;104:228–236. doi: 10.1016/j.rmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.van Noord JA, Cornelissen PJ, Aumann JL, Platz J, Mueller A, Fogarty C. The efficacy of tiotropium administered via Respimat Soft Mist inhaler or HandiHaler in COPD patients. Respir Med. 2009;103:22–29. doi: 10.1016/j.rmed.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Hohlfeld JM, Sharma A, van Noord JA, et al. Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol. 2014;54(4):405–414. doi: 10.1002/jcph.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehringer Ingelheim . Boehringer Ingelheim submits application in Europe to extend the indication for the use of tiotropium Respimat® to the treatment of asthma in adults aged 18 years and over [press release archive: asthma] Ingelheim, Germany: Boehringer Ingelheim; 2013. Nov 13, [Accessed December 15, 2014]. Available from: http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2013/13_november_2013_tiotropium.html. [Google Scholar]
- 17.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, UPLIFT Study Investigators Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:948–955. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 18.Boehringer Ingelheim . Tiotropium (Spiriva) Respimat: evaluation of fatal events. Ingelheim, Germany: Boehringer Ingelheim; [Accessed January 30, 2014]. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/Pooled%20analysis/PA_205.372_251_252_254_255_U10-3255-01.pdf. [Google Scholar]
- 19.Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax. 2013;68:48–56. doi: 10.1136/thoraxjnl-2012-201926. [DOI] [PubMed] [Google Scholar]
- 20.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD009285. doi: 10.1002/14651858.CD009285.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Loke YK, Singh S. Risks associated with tiotropium in chronic obstructive pulmonary disease: overview of the evidence to date. Ther Adv Drug Safe. 2012;3:123–131. doi: 10.1177/2042098612438388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2011;342:d3215. doi: 10.1136/bmj.d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr RG, Bourbeau J, Camargo CA, Ram FS. Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2006;61:854–862. doi: 10.1136/thx.2006.063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137:20–30. doi: 10.1378/chest.09-0011. [DOI] [PubMed] [Google Scholar]
- 25.Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695–1703. doi: 10.1378/chest.130.6.1695. [DOI] [PubMed] [Google Scholar]
- 26.Kesten S, Celli B, Decramer M, Leimer I, Tashkin D. Tiotropium HandiHaler in the treatment of COPD: a safety review. Int J Chron Obstruct Pulmon Dis. 2009;4:397–409. doi: 10.2147/copd.s4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo GJ, Castro-Rodriguez JA, Nannini LJ, Plaza Moral V, Schiavi EA. Tiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: systematic review with meta-analysis. Respir Med. 2009;103:1421–1429. doi: 10.1016/j.rmed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Salpeter SR. Do inhaled anticholinergics increase or decrease the risk of major cardiovascular events?: a synthesis of the available evidence. Drugs. 2009;69:2025–2033. doi: 10.2165/11318580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Wise RA, Anzueto A, Cotton D, et al. TIOSPIR Investigators Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 30.ICH harmonised tripartite guidelines. Clinical safety data management: definitions and standards for expidited reporting E2A; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; October 27; 1994. [Google Scholar]
- 31.Tashkin DP, Metzdorf N, Hallman C, Koenen-Bergmann M, Kupas K, Dahl R. Safety of tiotropium in renally impaired patients. Eur Respir J. 2014;44(Suppl 58) Abstract 923. [Google Scholar]
- 32.Jenkins CR. More than just reassurance on tiotropium safety. N Engl J Med. 2013;369(16):1555–1556. doi: 10.1056/NEJMe1310107. [DOI] [PubMed] [Google Scholar]
- 33.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149:380–390. doi: 10.7326/0003-4819-149-6-200809160-00004. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–1450. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 35.Voshaar T, Lapidus R, Maleki-Yazdi R, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs ipratropium pMDI in COPD. Respir Med. 2008;102:32–41. doi: 10.1016/j.rmed.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Bateman E, Singh D, Smith D, et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 37.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, INSPIRE Investigators The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 38.Keating GM. Tiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary disease. Drugs. 2012;72:273–300. doi: 10.2165/11208620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Church A, Beerahee M, Brooks J, Mehta R, Shah P. Dose response of umeclidinium administered once or twice daily in patients with COPD: a randomised cross-over study. BMC Pulm Med. 2014;14:2. doi: 10.1186/1471-2466-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 41.Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III follow-up study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannino DM, Doherty DE, Buist SA. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100:115–122. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax. 2003;58:855–860. doi: 10.1136/thorax.58.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNicholas WT, Calverley PM, Lee A, Edwards JC. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23:825–831. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 45.Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 47.Verkindre C, Bart F, Aguilaniu B, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration. 2006;73:420–427. doi: 10.1159/000089655. [DOI] [PubMed] [Google Scholar]
- 48.Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–1748. doi: 10.1378/chest.124.5.1743. [DOI] [PubMed] [Google Scholar]
- 49.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 50.Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127:809–817. doi: 10.1378/chest.127.3.809. [DOI] [PubMed] [Google Scholar]
- 51.Ambrosino N, Foglio K, Balzano G, Paggiaro PL, Lessi P, Kesten S, Tiotropium Multicentric Italian Study Group Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance. Int J Chron Obstruct Pulmon Dis. 2008;3:771–780. doi: 10.2147/copd.s3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M, TIPHON study group Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:301–310. doi: 10.2147/copd.s2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beeh KM, Beier J, Buhl R, Stark-Lorenzen P, Gerken F, Metzdorf N, ATEM-Studiegruppe Efficacy of tiotropium bromide (Spiriva) in patients with chronic-obstructive pulmonary disease (COPD) of different severities. Pneumologie. 2006;60:341–346. doi: 10.1055/s-2005-919145. [DOI] [PubMed] [Google Scholar]
- 54.Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT, SAFE Study Group A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J. 2007;14:465–472. doi: 10.1155/2007/192961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powrie DJ, Wilkinson TM, Donaldson GC, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30:472–478. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 56.Freeman D, Lee A, Price D. Efficacy and safety of tiotropium in COPD patients in primary care–the SPiRiva Usual CarE (SPRUCE) study. Respir Res. 2007;8:45. doi: 10.1186/1465-9921-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansson G, Lindberg A, Romberg K, Nordstrom L, Gerken F, Roquet A. Bronchodilator efficacy of tiotropium in patients with mild to moderate COPD. Prim Care Respir J. 2008;17:169–175. doi: 10.3132/pcrj.2008.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moita J, Barbara C, Cardoso J, et al. Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulm Pharmacol Ther. 2008;21:146–151. doi: 10.1016/j.pupt.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy. 2005;25:1708–1718. doi: 10.1592/phco.2005.25.12.1708. [DOI] [PubMed] [Google Scholar]
- 60.Criner GJ, Sharafkhaneh A, Player R, et al. Efficacy of tiotropium inhalation powder in african-american patients with chronic obstructive pulmonary disease. COPD. 2008;5:35–41. doi: 10.1080/15412550701815981. [DOI] [PubMed] [Google Scholar]
- 61.Magnussen H, Bugnas B, van NJ, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respir Med. 2008;102:50–56. doi: 10.1016/j.rmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Sciurba FC, Siafakas N, Troosters T, et al. The efficacy of safety of tiotropium HandiHaler®, 18 ìg, once daily plys prn salbutamol versus placebo plus prn salbutamolin COPD subjects naïve to maintenance therapy; American Thoracic Society International Conference; 2011 May 13–18; Denver, CO. Abstract 21005. [Google Scholar]
- 63.Cooper CB, Celli BR, Jardim JR, et al. Treadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomized trial. Chest. 2013;144:490–497. doi: 10.1378/chest.12-2613. [DOI] [PubMed] [Google Scholar]
- 64.Littner MR, van NJ, Moroni-Zentgraf P, Sigmund R, Joseph E, Karpel J. Phase IIB dose-finding study of BEA2180 via Respimat® in patients with chronic obstructive pulmonary disease (COPD) Respirology. 2012;17(Suppl 2):44. [Google Scholar]
- 65.Abrahams R, Moroni-Zentgraf P, Ramsdell J, Schmidt H, Joseph E, Karpel J. Safety and efficacy of the once-daily anticholinergic BEA2180 compared with tiotropium in patients with COPD. Respir Med. 2013;107:854–862. doi: 10.1016/j.rmed.2013.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
PV endpoint definition
| PV endpoint/SMQ | MedDRA PT |
|---|---|
| Abdominal pain | Abdominal pain; abdominal pain lower; abdominal pain upper; abdominal rebound tenderness; abdominal tenderness; epigastric discomfort; gastrointestinal pain |
| Aneurysm | Aneurysm; aneurysm arteriovenous; aneurysm ruptured; aortic aneurysm; aortic aneurysm rupture; aortic aneurysm syphilitic; aortic dissection; aortic dissection rupture; aortic intramural hematoma; aortic stent insertion; artery dissection; cardiac aneurysm; carotid aneurysm rupture; cerebral aneurysm ruptured syphilitic; Charcot–Bouchard microaneurysms; coronary artery aneurysm; coronary artery dissection; dissecting coronary artery aneurysm; femoral artery aneurysm; femoral artery dissection; hemorrhage coronary artery; hepatic artery aneurysm; infective aneurysm; intracranial aneurysm; mycotic aneurysm; peripheral artery aneurysm; peripheral artery dissection; pulmonary artery aneurysm; renal aneurysm; renal artery dissection; retinal aneurysm; ruptured cerebral aneurysm; splenic artery aneurysm; subclavian artery aneurysm; superior vena cava dilatation; venous aneurysm |
| Anxiety symptoms/fears | Acrophobia; activation syndrome; acute stress disorder; agitation; agitation neonatal; agoraphobia; animal phobia; anticipatory anxiety; anxiety; anxiety disorder; anxiety disorder due to a general medical condition; arachnophobia; autophobia; Burnout syndrome; claustrophobia; compulsions; compulsive hand washing; dysmorphophobia; emetophobia; fear; fear of animals; fear of closed spaces; fear of crowded places; fear of death; fear of disease; fear of eating; fear of falling; fear of needles; fear of open spaces; fear of pregnancy; fear of weight gain; generalized anxiety disorder; haphephobia; hydrophobia; limited symptom panic attack; nervousness; neurosis; noctiphobia; nocturnal fear; nosophobia; obsessive thoughts; obsessive-compulsive disorder; ochlophobia; osmophobia; pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection; panic attack; panic disorder; panic disorder with agoraphobia; panic disorder without agoraphobia; panic reaction; paruresis; performance fear; phagophobia; pharmacophobia; phobia; phobia of driving; phobia of exams; phobia of flying; phobic avoidance; phonophobia; photaugiaphobia; post-traumatic stress disorder; postpartum neurosis; postpartum stress disorder; social fear; social phobia; stress; tension; thanatophobia |
| Cardiac arrest | Atrioventricular dissociation; cardiac arrest; cardiac death; cardiac massage; cardiorespiratory arrest; cardiogenic shock; pulseless electrical activity; resuscitation |
| Cardiac failure (SMQ cardiac failure [narrow]) | Acute left ventricular failure; acute pulmonary edema; acute right ventricular failure; cardiac asthma; cardiac failure; cardiac failure acute; cardiac failure chronic; cardiac failure congestive; cardiac failure high output; cardiogenic shock; cardiopulmonary failure; cardiorenal syndrome; chronic left ventricular failure; chronic right ventricular failure; cor pulmonale; cor pulmonale acute; cor pulmonale chronic; ejection fraction decreased; hepatic congestion; hepatojugular reflux; left ventricular failure; low cardiac output syndrome; neonatal cardiac failure; pulmonary edema; pulmonary edema neonatal; right ventricular failure; ventricular failure |
| Cognitive impairment | Cognitive disorder |
| Confusion | Confusion postoperative; confusional state; disorientation |
| Constipation | Colonic pseudo-obstruction; constipation; feces hard; infrequent bowel movements; postprocedural constipation |
| COPD exacerbation | Chronic obstructive pulmonary disease; infective exacerbation of chronic obstructive airways disease; obstructive airways disorder |
| Dehydration | Dehydration |
| Depression and self-injury (SMQ depression and self-injury (excluding suicide and self-injury) (narrow) | Activation syndrome; adjustment disorder with depressed mood; adjustment disorder with mixed anxiety and depressed mood; agitated depression; anhedonia; antidepressant therapy; childhood depression; decreased interest; depressed mood; depression; depression postoperative; depressive symptom; dysphoria; dysthymic disorder; electroconvulsive therapy; feeling guilty; feeling of despair; feelings of worthlessness; major depression; menopausal depression; postpartum depression |
| Dizziness | Dizziness; dizziness exertional; dizziness postural; vertigo; vertigo positional |
| Dry mouth | Aptyalism; dry mouth; dry throat; lip dry |
| Dyspepsia (including reflux) | Abdominal discomfort; dyspepsia; epigastric discomfort; esophageal pain; esophagitis; gastritis; gastroesophageal reflux disease; reflux gastritis; reflux laryngitis |
| Dysphagia | Dysphagia; esophageal hypomotility; malignant dysphagia |
| Dysuria | Dysuria; strangury |
| Gastroesophageal reflux disease | Dyspepsia; esophageal pain; esophagitis; reflux gastritis; gastroesophageal reflux; disease; reflux laryngitis |
| Gastrointestinal obstruction (SMQ gastrointestinal perforation, ulceration, hemorrhage, or obstruction) | Anal stenosis; anastomotic stenosis; anastomotic ulcer, obstructive; anorectal stenosis; barium impaction; colonic obstruction; colonic stenosis; distal ileal obstruction syndrome; distal intestinal obstruction syndrome; duodenal obstruction; duodenal scarring; duodenal stenosis; duodenal ulcer perforation, obstructive; duodenal ulcer, obstructive; esophageal obstruction; esophageal stenosis; fibrosing colonopathy; gastric stenosis; gastric ulcer hemorrhage, obstructive; gastric ulcer perforation, obstructive; gastric ulcer, obstructive; gastrointestinal anastomotic leak; gastrointestinal hypomotility; gastrointestinal motility disorder; gastrointestinal obstruction; gastrointestinal stenosis; ileal stenosis; impaired gastric emptying; intestinal obstruction; intestinal stenosis; jejuna tenosis; large intestinal obstruction; large intestinal obstruction reduction; large intestinal stricture; necrotizing colitis; necrotizing gastritis; necrotizing esophagitis; neonatal intestinal obstruction; obstruction gastric; peptic ulcer perforation, obstructive; peptic ulcer, obstructive; prepyloric stenosis; rectal obstruction; rectal stenosis; small intestinal bacterial overgrowth; small intestinal obstruction; small intestinal stenosis |
| Glaucoma (SMQ glaucoma) (narrow) | Angle closure glaucoma; borderline glaucoma; developmental glaucoma; diabetic glaucoma; fundoscopy abnormal; glaucoma; glaucoma drug therapy; glaucoma surgery; glaucoma traumatic; glaucomatocyclitic crises; glaucomatous optic disc atrophy; gonioscopy abnormal; halo vision; intraocular pressure; fluctuation; intraocular pressure increased; intraocular pressure test abnormal; iridotomy; loss of visual contrast sensitivity; normal tension glaucoma; ocular hypertension; open angle glaucoma; ophthalmic fluid drainage; optic discs blurred; optic nerve cup/disc ratio increased; optic nerve cupping; phacolytic glaucoma; phacotrabeculectomy; pigmentary glaucoma; pupillary light reflex tests abnormal; slit-lamp tests abnormal; trabeculectomy; trabeculoplasty; visual field tests abnormal |
| Headache | Basilar migraine; chronic paroxysmal hemicrania; cluster headache; complicated migraine; drug withdrawal headache; headache; migraine; migraine with aura; migraine without aura; ophthalmoplegic migraine; post-traumatic headache; postictal headache; retinal migraine; SUNCT syndrome; sinus headache; status migrainosus; temporomandibular joint syndrome; tension headache; typical aura without headache |
| Hyperglycemia/new-onset diabetes mellitus (SMQ Hyperglycemia/new-onset diabetes mellitus) (narrow) | Blood 1,5-anhydroglucitol decreased; blood glucose increased; diabetes complicating pregnancy; diabetes mellitus; diabetes mellitus inadequate control; diabetes with hyperosmolarity; diabetic coma; diabetic hyperglycemic coma; diabetic hyperosmolar coma; diabetic ketoacidosis; diabetic ketoacidotic hyperglycemic coma; fructosamine increased; gestational diabetes; glucose tolerance impaired; glucose tolerance impaired in pregnancy; glucose urine present; glycosuria; glycosuria during pregnancy; glycosylated hemoglobin increased; hyperglycemia; hyperglycemic hyperosmolar nonketotic syndrome; impaired fasting glucose; insulin resistance; insulin resistance syndrome; insulin-resistant diabetes; insulin-requiring Type 2 diabetes mellitus; ketoacidosis; ketonuria; ketosis; latent autoimmune diabetes in adults; metabolic syndrome; neonatal diabetes mellitus; pancreatogenous diabetes; Type 1 diabetes mellitus; Type 2 diabetes mellitus; urine ketone body present |
| Hypertension | Accelerated hypertension; blood pressure ambulatory increased; blood pressure diastolic increased; blood pressure inadequately controlled; blood pressure increased; blood pressure orthostatic increased; blood pressure systolic increased; diastolic hypertension; endocrine hypertension; essential hypertension; hypertension; hypertensive angiopathy; hypertensive cardiomegaly; hypertensive cardiomyopathy; hypertensive crisis; hypertensive emergency; hypertensive encephalopathy; hypertensive heart disease; hypertensive nephropathy; labile hypertension; Liddle’s syndrome; malignant hypertension; malignant hypertensive heart disease; malignant renal hypertension; mean arterial pressure increased; neurogenic hypertension; orthostatic hypertension; paradoxical pressor response; procedural hypertension; renal hypertension; renovascular hypertension; secondary hypertension; systolic hypertension |
| Insomnia | Initial insomnia; insomnia; middle insomnia; poor quality sleep; sleep disorder; sleep phase rhythm disturbance; somnambulism |
| Ischemic heart disease (SMQ ischemic heart disease sub-SMQ other ischemic heart disease) (broad) | Angina pectoris; angina unstable; arteriogram coronary abnormal; arteriosclerosis coronary artery; arteriospasm coronary; cardiac stress test abnormal; computerized tomogram; coronary artery abnormal; coronary angioplasty; coronary arterial stent insertion; coronary artery bypass; coronary artery disease; coronary artery dissection; coronary artery insufficiency; coronary artery restenosis; coronary artery stenosis; coronary endarterectomy; coronary no-reflow phenomenon; coronary ostial stenosis; coronary revascularization; dissecting coronary artery aneurysm; electrocardiogram signs of myocardial; electrocardiogram ST segment depression; electrocardiogram ST–T segment abnormal; electrocardiogram ST–T segment depression; electrocardiogram T-wave abnormal; electrocardiogram T-wave inversion; exercise electrocardiogram abnormal; exercise test abnormal; external counterpulsation; hemorrhage coronary artery; in-stent coronary artery restenosis; ischemic cardiomyopathy; microvascular angina; myocardial ischemia; other ischemic heart disease (broad) ischemia; percutaneous coronary intervention; prinzmetal angina; stress cardiomyopathy; stress echocardiogram abnormal; subclavian coronary steal; syndrome; subendocardial ischemia |
| MI (SMQ ischemic heart disease sub-SMQ myocardial infarction) (broad) | Acute coronary syndrome; acute myocardial infarction; blood creatine phosphokinase; MB abnormal; blood creatine phosphokinase; MB increased; blood creatine phosphokinase abnormal; blood creatine phosphokinase increased; cardiac enzymes increased; coronary artery embolism; coronary artery occlusion; coronary artery reocclusion; coronary artery thrombosis; coronary bypass thrombosis; electrocardiogram electrically inactive area; electrocardiogram Q-wave abnormal; electrocardiogram ST segment abnormal; electrocardiogram ST segment elevation; electrocardiogram ST–T segment elevation; infarction; Kounis syndrome; myocardial infarction; myocardial reperfusion injury; myocardial stunning; papillary muscle infarction; postprocedural myocardial infarction; postinfarction angina; scan myocardial perfusion abnormal; silent myocardial infarction; troponin I increased; troponin T increased; troponin increased; vascular graft occlusion |
| Palpitations | Extrasystoles; palpitations; supraventricular extrasystoles; ventricular extrasystoles |
| Pneumonia | Atypical mycobacterial pneumonia; bronchopneumonia; congenital pneumonia; embolic pneumonia; Enterobacter pneumonia; lobar pneumonia; miliary pneumonia; neonatal pneumonia; pneumonia; pneumonia adenoviral; pneumonia anthrax; pneumonia bacterial; pneumonia blastomyces; pneumonia Bordetella; pneumonia Chlamydial; pneumonia Cryptococcal; pneumonia Cytomegaloviral; pneumonia Escherichia; pneumonia fungal; pneumonia hemophilus; pneumonia helminthic; pneumonia herpes viral; pneumonia influenzal; pneumonia Klebsiella; pneumonia Legionella; pneumonia measles; pneumonia Moraxella; pneumonia mycoplasmal; pneumonia necrotizing; pneumonia parainfluenzae viral; pneumonia pneumococcal; pneumonia primary atypical; pneumonia respiratory syncytial viral; pneumonia Salmonella; pneumonia Staphylococcal; pneumonia Streptococcal; pneumonia toxoplasmal; pneumonia Tularemia; pneumonia viral; post procedural pneumonia |
| Renal failure | Acute prerenal failure; anuria; cardiorenal syndrome; diabetic end-stage renal disease; hepatorenal failure; postoperative renal failure; renal failure; renal failure acute; renal failure chronic |
| Respiratory failure | Acute respiratory failure; cardiopulmonary failure; chronic respiratory failure; hypoxia; respiratory acidosis; respiratory failure; respiratory paralysis |
| Restlessness | Agitation; psychomotor hyperactivity; restlessness |
| Sleep disturbance | Abnormal dreams; insomnia; nightmare; poor quality sleep; sedation; sleep disorder; sleep phase rhythm disturbance; sleep talking; somnambulism; somnolence |
| Stomatitis | Aphthous stomatitis; mouth ulceration; oral mucosal eruption; oropharyngeal blistering; stomatitis; stomatitis hemorrhagic; stomatitis necrotizing |
| Stroke | Amaurosis fugax; basal ganglia hemorrhage; basilar artery occlusion; basilar artery thrombosis; brachiocephalic artery occlusion; brain stem hemorrhage; brain stem infarction; brain stem ischemia; brain stem stroke; brain stem thrombosis; carotid aneurysm rupture; carotid arterial embolus; carotid artery occlusion; carotid artery thrombosis; central nervous system hemorrhage; cerebellar artery occlusion; cerebellar artery thrombosis; cerebellar embolism; cerebellar hematoma; cerebellar hemorrhage; cerebellar infarction; cerebellar ischemia; cerebral arteriovenous malformation hemorrhagic; cerebral artery embolism; cerebral artery occlusion; cerebral artery thrombosis; cerebral hematoma; cerebral hemorrhage; cerebral hemorrhage fetal; cerebral hemorrhage neonatal; cerebral infarction; cerebral infarction fetal; cerebral ischemia; cerebral thrombosis; cerebrovascular accident; embolic cerebral infarction; embolic stroke; hemorrhage intracranial; hemorrhagic cerebral infarction; hemorrhagic stroke; hemorrhagic transformation stroke; intracranial hematoma; intracranial tumor hemorrhage; intraoperative cerebral artery occlusion; intraventricular hemorrhage; intraventricular hemorrhage neonatal; ischemic cerebral infarction; ischemic stroke; lacunar infarction; lateral medullary syndrome; pituitary hemorrhage; pituitary infarction; postprocedural stroke; precerebral artery occlusion; putamen hemorrhage; reversible ischemic neurological deficit; ruptured cerebral aneurysm; stroke in evolution; subarachnoid hemorrhage; subarachnoid hemorrhage neonatal; subdural hemorrhage neonatal; thalamic infarction; thalamus hemorrhage; thrombotic cerebral infarction; thrombotic stroke; transient ischemic attack; vertebral artery dissection; vertebral artery occlusion; vertebral artery thrombosis |
| Supraventricular tachycardia | Atrial tachycardia; postural orthostatic tachycardia syndrome; sinus tachycardia; supraventricular tachycardia; tachycardia paroxysmal |
| Syncope | Loss of consciousness; presyncope; syncope |
| Tachycardia | Heart rate increased; sinus tachycardia; tachycardia |
| Urinary retention | Bladder neck obstruction; residual urine volume increased; urinary hesitation; urinary retention; urinary retention postoperative; urinary tract obstruction; urine flow decreased |
| Urinary tract infection | Adenoviral hemorrhagic; cystitis; asymptomatic bacteriuria; bacterial pyelonephritis; bacteriuria; bladder candidiasis; cystitis; cystitis Escherichia; cystitis glandularis; cystitis gonococcal; cystitis hemorrhagic; cystitis interstitial; cystitis Klebsiella; cystitis noninfective; cystitis pseudomonal; cystitis radiation; cystitis ulcerative; cytomegalovirus urinary tract infection; emphysematous cystitis; eosinophilic cystitis; Escherichia urinary tract infection; fungal cystitis; genitourinary tract infection; kidney infection; perinephric abscess; pyelocystitis; pyelonephritis; pyelonephritis acute; pyelonephritis chronic; pyelonephritis mycoplasmal; pyonephrosis; renal abscess; renal cyst infection; schistosomiasis bladder; Streptococcal urinary tract infection; trigonitis; tuberculosis bladder; ureter abscess; ureteritis; urethral abscess; urethral carbuncle; urethral stricture post infection; urethritis; urinary bladder abscess; urinary tract abscess; urinary tract infection; urinary tract infection bacterial; urinary tract infection enterococcal; urinary tract infection fungal; urinary tract infection neonatal; urinary tract infection pseudomonal; urinary tract infection staphylococcal; urosepsis; viral hemorrhagic cystitis |
| Ventricular tachycardia/fibrillation | Ventricular fibrillation; ventricular tachycardia |
| Vision blurred | Accommodation disorder; altered visual depth perception; diplopia; erythropsia; glare; halo vision; hypermetropia; loss of visual contrast sensitivity; malignant myopia; myopia; night blindness; oscillopsia; phosphenes; photopsia; presbyopia; refraction disorder; tunnel vision |
Abbreviations: MB, creatine kinase-MB; MedDRA, Medical Dictionary for Regulatory Activities; MI, myocardial infarction; PT, preferred term; PV, pharmacovigilance; SMQ, standardized MedDRA queries.