Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a major public health problem worldwide. The aim of this study was to evaluate the rate of optic neuropathy in COPD patients.
Methods
Forty patients diagnosed with COPD and 60 healthy subjects as control group enrolled. After examination by a pulmonary subspecialist, patients were ranked by Global initiative for chronic Obstructive Lung Disease (GOLD) criteria, and patients with zero grades on GOLD criteria were excluded. Visual evoked potential by checkerboard (raster background) method with a frequency of 2 Hz were done for all participants. P-values less than 0.05 were considered as significant.
Results
Fifty-five percent of COPD patients had visual evoked potential abnormalities. Mean P100 latency in both eyes was significantly longer in COPD patients. Average P100/N140 amplitude in both eyes were insignificantly higher in COPD.
Conclusion
Higher P100 latency in COPD patients shows demyelinating type of optic nerve involvement; however, further investigation in this area is needed.
Keywords: visual evoked potential, neuropathy, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is a main cause of mortality in both developed and developing countries. COPD is a respiratory disease characterized by progressive and partially reversible airflow limitation, which results from an emphysematous destruction of the lung parenchyma and increased airway resistance due to inflammation, bronchospasm, and increased mucous production.1 There is an increasing need for awareness regarding COPD and to help the thousands of people who suffer from this disease and die prematurely from COPD or its associated complication(s). As a result, a committed group of scientists encouraged the US National Heart, Lung, and Blood Institute and the World Health Organization to form a Global initiative for chronic Obstructive Lung Disease (GOLD).2
Cardiovascular problems (eg, coronary heart disease), neurological impairments (eg, polyneuropathy), and metabolic disorders (eg, osteoporosis) are also commonly associated with COPD as are psychological alterations such as depression and anxiety.3 While the presentation, development, and manifestations of COPD are highly variable, as the patient ages, the disease typically gets more severe.
Many studies have shown peripheral neuropathy as an extra pulmonary manifestation of COPD.4,5 Many patients with COPD suffer subclinical neuropathy, and this type of neuropathy is correlated with cigarette consumption.6
Visual evoked potentials (VEP), the electrical potential difference generated in response to visual stimuli, provide a functional measurement of optical pathway. Moreover, this evaluation can provide valuable evidence of optic nerve involvement in the primary stages of ophthalmic disease.7,8 It has been recently declared that COPD may cause considerable retinal and optic nerve damage.9 Furthermore, nonarteritic anterior ischemic optic neuropathy in middle-aged people is associated with high prevalence of COPD.10 Therefore we aimed to study the rate of optic neuropathy in COPD patients by VEP evaluations.
Materials and methods
This study was conducted in the Neuroscience Research Center and Tuberculosis and Lung Diseases Research Center of Tabriz University of Medical Science. Forty COPD diagnosed patients were enrolled by a pulmonary subspecialist. In addition, 60 age-matched healthy volunteers were selected as the control group. None of the participants had ophthalmic signs or symptoms. All subjects were referred to a neurologist for the VEP test. After examination by a pulmonary subspecialist and completion of spirometry, patients were classified according to the GOLD criteria. Spirometry was conducted before and after bronchodilator usage, and pulse oximetry was done for all subjects. Patients with zero grades on GOLD criteria were excluded.
After respiratory examinations, the VEP test was performed (four-channel device, Toennis Neuroscreen R plus [Erich Jaeger, Inc., Hoechberg, Germany]) with the checkerboard (raster background) method with a frequency of 2 Hz and 200 irritations.
Participants with refractive error were excluded from the study. Ophthalmoscopic examination was performed bywell-trained personnel, and individuals with any retinal abnormality were excluded (eg, evidence of hypertensive/diabetic retinopathy).
Statistical analysis was performed with SPSS 16 software and P-values less than 0.05 were considered significant. Ethical approval was obtained from the medical ethics committee of the Tabriz University of Medical Sciences. In this study, no invasive intervention was performed, nevertheless written informed consent was given to each participant.
Results
The median age of patients was 60 years (range 32–82 years). Twenty-five percent of patients had a high-risk occupation, and 75% of them had a low-risk one. The duration of symptoms in all patients with COPD was at least 5 years. All healthy volunteers were nonsmokers and had no symptoms of lung disease. As expected, their spirometric indexes were statistically different from those of the COPD patients.
In the COPD group, 59.4% (19/32) of male and 37.5% (3/8) of female patients had optic nerve abnormalities on VEP. Results of VEP studies in two groups are shown in Table 1. Figures 1 and 2 show VEP wave patterns of a healthy individual in the control group and VEP of a patient with COPD, respectively.
Table 1.
Results of VEP studies in two groups
| VEP | COPD | Control | P-value |
|---|---|---|---|
| Right P100 latency, ms | 118.2±11.9 | 110.2±4.4 | 0.001 |
| Left P100 latency, ms | 119.7±12.4 | 110.6±4.9 | 0.001 |
| Right P100/N140 amplitude, mV | 10.9±5.0 | 9.3±5.8 | – |
| Left P100/N140 amplitude, mV | 9.5±4.1 | 8.5±4.8 | – |
Abbreviations: COPD, chronic obstructive pulmonary disease; VEP, visual evoked potential.
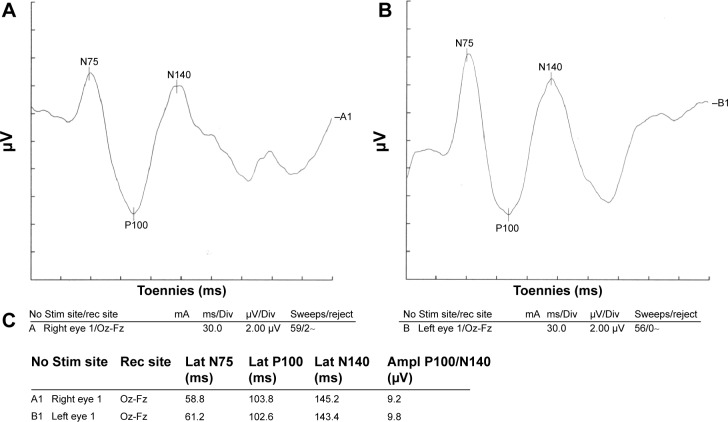
Figure 1.
Visual evoked potential of a healthy participant.
Notes: Normal visual evoked potential waveform parameters on both sides. (A) Visual evoked potential of the right eye. (B) Visual evoked potential of the left eye. (C) Parameters of both eyes.
Abbreviations: Ampl, amplitude; Div, division; Fz, lead placed at the mid line frontal; Lat, latency; Oz, lead placed at the mid line occipital; rec, recording; Stim, stimulating.
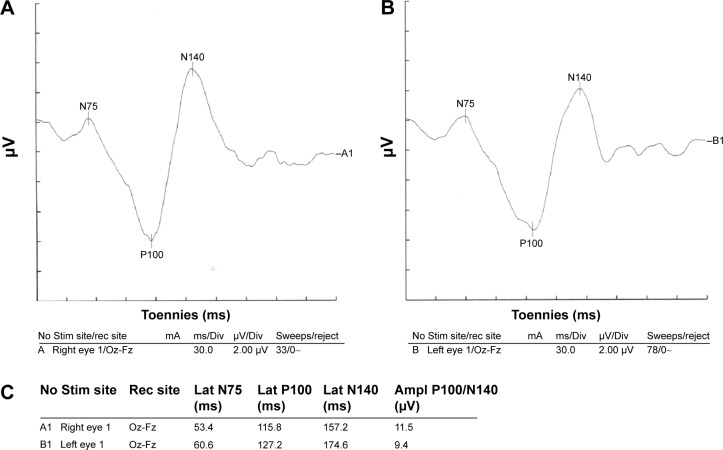
Figure 2.
Visual evoked potential of a patient with COPD.
Notes: Left side P100 latency was prolonged; Conclusion: normal right side visual evoked potential waveform parameters and mild demyelinating type lesion on left side optic pathway. (A) Visual evoked potential of the right eye. (B) Visual evoked potential of the left eye. (C) Parameters of both eyes.
Abbreviations: Ampl, amplitude; COPD, chronic obstructive pulmonary disease; Div, division; Fz, lead placed at the mid line frontal; Lat, latency; Oz, lead placed at the mid line occipital; rec, recording; Stim, stimulating.
Mean P100 latency in both eyes was significantly longer in COPD patients. However, the average P100/N140 amplitude in both eyes were higher in the COPD group. The amplitudes in both groups were in normal ranges; hence, this finding is clinically insignificant.
Discussion
Axonal degeneration, secondary demyelination, and abnormal endoneurial vessels characterize peripheral nerve neuropathy in respiratory insufficiency due to COPD.11–14 Hypoxemia causes peripheral nerve damage by harming vasa nervorum. In the early stages of ischemia, some mechanisms are activated to reduce peripheral nerve neuropathy, but these mechanisms become insufficient over time and obvious neuropathy is to be expected.13–16 However, unlike peripheral neuropathy, only a few studies have reported the frequency and the clinical or laboratory feature of cranial neuropathies in patients with COPD and severe hypoxemia.
In an Italian study, Nebbioso et al evaluated 22 eyes of cystic fibrosis patients. The visual field was tested by the frequency doubling technology (FDT). The result showed that there is a significant relationship between FDT parameters and spirometry findings of subjects. It can be concluded that hypoxia caused by cystic fibrosis may decrease the optic nerve activity.17 A similar mechanism of optic nerve involvement can be considered for COPD patients as both sufferers endure chronic hypoxia due to lung disorders.
In another study, Nebbioso et al showed that the traditional VEP is the most practical and sensitive diagnostic test in evaluating optic nerve. Even though this study compared FDT perimetry with VEP in patients with optic neuritis, it is comprehensible that VEP, as an accessible and noninvasive method, still has a place in evaluation of visual pathway.18
Appenzeller et al reported the existence of neuropathy in COPD patients for the first time.19 Gupta et al studied 80 men without clinical neuropathy and visual impairment. Forty of them had COPD and 40 of them were healthy. Their study showed statistically significant increase in latency and decrease in P100 amplitude in both eyes of COPD patients compared with the control group. This finding suggests both demyelinating and axonal disorders.7 However, in the present study we found prolonged P100 latency, but we did not observe any decrease in amplitude of VEP waves in COPD. In contrast to the Gupta study, the average amplitude in the COPD group was increased, but since all figures are within normal ranges, comparison between the two groups seems irrational.
Sohmer et al studied multimodality evoked potentials in cats. It was indicated that severe hypoxemia causes defects in VEP and brain-stem evoked potentials response. It was shown that decrease in arterial pressure can cause more depression in evoked potential.20 Kayacan et al studied neurophysiologic changes in COPD patients. After taking flash VEP tests, it was indicated that there was no statistically significant difference between the two groups.21 However, being less sensitive than pattern VEPs, flash VEPs are being used less frequently to determine the disorders of the visual pathway. Today, their clinical use is limited to subjects with severe refractive errors or opacity of ocular media, who cannot visually resolve a pattern stimulus, and very young or uncooperative patients.22
Ozge et al evaluated optic nerve involvement in 28 patients with severe COPD.23 They observed significant VEP abnormalities in COPD patients (82.1%) compared with healthy controls. However, seven of their COPD patients had subjective visual complaints including decreased visual acuity, decreased color vision, and attacks of short durations of vision loss. They showed that optic nerve is commonly involved in patients with severe COPD, possibly as part of polyneuropathy, and concluded that VEP abnormalities were related to acidosis, hypercarbia, and airway obstruction, but independent of disease duration, smoking, and age. Results of this study were similar with these studies, and it was shown that in comparison with normal healthy subjects, VEP abnormalities were more common in COPD patients.
Conclusion
In this study, higher P100 latency in COPD group showed demyelinating type of optic nerve involvement in these patients.
Footnotes
Disclosure
The authors reports no conflicts of interest in this work.
References
- 1.Anthonisen N. Chronic obstructive pulmonary disease. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier; 2007. pp. 619–626. [Google Scholar]
- 2.Jindal SK, Gupta D, Aggarwal AN, WHO-Government of India Biennium (2002–2003) Programme Guidelines for management of chronic obstructive pulmonary disease (COPD) in India: a guide for physicians (2003) Indian J Chest Dis Allied Sci. 2004;46(2):137–153. [PubMed] [Google Scholar]
- 3.Jann S, Gatti A, Crespi S, Rolo J, Beretta S. Peripheral neuropathy in chronic respiratory insufficiency. J Peripher Nerv Syst. 1998;3(1):69–74. [PubMed] [Google Scholar]
- 4.Jarratt JA, Morgan CN, Twomey JA, et al. Neuropathy in chronic obstructive pulmonary disease: a multicentre electrophysiological and clinical study. Eur Respir J. 1992;5(5):517–524. [PubMed] [Google Scholar]
- 5.Faden A, Mendoza E, Flynn F. Subclinical neuropathy associated with chronic obstructive pulmonary disease: possible pathophysiologic role of smoking. Arch Neurol. 1981;38(10):639–642. doi: 10.1001/archneur.1981.00510100067011. [DOI] [PubMed] [Google Scholar]
- 6.Oncel C, Baser S, Cam M, Akdağ B, Taspinar B, Evyapan F. Peripheral neuropathy in chronic obstructive pulmonary disease. COPD. 2010;7(1):11–16. doi: 10.3109/15412550903499480. [DOI] [PubMed] [Google Scholar]
- 7.Gupta PP, Sood S, Atreja A, Agarwal D. Assessment of visual evoked potentials in stable COPD patients with no visual impairment. Ann Thorac Med. 2010;5(4):222–227. doi: 10.4103/1817-1737.69111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göçmen AY, Celikbilek A, Hacıoğlu G, et al. The relationship between oxidative stress markers and visual evoked potentials in different hypertension models. Anadolu Kardiyol Derg. 2014;14(6):498–504. doi: 10.5152/akd.2014.4923. [DOI] [PubMed] [Google Scholar]
- 9.Demir HD, Inönü H, Kurt S, Doruk S, Aydın E, Etikan I. Evaluation of visual field parameters in patients with chronic obstructive pulmonary disease. Acta Ophthalmol. 2012;90(5):e349–e354. doi: 10.1111/j.1755-3768.2012.02432.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118(6):766–780. doi: 10.1016/s0002-9394(14)72557-7. [DOI] [PubMed] [Google Scholar]
- 11.Gupta PP, Agarwal D. Chronic obstructive pulmonary disease and peripheral neuropathy. Lung India. 2006;23:25–33. [Google Scholar]
- 12.Gupta PP, Sood S, Atreja A, Agarwal D. Evaluation of brain stem auditory evoked potentials in stable patients with chronic obstructive pulmonary disease. Ann Thorac Med. 2008;3(4):128–134. doi: 10.4103/1817-1737.42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal D, Vohra R, Gupta PP, Sood S. Subclinical peripheral neuropathy in stable middle-aged patients with chronic obstructive pulmonary disease. Singapore Med J. 2007;48(10):887–894. [PubMed] [Google Scholar]
- 14.Barnes PJ. Asthma. In: Fauci A, Braunwald E, Kasper D, et al., editors. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008. pp. 1596–1606. [Google Scholar]
- 15.Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995;8(8):1398–1420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 16.Farebrother MJ, Kelson MC, Heller RF. Death certification of farmer’s lung and chronic airway diseases in different countries of the EEC. Br J Dis Chest. 1985;79(4):352–360. doi: 10.1016/0007-0971(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 17.Nebbioso M, Quattrucci S, Leggieri E, Spadea L, Vingolo EM. Cystic fibrosis and new trends by ophthalmological evaluation: a pilot study. Biomed Res Int. 2014 Jul 15; doi: 10.1155/2014/580373. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nebbioso M, Steigerwalt RD, Pecori-Giraldi J, Vingolo EM. Multifocal and pattern-reversal visual evoked potentials vs. automated perimetry frequency-doubling technology matrix in optic neuritis. Indian J Ophthalmol. 2013;61(2):59–64. doi: 10.4103/0301-4738.99638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appenzeller O, Parks RD, MacGee J. Peripheral neuropathy in chronic disease of the respiratory tract. Am J Med. 1968;44(6):873–880. doi: 10.1016/0002-9343(68)90087-9. [DOI] [PubMed] [Google Scholar]
- 20.Sohmer H, Freeman S, Malachi S. Multi-modality evoked potentials in hypoxaemia. Electroencephalogr Clin Neurophysiol. 1986;64(4):328–333. doi: 10.1016/0013-4694(86)90156-2. [DOI] [PubMed] [Google Scholar]
- 21.Kayacan O, Beder S, Deda G, Karnak D. Neurophysiological changes in COPD patients with chronic respiratory insufficiency. Acta Neurol Belg. 2001;101(3):160–165. [PubMed] [Google Scholar]
- 22.American Clinical Neurophysiology Society . Guideline 9B: Guidelines on Visual Evoked Potentials. Milwaukee, WI: American Clinical Neurophysiology Society; 2008. [Accessed November 15, 2014]. Available from: http://www.acns.org/pdf/guidelines/Guideline-9B.pdf. [DOI] [PubMed] [Google Scholar]
- 23.Ozge C, Ozge A, Yilmaz A, Yalçinkaya DE, Calikoğlu M. Cranial optic nerve involvement in patients with severe COPD. Respirology. 2005;10(5):666–672. doi: 10.1111/j.1440-1843.2005.00766.x. [DOI] [PubMed] [Google Scholar]