Abstract
IMPORTANCE
Cigarette smoking adds an estimated $100 billion in annual incremental direct health care costs nationwide. Cigarette smoking increases complication risk in surgical patients, but the potential effects of smoking status on perioperative health care costs are unclear.
OBJECTIVE
To test the hypothesis that current and former smoking at the time of admission for inpatient surgery, compared with never smoking, are independently associated with higher incremental health care costs for the surgical episode and the first year after hospital discharge.
DESIGN, SETTING, AND PARTICIPANTS
This population-based, propensity-matched cohort study, with cohort membership based on smoking status (current smokers, former smokers, and never smokers) was performed at Mayo Clinic in Rochester (a tertiary care center) and included patients at least 18 years old who lived in Olmsted County, Minnesota, for at least 1 year before and after the index surgery.
EXPOSURE
Undergoing an inpatient surgical procedure at Mayo Clinic hospitals between April 1, 2008, and December 31, 2009.
MAIN OUTCOMES AND MEASURES
Total costs during the index surgical episode and 1 year after hospital discharge, with the latter standardized as costs per month. Costs were measured using the Olmsted County Healthcare Expenditure and Utilization Database, a claims-based database including information on medical resource use, associated charges, and estimated economic costs for patients receiving care at the 2 medical groups (Mayo Clinic and Olmsted Medical Center) that provide most medical services within Olmsted County, Minnesota.
RESULTS
Propensity matching resulted in 678 matched pairs in the current vs never smoker grouping and 945 pairs in the former vs never smoker grouping. Compared with never smokers, adjusted costs for the index hospitalization did not differ significantly for current or former smokers. However, the adjusted costs in the year after hospitalization were significantly higher for current and former smokers based on regression analysis (predicted monthly difference of $400 [95% CI, $131-$669] and $273 [95% CI, $56-$490] for current and former smokers, respectively).
CONCLUSIONS AND RELEVANCE
Compared with never smokers, health care costs during the first year after hospital discharge for an inpatient surgical procedure are higher in both former and current smokers, although the cost of the index hospitalization is not affected by smoking status.
Cigarette smoking adds approximately $100 billion in annual incremental direct health care costs nationwide.1 Most studies addressing this topic estimate costs by combining data regarding the relative risk of smoking for a disease state and costs associated with that condition.1-3 Few studies have directly measured the effects of smoking status on health care costs in individual patients or analyzed the cause of increased costs.4-6
One potential source of increased costs associated with smoking is increased use of surgery associated with smoking-related diseases (eg, coronary artery bypass grafting). In addition, after surgery, smokers are more likely to experience surgical complications, such as myocardial infarction, pneumonia, and wound infections,7 although the absolute incidence of these complications is relatively low.8,9 Consequently, smokers may receive more intensive postoperative medical care, have longer hospital stays, and require more readmissions. Thus, in the immediate postoperative period, smokers may have higher health care use and costs. A recent study of veterans undergoing general surgical procedures provided the first evidence supporting this hypothesis, finding a modest increase in direct inpatient costs in patients who had smoked within 1 year of surgery compared with never smokers.10 Beyond helping to assess the economic effects of smoking on surgical costs and use, knowing the incremental costs and increased use associated with smoking could provide an estimate of the potential savings if effective tobacco use interventions were implemented as a part of routine surgical care.
The purpose of this investigation was to test the hypothesis that current and former smoking at the time of admission for inpatient surgery are independently associated with higher incremental health care costs for the surgical episode and the first year after hospital discharge. This hypothesis was tested using a population-based, matched cohort design. Cohorts were constructed from patients who lived in Olmsted County, Minnesota. Health care costs and use were measured directly using a unique resource, the Olmsted County Health care Expenditure and Utilization Database (OCHEUD).
Methods
This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center.
Study Population and Setting
Starting in 2004, all patients admitted to the 2 Mayo Clinic Rochester hospitals have been assessed by the nursing staff for tobacco use.11 Information collected includes tobacco use status (current, former, or never tobacco user), type of tobacco used, and the current quantity of tobacco used. Patients were eligible for this study if they (1) underwent an inpatient surgical procedure at 1 of the 2 Mayo Clinic hospitals in Rochester, Minnesota, between April 1, 2008, and December 31, 2009; (2) had a documented tobacco status assessment (currently 100% of all admissions); (3) were residents of Olmsted County for at least 1 year before and after the index procedure; and (4) were at least 18 years old. The first surgical date within the above-mentioned period was defined as the index date. The index surgical episode was defined as starting 7 days before index hospital admission and ending at hospital discharge (Figure). This period was chosen so that costs associated with hospital admission (eg, preoperative evaluation and emergency department admission) could potentially be included as part of the index costs. The baseline period included 1 year before the start of the index episode. Patients were followed up for 1 year after the end of the index episode. Patients who underwent an inpatient surgical procedure within 1 year before the index date, died during the index hospitalization, or declined research authorization as per Minnesota statutes were excluded from study.
Figure. Study Time Frame, Including Baseline, Index Surgical Episode, and Follow-up.
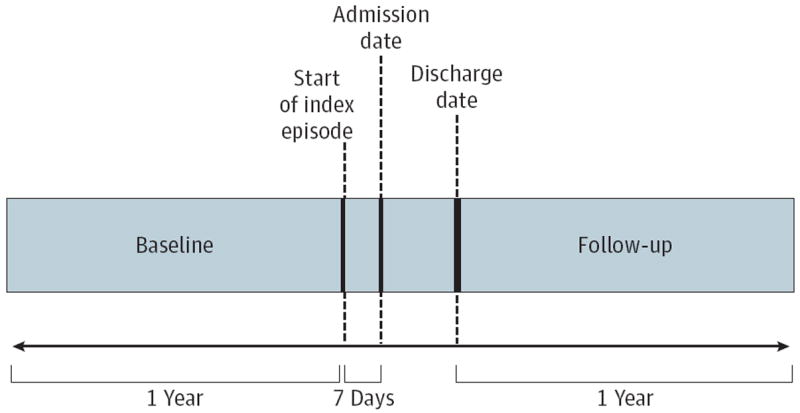
The index surgical episode was defined as starting 7 days before index hospital admission and ending at hospital discharge. This period was chosen so that costs associated with hospital admission (eg, preoperative evaluation and emergency department admission) were included as part of the index costs. The baseline period included 1 year before the start of the index episode. Patients were followed up for 1 year after the end of the index episode. Patients who had an inpatient surgical procedure within 1 year before the index date, died during the index hospitalization, or declined research authorization as per Minnesota statutes were excluded from study.
Data Sources
All data used were retrospectively gathered from multiple electronic data sources. Patient discharge information for the index inpatient surgical episode was obtained from billing data. Surgery dates from the electronic medical record were linked to discharge information. Smoking status was obtained for the linked hospital discharge information from the nursing tobacco status protocol database.
Cost and utilization data were obtained from the OCHEUD. This claims-based database houses information on medical resource use, associated charges, and estimated economic costs for patients receiving care at the 2 medical groups (Mayo Clinic and Olmsted Medical Center) that provide most medical services within Olmsted County, Minnesota. The OCHEUD provides a standardized inflation-adjusted cost estimate for each service or procedure provided locally in constant dollars. Specifically, a “bottom-up” costing approach is used to group services into the Medicare Part A and B classification system. This approach adjusts Part A hospital-billed charges with Medicare cost report department-level cost-to-charge ratios. Part B physician services are proxied with Medicare reimbursement rates. Although the types of services provided represent clinical practice patterns of local providers, the value of each unit of service has been adjusted to reflect national norms by the use of widely accepted valuation techniques.12 The OCHEUD provides an estimated economic cost for each line item in the billing record, allowing the aggregation of costs into categories deemed relevant to a particular study. To ensure that complete costs are captured, residency in Olmsted County was verified by the Rochester Epidemiology Project.13 More than 80% of the residents of Olmsted County are seen at least once annually at 1 of the 2 health care organizations included in the OCHEUD.14
Outcome Measures
The primary outcomes of the study were total costs during the index surgical episode and the 1-year follow-up period. The Figure illustrates the study time frame. To account for differing lengths of follow-up due to deaths during the follow-up period, the outcome measures for the follow-up period were standardized as costs per month. Descriptive analyses were conducted to compare unadjusted baseline costs, emergency department use, hospital use, and mean length of hospitalization (in days) during follow-up.
Statistical Analysis
Three patient cohorts were constructed from eligible patients based on the admission tobacco use assessments: current cigarette smokers (daily or less than daily), former smokers (past cigarette smoker but “not currently using”), and never smokers. Based on these cohorts, the analyses focused on 3 groupings of comparisons: current smokers vs never smokers, current smokers vs former smokers, and former smokers vs never smokers. Each analysis grouping used propensity score matching to control differences in the baseline characteristics between the 2 cohorts.15 For any given analysis grouping, the propensity score is the conditional probability that a patient was of a particular smoking status given observed covariates. Propensity scores were generated using logistic regression models controlling for age, sex, emergency department admission, patient severity, hospital discharge location, payer type, marital status, and principal index procedure captured through Clinical Classification Software categories.16 Patient severity was measured using the All-Patient Refined Diagnosis Related Groups Severity of Illness scale, a 4-level measure classifying the severity of patients’ illness as minor, moderate, major, and extreme.17 Hospital discharge location was classified as home, home with home health services, skilled nursing facility, or other. Payer type was classified as private insurance, government insurance, or no insurance. Marital status was classified as married/life partner, divorced/separated, single, or other.
One-to-one propensity score matching was implemented in Stata software.18 Common support was implemented, requiring the empirical distributions for outcomes in the 2 cohorts to have sufficient overlap.19,20 The C statistic was used to assess goodness of fit of each propensity score model. Patient characteristics of unmatched and matched cohorts were compared using standardized differences.21
To account for the skewed nature of cost data, patient costs were modeled using a generalized linear model, with an appropriate distribution (based on modified Park test) and logarithmic link function, which was confirmed by the Pregibon link test.22,23 When appropriate, 2-part regression was conducted, first using a logistic regression modeling the probability of having nonzero costs in baseline and then using generalized linear modeling in those patients with nonzero costs.24 The generalized linear regression models adjusted for Elixhauser comorbid conditions identified during the baseline period and patient characteristics included in propensity models, which are expected to remove the residual bias after propensity score matching.25,26 Predicted differences in costs between the 2 cohorts were obtained through recycled predictions to account for the skewness of cost data, with 200 boot-strap replications.27-29 To investigate drivers of cost differences in the primary outcomes, costs were categorized by using the Berenson-Eggers Type of Service (BETOS) classifications for Medicare Part B costs.30
All calculated P values were 2 sided. Statistical analyses were performed using SAS (version 9.2; SAS Institute) and Stata (version 12.1; StataCorp) software.
Results
A total of 681 current smokers, 946 former smokers, and 5093 never smokers were available for matching after inclusion and exclusion criteria were applied. Comparisons of patient characteristics of each unmatched analysis group are described in Table 1. The unmatched groups were dissimilar in many respects, including age, sex, marital status, payer type, and surgical category (Table 1). Propensity matching resulted in 678 matched pairs in the current vs never smoker grouping, 665 in the current vs former smoker grouping, and 945 in the former vs never smoker grouping (Table 2). Covariate balance in the matched cohorts was fairly good, although the current vs former smoker grouping had the most variable categories with residual differences after matching, with former smokers being older and more likely to be married and to be undergoing cardiovascular surgery. The C statistics of the current smoker vs never smoker, current smoker vs former smoker, and former smoker vs never smoker propensity models were 0.804, 0.796, and 0.713, respectively. Mortality the year after discharge did not differ according to smoking status in any analysis group (Table 2).
Table 1.
Unmatched Comparisons of Paired Groupingsa
| Characteristic | Current vs Never Smokers
|
Current vs Former Smokers
|
Former vs Never Smokers
|
|||
|---|---|---|---|---|---|---|
| Current (n = 681) | Never (n = 5093) | Current (n = 681) | Former (n = 946) | Former (n = 946) | Never (n = 5093) | |
| Age, mean (SD), y | 46.3 (17.0) | 49.8 (20.7) | 46.3 (17.0) | 61.4 (18.2) | 61.4 (18.2) | 49.8 (20.7) |
|
| ||||||
| Female sex, % | 50.2 | 72.3 | 50.2 | 47.7 | 47.7 | 72.3 |
|
| ||||||
| Marital status, % | ||||||
|
| ||||||
| Married/life partner | 39.5 | 71.5 | 39.5 | 66.8 | 66.8 | 71.5 |
|
| ||||||
| Divorced/separated | 20.0 | 5.9 | 20.0 | 11.0 | 11.0 | 5.9 |
|
| ||||||
| Single | 35.5 | 15.3 | 35.5 | 11.5 | 11.5 | 15.3 |
|
| ||||||
| Other | 5.0 | 7.3 | 5.0 | 10.7 | 10.7 | 7.3 |
|
| ||||||
| Payer type, % | ||||||
|
| ||||||
| Private | 44.3 | 62.0 | 44.3 | 38.7 | 38.7 | 62.0 |
|
| ||||||
| Government | 52.0 | 37.1 | 52.0 | 60.9 | 60.9 | 37.1 |
|
| ||||||
| No insurance | 3.7 | 0.9 | 3.7 | 0.4 | 0.4 | 0.9 |
|
| ||||||
| APR DRG severity, % | ||||||
|
| ||||||
| 1 | 39.2 | 47.3 | 39.2 | 37.7 | 37.7 | 47.3 |
|
| ||||||
| 2 | 38.8 | 36.9 | 38.8 | 37.6 | 37.6 | 36.9 |
|
| ||||||
| 3 | 15.9 | 12.4 | 15.9 | 19.0 | 19.0 | 12.4 |
|
| ||||||
| 4 | 6.2 | 3.4 | 6.2 | 5.7 | 5.7 | 3.4 |
|
| ||||||
| CCS category, %b | ||||||
|
| ||||||
| Operations | ||||||
|
| ||||||
| Nervous system | 6.6 | 4.3 | 6.6 | 6.2 | 6.2 | 4.3 |
|
| ||||||
| Endocrine system | 1.3 | 0.8 | 1.3 | 0.3 | 0.3 | 0.8 |
|
| ||||||
| Eye | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||||
| Ear | 0.0 | <0.1 | 0.0 | 0.1 | 0.1 | <0.1 |
|
| ||||||
| Nose, mouth, and pharynx | 1.2 | 0.3 | 1.2 | 0.8 | 0.8 | 0.3 |
|
| ||||||
| Respiratory system | 3.7 | 1.3 | 3.7 | 3.8 | 3.8 | 1.3 |
|
| ||||||
| Cardiovascular system | 20.6 | 11.9 | 20.6 | 25.1 | 25.1 | 11.9 |
|
| ||||||
| Hemic and lymphatic system | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
|
| ||||||
| Digestive system | 14.8 | 11.7 | 14.8 | 13.2 | 13.2 | 11.7 |
|
| ||||||
| Urinary system | 2.3 | 2.7 | 2.3 | 2.6 | 2.6 | 2.7 |
|
| ||||||
| Urinary system | 1.2 | 1.4 | 1.2 | 2.3 | 2.3 | 1.4 |
|
| ||||||
| Female genital organs | 5.4 | 5.7 | 5.4 | 3.9 | 3.9 | 5.7 |
|
| ||||||
| Obstetrical procedures | 13.4 | 32.7 | 13.4 | 9.7 | 9.7 | 32.7 |
|
| ||||||
| Musculoskeletal system | 18.8 | 18.5 | 18.8 | 21.0 | 21.0 | 18.5 |
|
| ||||||
| Integumentary system | 1.2 | 3.7 | 1.2 | 3.1 | 3.1 | 3.7 |
|
| ||||||
| Miscellaneous diagnostic and therapeutic procedures | 8.8 | 4.1 | 8.8 | 7.0 | 7.0 | 4.1 |
|
| ||||||
| Discharge location, % | ||||||
|
| ||||||
| Home | 88.3 | 88.0 | 88.3 | 83.3 | 83.3 | 88.0 |
|
| ||||||
| Home with home health services | 4.6 | 2.8 | 4.6 | 4.3 | 4.3 | 2.8 |
|
| ||||||
| Skilled nursing facility | 5.4 | 8.9 | 5.4 | 11.6 | 11.6 | 8.9 |
|
| ||||||
| Other | 1.8 | 0.3 | 1.8 | 0.7 | 0.7 | 0.3 |
|
| ||||||
| Admission through ED, % | 43.8 | 21.1 | 43.8 | 33.2 | 33.2 | 21.1 |
|
| ||||||
| Baseline costs, mean (SD), $b | 6743 (12 607) | 5430 (10 857) | 6743 (12 607) | 7911 (13 514) | 7911 (13 514) | 5430 (10 857) |
Abbreviations: APR DRG, All-Patient Refined Diagnosis Related Groups; CCS, Clinical Classification Software; ED, emergency department.
Boldface values represent significant differences (P < .05).
Not used in propensity matching.
Table 2.
Matched Comparisons of Paired Groupsa
| Characteristic | Current vs Never Smokers
|
Current vs Former Smokers
|
Former vs Never Smokers
|
|||
|---|---|---|---|---|---|---|
| Current (n = 678) | Never (n = 678) | Current (n = 665) | Former (n = 665) | Former (n = 945) | Never (n = 945) | |
| Age, mean (SD), y | 46.3 (17.0) | 44.8 (19.6) | 46.8 (16.8) | 55.2 (17.6) | 61.4 (18.2) | 61.2 (19.1) |
|
| ||||||
| Female sex, % | 50.4 | 52.7 | 50.2 | 51.1 | 47.6 | 46.6 |
|
| ||||||
| Marital status, % | ||||||
|
| ||||||
| Married/life partner | 39.7 | 39.5 | 40.5 | 61.1 | 66.9 | 66.2 |
|
| ||||||
| Divorced/separated | 19.8 | 16.5 | 20.3 | 14.3 | 10.9 | 10.1 |
|
| ||||||
| Single | 35.5 | 39.4 | 34.1 | 15.8 | 11.5 | 13.1 |
|
| ||||||
| Other | 5.0 | 4.6 | 5.1 | 8.9 | 10.7 | 10.6 |
|
| ||||||
| Payer type, % | ||||||
|
| ||||||
| Private | 44.4 | 49.4 | 45.3 | 49.0 | 38.7 | 39.5 |
|
| ||||||
| Government | 51.9 | 47.8 | 52.2 | 50.4 | 60.8 | 60.3 |
|
| ||||||
| No insurance | 3.7 | 2.8 | 2.6 | 0.6 | 0.4 | 0.2 |
|
| ||||||
| APR DRG severity, % | ||||||
|
| ||||||
| 1 | 39.4 | 39.4 | 39.1 | 38.8 | 37.8 | 38.2 |
|
| ||||||
| 2 | 38.6 | 38.5 | 38.9 | 39.7 | 37.7 | 37.8 |
|
| ||||||
| 3 | 15.9 | 15.0 | 15.8 | 15.5 | 18.8 | 17.7 |
|
| ||||||
| 4 | 6.0 | 7.1 | 6.2 | 6.0 | 5.7 | 3.4 |
|
| ||||||
| CCS category, % | ||||||
|
| ||||||
| Operations | ||||||
|
| ||||||
| Nervous system | 6.5 | 7.8 | 6.8 | 6.3 | 6.2 | 6.5 |
|
| ||||||
| Endocrine system | 1.3 | 1.2 | 1.1 | 0.5 | 0.3 | 0.2 |
|
| ||||||
| Eye | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
|
| ||||||
| Ear | 0 | 0 | 0 | 0 | 0.1 | 0.0 |
|
| ||||||
| Nose, mouth, and pharynx | 1.2 | 0.7 | 1.2 | 0.9 | 0.8 | 1.0 |
|
| ||||||
| Respiratory system | 3.4 | 4.3 | 3.6 | 3.5 | 3.7 | 4.3 |
|
| ||||||
| Cardiovascular system | 20.3 | 19.0 | 20.9 | 25.9 | 25.1 | 24.9 |
|
| ||||||
| Hemic and lymphatic system | 0.6 | 0.0 | 0.6 | 0.6 | 0.6 | 0.6 |
|
| ||||||
| Digestive system | 14.9 | 17.3 | 14.6 | 5.1 | 13.2 | 12.2 |
|
| ||||||
| Urinary system | 2.4 | 1.9 | 2.4 | 1.7 | 2.6 | 2.6 |
|
| ||||||
| Urinary system | 1.2 | 0.3 | 1.2 | 2.1 | 2.3 | 2.9 |
|
| ||||||
| Female genital organs | 5.5 | 6.6 | 5.3 | 5.0 | 3.9 | 3.4 |
|
| ||||||
| Obstetrical procedures | 13.4 | 13.4 | 13.7 | 13.8 | 9.7 | 9.5 |
|
| ||||||
| Musculoskeletal system | 18.9 | 16.5 | 18.6 | 19.7 | 21.1 | 21.6 |
|
| ||||||
| Integumentary system | 1.2 | 1.5 | 1.2 | 0.9 | 3.1 | 3.0 |
|
| ||||||
| Miscellaneous diagnostic and therapeutic procedures | 8.8 | 9.3 | 8.7 | 8.0 | 7.0 | 7.3 |
|
| ||||||
| Discharge location, % | ||||||
|
| ||||||
| Home | 88.5 | 89.7 | 88.4 | 87.2 | 83.4 | 81.2 |
|
| ||||||
| Home with home health services | 4.6 | 4.3 | 4.7 | 3.9 | 4.3 | 4.8 |
|
| ||||||
| Skilled nursing facility | 5.5 | 5.2 | 5.6 | 7.8 | 11.6 | 13.4 |
|
| ||||||
| Other | 1.5 | 0.9 | 1.4 | 1.1 | 0.6 | 0.6 |
|
| ||||||
| Admission through ED, % | 43.5 | 40.2 | 42.7 | 34.6 | 33.1 | 31.9 |
|
| ||||||
| Baseline costs, mean (SD), $ | 6758 (12 633) | 7617 (17 948) | 6759 (12 603) | 7370 (13 616) | 7911 (13 521) | 6725 (11 746) |
|
| ||||||
| ED during follow-up, % | 43.7 | 35.5 | 43.3 | 36.5 | 39.4 | 38.9 |
|
| ||||||
| ED visits during follow-up, mean (SD) | 1.3 (2.7) | 0.7 (1.3) | 1.3 (2.7) | 0.9 (2.5) | 0.9 (2.2) | 0.7 (1.2) |
|
| ||||||
| Any hospitalization during follow-up, % | 30.7 | 26.4 | 31.1 | 28.3 | 30.7 | 27.4 |
|
| ||||||
| No. of hospital stays during follow-up, mean (SD) | 0.6 (1.2) | 0.4 (0.9) | 0.6 (1.2) | 0.6 (1.4) | 0.6 (1.3) | 0.5 (1.0) |
|
| ||||||
| Length of hospitalization during follow-up, mean (SD), d | 3.3 (9.8) | 2.7 (7.4) | 3.9 (12.2) | 3.4 (10.1) | 3.8 (11.2) | 2.8 (7.9) |
|
| ||||||
| Mortality rate during follow-up, % | 3.8 | 4.0 | 3.9 | 4.7 | 5.4 | 5.5 |
Abbreviations: APR DRG, All-Patient Refined Diagnosis Related Groups; CCS, Clinical Classification Software; ED, emergency department.
Boldface values represent significant differences (P < .05).
Tables 3, 4, and 5 present both the observed and adjusted costs for each matched comparison according to smoking status. Compared with costs in never smokers, adjusted costs for the index hospitalization did not differ significantly in current smokers (Table 3). However, the adjusted costs the year after hospitalization were significantly higher for current smokers based on regression analysis, with a predicted monthly difference of $400 (95% CI, $131-$669). Compared with costs in former smokers, neither the adjusted costs for index hospitalization nor costs the year after hospitalization significantly differed in current smokers (Table 4). Compared with costs in never smokers, adjusted costs for the index hospitalization did not differ significantly in former smokers (Table 5), but costs the year after hospitalization were significantly higher in the former smokers at adjusted analysis, with a predicted monthly difference of $273 (95% CI, $56-$490). Unadjusted baseline costs did not differ according to smoking status in any of the 3matched groups (Table 2).
Table 3.
Observed and Adjusted Costs in Matched Analyses for Current and Never Smokersa
| Cost Period | Observed Cost, $ (95% CI)
|
Adjusted Cost, $ (95% CI)
|
||||
|---|---|---|---|---|---|---|
| Current Smokers | Never Smokers | Current Smokers | Never Smokers | Predicted Difference | ||
| Index hospitalization, total cost | 19 050 (17 182 to 20 917) | 20 422 (18 031 to 22 813) | 19 542 (18 117 to 20 968) | 19 337 (17 759 to 20 914) | 205.87 (−771 to 1183) | |
|
| ||||||
| Postdischarge follow-up, monthly cost | 1714 (1033 to 2395) | 1510 (1039 to 1980) | 1434 (1139 to 1730) | 1034 (933 to 1136) | 400 (131 to 669)b | |
Adjusted costs were based on a generalized linear model with inverse gaussian distribution and logarithmic link, which were confirmed with modified Park and Pregibon link tests, respectively. These analyses were based on propensity score–matched data; 95%CIs were based on 200 bootstrap replications. Monthly costs were calculated as total costs divided by person-months observed to account for the differential follow-up time due to deaths in each of the groups after surgery.
P < .001.
Table 4.
Observed and Adjusted Cost Outcomes of Matched Analyses for Current and Former Smokersa
| Cost Period | Observed Cost, $ (95% CI)
|
Adjusted Cost, $ (95% CI)
|
||||
|---|---|---|---|---|---|---|
| Current Smokers | Former Smokers | Current Smokers | Former Smokers | Predicted Difference | ||
| Index hospitalization, total cost | 19 183 (17 503 to 20 862) | 19 875 (18 063 to 21 686) | 19 248 (17 856 to 20 640) | 19 353 (18 028 to 20 677) | −105 (−1235 to 1026) | |
|
| ||||||
| Postdischarge follow-up, monthly cost | 1745 (1130 to 2361) | 2152 (1509 to 2794) | 2022 (1433 to 2612) | 1939 (1339 to 2540) | 83 (−444 to 610) | |
Methods of analysis are explained in Table 3.
Table 5.
Observed and Adjusted Costs in Matched Analyses for Former and Never Smokersa
| Cost | Observed Cost, $ (95% CI)
|
Adjusted Cost, $ (95% CI)
|
||||
|---|---|---|---|---|---|---|
| Former Smoker | Never Smokers | Former Smokers | Never Smokers | Predicted Difference | ||
| Index hospitalization, total cost | 19 888 (18 451 to 21 324) | 19 994 (18 363 to 21 626) | 19 880 (18 784 to 20 975) | 19 536 (18 448 to 20 624) | 344 (−503 to 1190) | |
|
| ||||||
| Postdischarge follow-up, monthly cost | 2221 (1651 to 2790) | 1555 (1204 to 1906) | 1397 (1163 to 1631) | 1123 (1015 to 1231) | 273 (56 to 490)b | |
Methods of analysis are explained in Table 3.
P < .05.
Further analyses were used to explore the potential causes of observed cost differences. In the year after discharge, current smokers were more likely than never smokers to visit the emergency department at least once and had more emergency department visits (Table 2). The proportion of patients requiring hospitalization at least once did not differ significantly according to smoking status, but the number of hospitalizations was significantly higher among current smokers, and the length of hospitalization tended to be longer (but not significantly so). Accordingly, the unadjusted hospital-related (Part A) costs, the primary component of postdischarge costs, were significantly higher in current smokers than in never smokers ($1096 vs $965 per month, respectively; P < .01; see eTable 1 in Supplement for results in other cost categories). This was also true for follow-up costs in former compared with never smokers (Part A monthly costs of $1454 and $961, respectively; P = .03), probably reflecting the tendency toward increased length of stay, although this difference did not reach statistical significance (see eTable 2 in Supplement for results in other cost categories).
Discussion
Compared with costs in never smokers, health care costs during the first year after hospital discharge for an inpatient surgical procedure are higher in both former and current smokers, although the cost of the index hospitalization is not affected by smoking status.
A key feature of our study design was a population-based analysis based on geography, including residents of Olmsted County, Minnesota. This conferred several advantages, including a reduced potential for referral bias and inclusion of the full spectrum of surgical procedures, providing a true population-based estimate of costs. The design also took advantage of a unique billing resource that allowed thorough ascertainment of cost and use of procedures. We are unaware of any prior studies that have measured incremental costs associated with smoking in a geographically defined population. The degree to which the Olmsted County population is representative of the general United States population has been reviewed elsewhere; in general, this population is more affluent and less diverse than the overall population, which may affect the generalizability of our results.14 In addition, medical care delivered by the 2 major providers in Olmsted County, Mayo Clinic and Olmsted Medical Center, may not be representative of the nation.
When all eligible participants were compared by smoking status, there were grouping differences in several potential confounders known to be associated with smoking status, including sex, age, insurance type, and type of surgery.31 For example, cardiovascular surgery was more common in former and current smokers, probably reflecting smoking-related disease that provided the indication for surgery. Because costs vary widely by procedure, these and other confounders must be addressed in analyses, accomplished in this study through propensity matching. Matching was more challenging when comparing current and former smokers; even after matching, former smokers were older and more likely to need cardiovascular surgery, 2 factors that can be associated with higher rates of resource use.
After adjustment, there were no significant differences in index hospitalization costs among any group. In the only other study to specifically examine the effects of smoking status on inpatient surgical costs, in patients who smoked within 1 year before surgery, compared with never smokers, Kamath et al10 found a small (approximately 4%) increase in direct inpatient costs (during and within 30 days of the index hospitalization), mediated partially by an increase in postoperative respiratory complications. Their analysis differed from ours in several respects, including surgical procedure type (general surgery only), population studied (Veterans Administration patients, predominantly male), and definition of smoking status (eg, they had no information regarding current smoking status at the time of surgery, thus their “current smokers” may have included those whom we would classify as former smokers). Furthermore, because their study included many more patients than ours (approximately 5000 current smokers), our study was probably not sufficiently powered to detect the relatively small difference they observed. Finally, because we were interested in costs the year after surgery, we excluded patients who died during hospitalization, which could affect the costs of inpatient hospitalization. However, Kamath et al10 found increased inpatient costs even after excluding those who died within 24 hours after surgery.
In contrast to results for index hospitalization, smoking status significantly affected costs in the year after index hospitalization, for both current and former smokers compared with never smokers. In secondary analysis, this seems to be associated primarily with increased hospital use, although we did not determine whether this use was related specifically to the consequences of surgery. These differences could reflect relatively poorer health in current and former smokers, apart from any factor associated specifically with surgery, such as postoperative complications. However, there was no evidence for baseline differences in health care costs in the year before surgery, suggesting that these costs were associated with the surgical episode.
Quitting smoking can reduce the rate of perioperative complications,32-34 and the findings of 1 small study suggest that this improvement in outcome is associated with decreased costs.35 We did not observe significant differences in direct costs between current and former smokers, but for several reasons these data cannot be interpreted in terms of potential effects of preoperative smoking cessation on perioperative costs. First, even after matching, former smokers were about 8 years older and more likely to have high-risk, high-cost surgery, such as cardiovascular surgery. It may be difficult for even adjusted analysis to account for these fundamental differences. Second, the former smokers may include those who quit only recently, within the several weeks that is apparently necessary for maximum risk reduction after quitting.36 Third, because these are observational data only, there is the potential for “healthy smoker” effects, because sicker patients are more likely to quit in the perioperative period, which may bias the former smokers toward increased risk. Finally, postoperative smoking behavior may also affect risk,37 and it is possible that those who self-designated as former smokers resumed smoking after hospital discharge. Although the potential effects of preoperative smoking cessation cannot be estimated from these data, the findings suggest that even in former smokers, smoking-related diseases may have continued effects on surgical risk and costs.
Given that our data are population based, the potential effects of the increased costs associated with smoking status in the postoperative period can be estimated. Based on these results, in Olmsted County, the estimated annual incremental cost (based on annualized number of surgical patients who smoke) is approximately $2 million for the current smokers eligible for study and $1.4 million for former smokers. Recognizing the potential limitations of extrapolation to the US population for the reasons already noted, approximately 12 million inpatient surgical procedures are performed annually in the United States.38,39 With a conservative population-based smoking prevalence for the mean age of study participants estimated at 16%,40 approximately 2 million inpatient procedures are thus performed in adult smokers annually. If there are $4800 in annual excess postoperative costs per procedure, approximately $10 billion in annual excess postoperative costs within the first year after surgery are associated with inpatient procedures for current smokers and $7 billion for former smokers. This is a substantial fraction of current estimates for the incremental increase in direct medical costs caused by smoking in the general population (approximately $100 billion).1 Thus, the increased costs that we have identified have significant public health consequences. It remains to be determined whether systematic efforts to promote perioperative smoking abstinence in surgical patients could reduce these costs,41 but these data provide a rationale for studying this issue.
Conclusions
Compared with those in never smokers, health care costs during the first year after hospital discharge for an inpatient surgical procedure are higher in both current and former smokers, although the cost of the index hospitalization is not affected by smoking status. These excess costs add an estimated $17 billion annually to direct medical costs in the United States.
Supplementary Material
Acknowledgments
Funding/Support: The data analysis was supported by funds from the Mayo Foundation. Study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (grant R01 AG034676).
Role of the Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions:
DrWarner and Mr Moriarty had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Warner, Borah, Schroeder, Shi, Shah.
Acquisition of data: Moriarty, Shah.
Analysis and interpretation of data:Warner, Borah, Moriarty, Schroeder, Shah.
Drafting of the manuscript:Warner, Moriarty, Shi.
Critical revision of the manuscript for important intellectual content:Warner, Borah, Moriarty, Schroeder, Shah.
Statistical analysis: Borah, Moriarty, Schroeder, Shi.
Obtained funding:Warner, Shah.
Study supervision:Warner, Shah.
Supplemental content at jamasurgery.com
Conflict of Interest Disclosures: None reported.
Contributor Information
David O. Warner, Department of Anesthesiology, Mayo Clinic, Rochester, Minnesota.
Bijan J. Borah, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
James Moriarty, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
Darrell R. Schroeder, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
Yu Shi, Department of Anesthesiology, Mayo Clinic, Rochester, Minnesota.
Nilay D. Shah, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
References
- 1.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.Barendregt JJ, Bonneux L, van der Maas PJ. The health care costs of smoking. N Engl J Med. 1997;337(15):1052–1057. doi: 10.1056/NEJM199710093371506. [DOI] [PubMed] [Google Scholar]
- 3.Warner KE, Hodgson TA, Carroll CE. Medical costs of smoking in the United States: estimates, their validity, and their implications. Tob Control. 1999;8(3):290–300. doi: 10.1136/tc.8.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman PA, Khan ZM, Thompson EE, Curry SJ. Health care costs among smokers, former smokers, and never smokers in an HMO. Health Serv Res. 2003;38(2):733–749. doi: 10.1111/1475-6773.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner EH, Curry SJ, Grothaus L, Saunders KW, McBride CM. The impact of smoking and quitting on health care use. Arch Intern Med. 1995;155(16):1789–1795. [PubMed] [Google Scholar]
- 6.Moriarty JP, Branda ME, Olsen KD, et al. The effects of incremental costs of smoking and obesity on health care costs among adults: a 7-year longitudinal study. J Occup Environ Med. 2012;54(3):286–291. doi: 10.1097/JOM.0b013e318246f1f4. [DOI] [PubMed] [Google Scholar]
- 7.Warner DO. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104(2):356–367. doi: 10.1097/00000542-200602000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Houston TK, Ponce BA, et al. Smoking as a risk factor for short-term outcomes following primary total hip and total knee replacement in veterans. Arthritis Care Res (Hoboken) 2011;63(10):1365–1374. doi: 10.1002/acr.20555. [DOI] [PubMed] [Google Scholar]
- 9.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J AmColl Surg. 2007;204(6):1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Kamath AS, Vaughan Sarrazin M, Vander Weg MW, Cai X, Cullen J, Katz DA. Hospital costs associated with smoking in veterans undergoing general surgery. J Am Coll Surg. 2012;214(6):901–908. doi: 10.1016/j.jamcollsurg.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Zarling KK, Burke MV, Gaines KA, Gauvin TR. Registered nurse initiation of a tobacco intervention protocol: leading quality care. J Cardiovasc Nurs. 2008;23(5):443–448. doi: 10.1097/01.JCN.0000317451.64778.e9. [DOI] [PubMed] [Google Scholar]
- 12.Lipscomb J, Ancukiewicz M, Parmigiani G, Hasselblad V, Samsa G, Matchar DB. Predicting the cost of illness: a comparison of alternative models applied to stroke. Med Decis Making. 1998;18(2 suppl):S39–S56. doi: 10.1177/0272989X98018002S07. [DOI] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 16.Healthcare Cost and Utilization Project (HCUP) [August 7, 2012];Clinical Classifications Software (CCS) for ICD-90CM. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 17.Healthcare Cost and Utilization Project (HCUP) [August 7, 2012];Overview of The Nationwide Inpatient Sample. http://hcup-us.ahrq.gov/nisoverview.jsp.
- 18.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. [November 19, 2013];2003 Revised July 19, 2012. http://ideas.repec.org/c/boc/bocode/s432001.html.
- 19.Dehejia RH, Wahba S. Matching Methods for Estimating Causal Effects in Non-experimental Studies. Cambridge, MA: National Bureau of Economic Research; 1998. [Google Scholar]
- 20.Dehejia RH, Wahba S. Propensity score-matching for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151–161. [Google Scholar]
- 21.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 22.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 23.Pregibon D. Goodness of link tests for generalized linear models. Appl Stat. 1980;29(1):15–24. [Google Scholar]
- 24.Duan N, Manning WG, Morris C, Newhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1:115–126. [Google Scholar]
- 25.Rubin DB. The use of matched sampling and regression adjustment to remove bias in observational studies. Biometrics. 1973;29:185–203. [Google Scholar]
- 26.Rubin DB, Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. J Am Stat Assoc. 2000;95(450):573–585. [Google Scholar]
- 27.Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93–109. doi: 10.1093/biostatistics/kxh020. [DOI] [PubMed] [Google Scholar]
- 28.Polsky D, Glick HA, Willke R, Schulman K. Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ. 1997;6(3):243–252. doi: 10.1002/(sici)1099-1050(199705)6:3<243::aid-hec269>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Barber JA, Thompson SG. Analysis and interpretation of cost data in randomised controlled trials: review of published studies. BMJ. 1998;317(7167):1195–1200. doi: 10.1136/bmj.317.7167.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. [November 19, 2013];Berenson-Eggers Type of Service (BETOS) http://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/BETOS.html.
- 31.Warner DO, Patten CA, Ames SC, Offord K, Schroeder D. Smoking behavior and perceived stress in cigarette smokers undergoing elective surgery. Anesthesiology. 2004;100(5):1125–1137. doi: 10.1097/00000542-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 33.Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control. 2006;15(5):352–358. doi: 10.1136/tc.2005.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen T, Tønnesen H, Møller AM. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg. 2009;96(5):451–461. doi: 10.1002/bjs.6591. [DOI] [PubMed] [Google Scholar]
- 35.Møller AM, Kjellberg J, Pedersen T. Health economic analysis of smoking cessation prior to surgery—based on a randomised trial [in Danish] Ugeskr Laeger. 2006;168(10):1026–1030. [PubMed] [Google Scholar]
- 36.Warner DO. Preoperative smoking cessation: how long is long enough? Anesthesiology. 2005;102(5):883–884. doi: 10.1097/00000542-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335–1342. doi: 10.2106/JBJS.I.00627. [DOI] [PubMed] [Google Scholar]
- 38.Russo A, Elixhauser A, Steiner C, Wier L. Hospital-Based Ambulatory Surgery, 2007: Statistical Brief #86. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Statistical Briefs; 2007. [Google Scholar]
- 39.Tzong KY, Han S, Roh A, Ing C. Epidemiology of pediatric surgical admissions in US children: data from the HCUP kids inpatient database. J Neurosurg Anesthesiol. 2012;24(4):391–395. doi: 10.1097/ANA.0b013e31826a0345. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 41.Warner DO. Surgery as a teachable moment: lost opportunities to improve public health. Arch Surg. 2009;144(12):1106–1107. doi: 10.1001/archsurg.2009.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


