Abstract
BACKGROUND
Pulmonary metastasectomy is an acceptable treatment option in various metastatic lesions. The role of minimally invasive surgery for metastasectomy remains controversial. We report on a recently described hybrid video-assisted thoracoscopic surgery (hVATS) technique in the community hospital setting.
METHODS
Using a retrospective study design, data on 61 patients undergoing 67 resections between April 2000 and January 2008 was collected at a single institution. Patient demographics, pathology, and clinical outcome data were recorded. Kaplan Meier estimates and multivariate Cox regression were used to assess survival and prognostic factors, respectively.
RESULTS
Mean patient age was 61.7 years. The majority of lesions were solitary, unilateral, and genitourinary or gastrointestinal in origin (69%). R0 resection was achieved in 97% of cases with the most common operation being lobectomy. Mean length of stay was 4.4 days. Mean follow-up was 39.7 months and 5-year overall survival was 63.2% for the cohort; median survival was not reached. The number of lesions (univariate only) and tumor size over 4 cm influenced overall survival.
CONCLUSIONS
Hybrid VATS is a safe and feasible technique in the community medical center setting and warrants additional investigation as an alternative strategy in the management of pulmonary metastases.
Keywords: hybrid surgery, minimally invasive surgery, pulmonary metastases, VATS
I. Introduction
Pulmonary metastasectomy is a widely accepted treatment option at nearly all major medical institutions. It was described by Charles Emmanuel Sedillot in 1855 during a resection of an invasive chest wall tumor [1]. More widespread adoption of the technique, however, did not emerge until nearly a century later, in 1947, when Alexander et al demonstrated a significant three-year survival of 45% among 24 patients [2]. Thomford et al published the results of pulmonary 205 metastasectomies in 1965 with five-year survival exceeding 30% [3]. Since then, numerous studies have cited equal or better outcomes, ranging between 30 and 50% [4]. These reports, however, described mainly standard open approaches via thoracotomy or median sternotomy.
The role of minimally invasive operative techniques in metastasectomy, while accepted by some, still remains controversial [5]. Video-assisted thoracoscopic surgery (VATS) for metastasectomy was first described by Dowling et al in 1992 [6]. Opponents of this approach have raised concerns over limitations stemming from loss of manual palpation, the potential for inadequate resection, limited extent of resection, and inaccessibility to centrally-located lesions [7, 8]. More recent literature has reported on hybrid VATS approaches that incorporate minimally invasive principles and open techniques. We present our experience with pulmonary metastasectomy using a previously described hybrid VATS (hVATS) in the community hospital setting [9].
II. Methods
Patients
A retrospective review was conducted of all hVATS for lesions metastatic to the lungs performed at a single community medical center (Chippenham Medical Center, Richmond, Virginia) between April 2000 and January 2008. All procedures were performed by a single surgeon (C.G.L.). Approval by the Institutional Review Board was obtained prior to data collection and all ensuing protocols were followed.
Surgical Technique
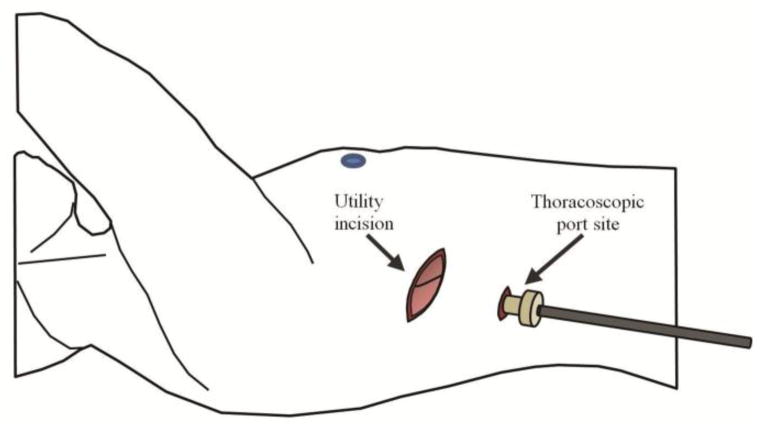
The hVATS technique has been previously described 10. Briefly, a 1 cm thoracoscopy incision is made in the eighth interspace. After port placement, thoracoscopic evaluation of the pleural surface is performed and the lesion(s) of interest is (are) identified. Thereafter, an 8 to 10 cm lateral “utility” incision is made over the region of focus (Figure 1). The utility incision allows for manual palpation of the lung parenchyma, better visualization, appropriate instrumentation for resection, delivery of the specimen, and emergency access. Hilar lymph node dissection is performed if applicable. The utility incision is closed in the standard fashion and the thoracoscopic site is used for chest tube placement. Rib resection is not performed.
Fig. 1.
Schematic description of hybridized video-assisted thoracoscopic surgery (hVATS). An 8 to 10 cm “utility” incision is made in addition to thoracoscopic port placement. This utility incision allows for increased visualization, better mobilization, and instrumentation of the target lesion and surrounding lung parenchyma.
Outcomes and Statistical Analyses
The following outcomes were evaluated: patient demographics; pathologic diagnosis; lesion number and size; extent of resection; operative time; length of hospital and intensive care unit stay; duration of chest tube; peri-operative mortality and morbidity; and five-year overall survival. Kaplan-Meier analysis was used to assess overall survival. Cox proportional hazard modeling was used to evaluate the effect of various patient, pathologic, and operative variables. Significance was set at p <0.05. All analyses were performed using IBM SPSS Statistics® Version 22.0 (New York, NY).
III. Results
The Demographics and Tumor Pathology
During the study period, 61 patients underwent 67 hVATS procedures. The mean age was 61.7 years with males accounting for 52% of subjects. The demographic characteristics for the patient population is summarized in Table 1.
Table 1.
Demographics of Patients Undergoing Hybrid Video Assisted Thoracoscopic Surgery for Pulmonary Metastasectomy
| Number of Patients | 61 |
| Mean age | 61.7 (range 23–85) |
| Gender | |
| Male | 32 (52%) |
| Female | 29 (48%) |
| Ethnicity | |
| Caucasian | 38 (62%) |
| African American | 19 (31%) |
| Other | 4 (7%) |
The majority of metastatic pulmonary lesions were genitourinary or gastrointestinal in origin, accounting for 37.7% and 31.2%, respectively (Table 2). Gastrointestinal lesions included 18 metastatic colorectal tumors and one originating from the gastro-esophageal junction. Genitourinary lesions included 18 renal cell cancers, 2 prostate cancers, 2 bladder carcinomas, and 1 testicular tumor. Breast cancer metastases accounted for 9.8% while melanomas included 7 cases. Sarcomas, gynecological tumors, and head and neck primaries made up 6.5%. In two cases, the resected nodules were described as “metastatic lesions of unknown primary.” The average tumor size was 2.43 cm with a range of 0.3 to 5 cm. Data pertaining to the number of lesions resected was available in 66 of 67 resections with 87.9% being solitary.
Table 2.
Pathology of Lesions Resected Using Hybrid Video Assisted Thoracoscopic Surgery
| TUMOR TYPE | NUMBER | PERCENTAGE |
|---|---|---|
| Genitourinary | 23 | 37.7 |
| Gastrointestinal | 19 | 31.2 |
| Melanoma | 7 | 11.5 |
| Breast | 6 | 9.8 |
| Other Tumor Types | 6 | 9.8 |
| Sarcoma | 2 | 3.3 |
| Unknown | 2 | 3.3 |
| Gynecologic | 1 | 1.6 |
| Head Neck | 1 | 1.6 |
| 61 | ||
| TUMOR SIZE (CM) | 2.43 (0.3 – 5) | |
| NUMBER LESIONS | ||
| 1 | 58 | |
| 2 | 6 | |
| 3 OR MORE | 2 | |
| UNKNOWN | 1 | |
| 67 | ||
| NODAL DISSECTION | ||
| Yes / No | 27/40 | |
| Avg No. Nodes | 9.1(1–37) | |
| Metastatic nodes | 7/247 |
Operative Details
The most common resection performed was lobectomy (43%), followed by segmentectomy (30%) and wedge resection (26%) (Table 3). A R0 resection was obtained in 59/61 (96.7%) of the primary resections. A second resection was performed in four patients and one went onto undergo a third resection; all these patients had a previous R0 resection. The mean time to a second operation was 60 months (range, 16 to 100). The mean tumor size for secondary resections was 1.2 cm; a solitary lesion was encountered in all cases. A third resection (lobectomy) was performed in one patient 7.7 months after his second operation and 17.6 months after his primary lung resection; five lesions were found, with the largest one being 1.3cm. Lymph node dissection was performed in 40% of resections. The mean operative time was 50 minutes. No intra-operative deaths occurred and no cases were converted to open thoracotomy.
Table 3.
Operative Details and Procedures Performed.
| PROCEDURE | |||
|---|---|---|---|
| Primary resection | Second resection | Third resection | |
| Lobectomy | 26 | 1 | |
| Segmentectomy | 18 | 3 | |
| Wedge resection | 16 | 2 | |
| Pneumonectomy | 1 | ||
| Total | 61 | 5 | 1 |
| OPERATIVE TIME (MIN) | 50 (19–139) |
Survival
The clinical outcomes for the patient population are summarized in Table 4. The mean follow-up for all patients was 39.7 months (0.3–129.4); fourteen patients were lost-to-follow-up with no clinical information available after initial hospital discharge. The mean length of stay was 4.4 days with only 6 patients requiring an ICU stay. The mean number of days with a chest tube was 2.9 days. Mortality within the first 30 days post-operatively was 6% (4 cases). Thirteen patients had 16 complications.
Table 4.
Selected Clinical Outcomes Among 61 Patients Undergoing Hybrid Video Assisted Thoracoscopic Surgery for Pulmonary Metastasectomy
| MEAN FOLLOW-UP (MOS.) | 39.7 (0.3–129.4) |
| LENGTH OF STAY (DAYS) | |
| ICU | 3.2 (1–6) |
| Overall | 4.4 (2–27) |
| DAYS with CHEST TUBE | 2.9 (2 – 670) |
| 30 DAY MORTALITY | 4 (6%) |
| COMPLICATIONS | 16 |
| Arrhythmia | 4 |
| Myocardial infarction | 2 |
| Pleural effusion | 4 |
| Empyema / Pneumonia | 3 |
| Neuropraxia | 2 |
| Chest Tube Site Granulation | 1 |
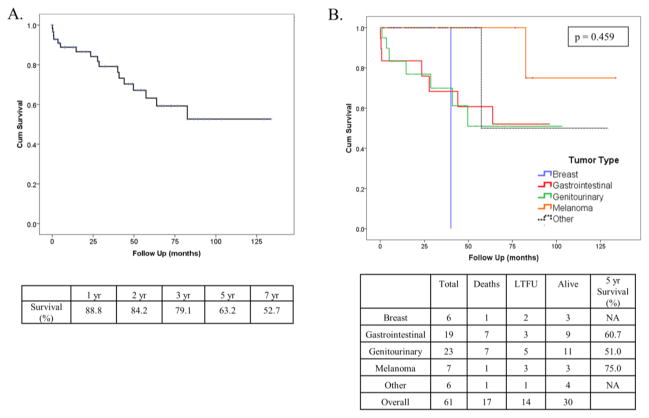
There were 17 known deaths during follow-up. On Kaplan-Meier analysis, median survival for the cohort was not reached; mean survival was 88.4 +/− 8.5 months (Figure 2A). One, two, five, and seven-year survival estimates were 88.8, 79.1, 63.2, and 52.7%, respectively. Although not statistically significant, patients with lesions of gastrointestinal origin, genitourinary origin, or melanoma had prolonged five-year survivals of 60.7, 51.0, and 75%, respectively (Figure 2B); median survival was not reached. Patients with metastatic breast cancers had a median estimate of 40.1 months and those in the “Other” cohort including sarcomas, head and neck tumors, gynecological lesions, and tumors of indeterminate etiology, had a median survival of 57.3 months. The number of tumors, specifically solitary lesions, was associated with longer survival compared to having 2 or more lesions (Figure 3A). Likewise, patients with smaller lesions under 4 cm had longer estimated survival than those with larger tumors (Figure 3B). Repeated resection, in our series, did not improve survival (p = 0.281).
Fig. 2.
(A) Overall survival. (B) Overall Survival By tumor type.
Fig. 3.
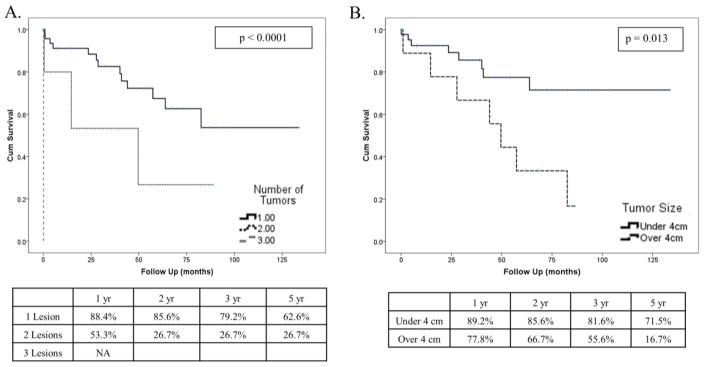
(A) Overall Survival By Number of Lesions and (B) Tumor Size.
Multivariate Analysis
Multivariate Cox-regression was performed on patient demographics, lesion size, tumor number, extent of resection, number of resections, and tumor biology (Table 5). A tumor size under 4 cm was the only covariate significantly associated with improved overall survival (HR 0.264, p 0.043, CI: 0.730 – 0.956).
Table 5.
Cox Proportional Hazard Univariate (A) and (B) Multivariate Regression.
| A. Univariate Analysis | |||
|---|---|---|---|
| Variable | Hazard Ratio | p Value | Confidence Interval (95%) |
| Age (yrs) | 0.99 | 0.774 | 0.948– 1.040 |
| Gender (M/F) | 2.10 | 0.145 | 0.774 – 5.720 |
| Tumor Type | |||
| Breast | 2.34 | 0.551 | 0.142 – 38.613 |
| Gastrointestinal | 2.16 | 0.472 | 0.265 – 17.635 |
| Genitourinary | 2.41 | 0.412 | 0.295–19.677 |
| Melanoma | 0.44 | 0.559 | 0.027 – 7.043 |
| Number of Resections | 2.91 | 0.303 | 0.381 – 22.250 |
| Extent of Resection (R0/R1) | 21.01 | 0.825 | 0.000 – 1.031E+13 |
| Number of lesions | 4.42 | 0.004 | 1.613–12.108 |
| Tumor < 4cm | 0.30 | 0.020 | 0.108 – 0.825 |
| B. Multivariate Analysis | |||
|---|---|---|---|
| Variable | Hazard Ratio | p Value | Confidence Interval (95%) |
| Age (yrs) | 1.023 | 0.474 | 0.962 – 1.087 |
| Gender (M/F) | 2.220 | 0.189 | 0.676 – 7.293 |
| Tumor Type | |||
| Breast | 8.383 | 0.201 | 0.324 – 214.421 |
| Gastrointestinal | 2.319 | 0.480 | 0.225 – 23.944 |
| Genitourinary | 4.893 | 0.210 | 0.409–58.590 |
| Melanoma | 0.458 | 0.614 | 0.022 – 9.534 |
| Number of Resections | 2.160 | 0.502 | 0.228 – 20.468 |
| Number of lesions | 1.370 | 0.687 | 0.296 – 6.343 |
| Tumor < 4cm | 0.264 | 0.043 | 0.073 – 0.956 |
IV. Discussion
Video-assisted thoracoscopic surgery for cancer was first described in the early 1990s [11, 12]. Clear benefits emerged from its application stemming from decreased pain, faster recovery, shorter length of stay, and reduced cost [13]. In terms of oncologic and clinical outcomes, the Z0030 randomized clinical trial demonstrated that patients who underwent VATS for primary lung cancer had similar operative mortality (0 vs 1.6%), lymph node retrieval rates (15 vs 19), and R1/2 resections (0 vs 2.3%) as those undergoing thoracotomy; the thoracoscopic group was also found to have fewer respiratory complications and need for invasive procedures including bronchoscopy (0 vs 6.3%) [14]. A subsequent meta-analysis compared 2106 VATS and 2661 thoracotomy patients; subjects who had thoracoscopic surgery had a five percent advantage in five-year overall survival [15].
Dowling et al reported on the first case series of thoracoscopic metastasectomy among thirteen patients; since then various longitudinal reports have supported its efficacy and feasibility among highly selected patients [6, 16]. Carballo et al compared 21 patients undergoing resection with VATS to 21 having an open thoracotomy primarily for breast and colorectal metastases [17]. Five-year overall survival was found to be similar between groups (54 vs 78%). Likewise, there was no difference in recurrence free survival (53 vs 57%); VATS patients, however, had non-significant trend towards decreased recurrence by 12.5%. A subsequent report among 186 patients (36 VATS and 135 open procedures) demonstrated similar 5-year overall and recurrence free survival rates (67 vs 51%) [18]. Patients undergoing thoracoscopic resection, however, were more likely to have unilateral lesions or single lesions. Gassot el al reported on 31 and 29 patients with sarcoma who, respectively, underwent VATS and thoracotomy for fewer than 2 nodules. Disease free survival, overall survival, and complication rates were equivalent, while length of stay favored the minimally invasive approach [19]. Limited reports concerning resections of gynecological pulmonary metastases have yielded similar outcomes [20].
Resections of colorectal lesions have yielded promising results. Lo Faso et al reported on 164 patients who underwent thoracoscopic resection [21]. While the cohort contained mixed tumor biology, the majority (60%) of lesions were colorectal with more than half of patients surviving the follow-up period. Nakas et al compared 25 VATS patients to 27 subjects undergoing open resection. Surprisingly, nearly a third of patients in each cohort had prior liver lesions that were resected. The VATS group had a trend towards shorter disease free interval from the resection of the primary cancer or liver tumor (19.2 vs 32.3 months). Short term survival at 2 years was not statistically different (72 vs 90%) [22]. Nakajima et al reported 72 and 71 patients undergoing VATS or thoracotomy, respectively, for colorectal cancer and found improved recurrence free survival (34 vs 21%) in the thoracoscopic approach. A non-significant trend towards improved survival was noted as well (49.3 vs 39.5%); the VATS group, however, was more likely to have smaller or fewer lesions and periphery located metastases. Factors negatively affecting survival included pulmonary lesion diameter and surgical resection by wedge resection. Recurrence was heavily influenced by multiple pulmonary metastases and nodal involvement [23]. Chao et al compared 70 patients undergoing either VATS or open thoracotomy for pulmonary colorectal disease using a matched case-control design; tumor number, size, and extent of resection were matched. No difference was noted in hospital mortality, recurrence, or five-year survival [24].
A hybrid approach to video-assisted thoracoscopic surgery was first extensively described by Okada et al in 200525. While similar techniques had been present earlier [26, 27], Okada et al first coined the term “hybrid VATS” and defined it to include a “minithoracotomy with television monitoring and direct visualization.” During a six year period, 570 hVATS procedures (405 lobectomies and 165 segmentectomies) were performed for primary lung cancer. Compared with traditional operative approaches, hVATS was shown to have equivalent pathological outcomes, survival, and morbidity. Kim et al described a similar technique (see Methods) and demonstrated its applicability for both benign and malignant disease among 1170 patients in a community hospital setting [9]. He et al assessed hVATS in complex resections including sleeve lobectomies for non-small cell lung cancer and demonstrated modest long-term survival (54.2% at 5 years) among 148 patients [28].
A version of hybrid VATS for pulmonary metastasectomy was first described by d’Amato et al in 2001 [29]. While being primarily reported for lung volume reduction surgery, six patients with bilateral pulmonary metastases were assessed. The procedure entailed performing a 6-cm anterolateral thoracotomy and manually inspecting one hemi-thorax for disease that would preclude a therapeutic resection. If a resection was feasible and was performed, the contralateral lung then would undergo inspection via a second thoracotomy incision. In this series, the average number of lesions was four and one additional lesion was identified though manual palpation. The reported length of stay was 8 days with a mean ICU stay of 2 days, and chest tube duration of 6 days. No long-term oncologic data was provided. Various other reports have described a transxiphoid or transdiagphramtic approach. Mineo et al published a series in 1999 among 13 patients undergoing metastasectomy via a thoracosopcic and subcostal incision with partial xiphoid resection [30]. The mean nodule size was 1.5 cm and 2 to 6 lesions were encountered per patient. No operative deaths were reported and follow-up was limited to only 10 months. Wright et al described a similar technique in 46 patients and described its limited applicability in obese patients and posterior lesions; no outcome data was provided [31]. Long et al reported on “hand-assisted thoracoscopic surgery” (HATS) in 2011 [32]. Like previous approaches, an epigastric incision was made, in addition to thoracoscopic port placement, and the thoracic cavity was entered via the sternocostal triangle. Here, 55 patients primarily underwent HATS for colorectal or hepatic malignancies. One lobectomy and 201 minimal resections were reported. Three-year and five-year overall survival rates were 60% and 47%, respectively.
In our series, we report on 61 patients undergoing 67 hVATS procedures for metastatic pulmonary disease in a community hospital setting. The overall survival for this cohort was 63.7% at five years. The reported overall survival among VATS only resections has ranged between 49.3% and 69.6% [17–19, 23]; any direct comparison remains difficult due to heterogeneous population, disease distribution, and varied technique. All lesions were assessed radiographically pre-operatively; the vast majority were unilateral and single (96.7% and 86.5%, respectively). This likely reflects the selection criteria of the practicing surgeon. In our series, five year estimated survival for solitary lesions was 62.6% compared to 26.7% for two lesions. The number of lesions has previously been cited as a strong determinant of prolonged survival [33–36]. In a prognostic analyses on a large cohort of 5206 cases, patients with solitary metastatic lesions had significant improved 5 year and 10 year survival (43 and 31% vs 34 and 24%, respectively) [37]. Similarly, unilateral disease has been associated with longer outcomes [38, 39]. The majority of patients in our study were able to achieve R0 resection (96.7%) after primary resection. Complete removal of pulmonary metastases is a recognized prognostic factor associated with survival [33, 37, 40]. Univariate analyses failed to recognize extent (R0 vs R1) of resection as being statistically significant due to only two patients in the latter group; no patient in the R1 cohort, however, survived beyond 3 months. Lastly, we demonstrated that lesion size (< 4 cm) was associated with overall survival. Larger tumor size has been associated with prolonged outcomes. Choong et al found lesions greater than 2 cm were independently predictive of mortality, while others have demonstrated a size greater than 3 cm as being prognostic [41–43]. Others have demonstrated a more inconsistent relationship with survival [44, 45]. Regardless, it appears that tumor size is not well characterized in some studies [18, 22, 23, 37], even larger series, making its contribution towards survival promising but inconclusive.
We also demonstrated that the hVATS technique allows for major pulmonary resections. On other published series, the most frequently performed resections for metastasectomy using a hybrid technique were wedge resection or segmentectomy [29, 32]. In our series, 27 lobectomies and 1 pneumonectomy were feasible without conversion to a traditional open resection. The utility incision allows for better visualization and instrumentation during mobilization and allows for such extended resections and delivery of a larger specimen. The mean length of stay was 4.4 days and is comparable to data reported from both VATS only and hybrid techniques [22, 28, 32].
Limitations to our study include its retrospective nature, lack of disease free interval from the primary tumor resection, and single surgeon practice. The community medical center in our study incorporates a broad, non-centralized system of medical oncologists and primary care physicians making availability of some clinical data limited. Clearly factors such as disease free interval are highly predictive of survival [37] but were largely unavailable during data collection. The selection of patients for hybrid VATS pulmonary metastasectomy was at the discretion of a single clinician who employed a rigorous schema in choosing patients who would most benefit from resection. Such factors favored those patients with unilateral disease and solitary lesions and in whom complete resection was highly likely. While some may argue selection bias, others would agree that careful weighing of such characteristics are crucial in optimizing long-term survival outcomes. Here, we demonstrate a five year survival of 63.2% in carefully selected patients.
V. Conclusions
The hybrid VATS approach is a minimally invasive technique with the ability to offer greater manipulation and resection of lesions, while allowing access during emergent situations. This technique is safe and feasible in the community hospital setting, affording a shorter length of stay with acceptable long-term survival in selected patients. Like traditional VATS, its routine applicability has yet to be determined; nonetheless, it may be a feasible alternative in the management of patients with pulmonary metastases.
Acknowledgments
This work was supported in part by the US National Institute of Health (NIH) under Grant R01CA160688 to K.T..
We appreciate the assistance of Mr. George Orr of the Chippenham Medical Center Institutional Review Board (Richmond, Virginia, USA).
Abbreviations
- hVATS
Hybrid video-assisted thoracoscopic surgery
References
- 1.Billmann F. A pioneer in medicine and surgery: Charles Sedillot (1804–1883) Int J Surg. 2012;10(9):542–546. doi: 10.1016/j.ijsu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Haight C. Pulmonary resection for solitary metastatic sarcomas and carcinomas. Surgery, gynecology & obstetrics. 1947;85(2):129–146. [PubMed] [Google Scholar]
- 3.Thomford NR, Woolner LB, Clagett OT. The Surgical Treatment of Metastatic Tumors in the Lungs. The Journal of thoracic and cardiovascular surgery. 1965;49:357–363. [PubMed] [Google Scholar]
- 4.Hornbech K, Ravn J, Steinbruchel DA. Current status of pulmonary metastasectomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;39(6):955–962. doi: 10.1016/j.ejcts.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Asbun HJ, Straznicka M, Strong VE. The role of minimal access surgery for metastasectomy and cytoreduction. Surgical oncology clinics of North America. 2007;16(3):607–625. ix. doi: 10.1016/j.soc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Dowling RD, Ferson PF, Landreneau RJ. Thoracoscopic resection of pulmonary metastases. Chest. 1992;102(5):1450–1454. doi: 10.1378/chest.102.5.1450. [DOI] [PubMed] [Google Scholar]
- 7.McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. The Annals of thoracic surgery. 1996;62(1):213–216. doi: 10.1016/0003-4975(96)00253-6. discussion 216–217. [DOI] [PubMed] [Google Scholar]
- 8.Mutsaerts EL, Zoetmulder FA, Meijer S, Baas P, Hart AA, Rutgers EJ. Outcome of thoracoscopic pulmonary metastasectomy evaluated by confirmatory thoracotomy. The Annals of thoracic surgery. 2001;72(1):230–233. doi: 10.1016/s0003-4975(01)02629-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim RH, Takabe K, Lockhart CG. Outcomes of a hybrid technique for video-assisted thoracoscopic surgery (VATS) pulmonary resection in a community setting. Journal of thoracic disease. 2010;2(4):210–214. doi: 10.3978/j.issn.2072-1439.2010.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim RH, Takabe K, Lockhart CG. A hybrid technique: video-assisted thoracoscopic surgery (VATS) pulmonary resections for community-based surgeons. Surg Endosc. 2010;24(3):700–704. doi: 10.1007/s00464-009-0615-z. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. Video-assisted thoracic surgical resection of malignant lung tumors. The Journal of thoracic and cardiovascular surgery. 1992;104(6):1679–1685. discussion 1685–1677. [PubMed] [Google Scholar]
- 12.Roviaro G, Rebuffat C, Varoli F, Vergani C, Mariani C, Maciocco M. Videoendoscopic pulmonary lobectomy for cancer. Surgical laparoscopy & endoscopy. 1992;2(3):244–247. [PubMed] [Google Scholar]
- 13.Swanson SJ, Batirel HF. Video-assisted thoracic surgery (VATS) resection for lung cancer. The Surgical clinics of North America. 2002;82(3):541–559. doi: 10.1016/s0039-6109(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 14.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. The Journal of thoracic and cardiovascular surgery. 2010;139(4):976–981. doi: 10.1016/j.jtcvs.2009.11.059. discussion 981–973. [DOI] [PubMed] [Google Scholar]
- 15.Taioli E, Lee DS, Lesser M, Flores R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013;44(4):591–597. doi: 10.1093/ejcts/ezt051. [DOI] [PubMed] [Google Scholar]
- 16.Lin JC, Wiechmann RJ, Szwerc MF, et al. Diagnostic and therapeutic video-assisted thoracic surgery resection of pulmonary metastases. Surgery. 1999;126(4):636–641. discussion 641–632. [PubMed] [Google Scholar]
- 17.Carballo M, Maish MS, Jaroszewski DE, et al. Video-assisted thoracic surgery (VATS) for resection of metastatic adenocarcinoma as an acceptable alternative. Surg Endosc. 2009;23(9):1947–1954. doi: 10.1007/s00464-008-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carballo M, Maish MS, Jaroszewski DE, Holmes CE. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg. 2009;4:13. doi: 10.1186/1749-8090-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? The Annals of thoracic surgery. 2009;87(1):238–243. doi: 10.1016/j.athoracsur.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Lim MC, Lee HS, Seo SS, et al. Pathologic diagnosis and resection of suspicious thoracic metastases in patients with cervical cancer through thoracotomy or video-assisted thoracic surgery. Gynecologic oncology. 2010;116(3):478–482. doi: 10.1016/j.ygyno.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Lo Faso F, Solaini L, Lembo R, et al. Thoracoscopic lung metastasectomies: a 10-year, single-center experience. Surg Endosc. 2013;27(6):1938–1944. doi: 10.1007/s00464-012-2691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakas A, Klimatsidas MN, Entwisle J, Martin-Ucar AE, Waller DA. Video-assisted versus open pulmonary metastasectomy: the surgeon’s finger or the radiologist’s eye? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009;36(3):469–474. doi: 10.1016/j.ejcts.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima J, Murakawa T, Fukami T, Takamoto S. Is thoracoscopic surgery justified to treat pulmonary metastasis from colorectal cancer? Interactive cardiovascular and thoracic surgery. 2008;7(2):212–216. doi: 10.1510/icvts.2007.167239. discussion 216–217. [DOI] [PubMed] [Google Scholar]
- 24.Chao YK, Chang HC, Wu YC, et al. Management of lung metastases from colorectal cancer: video-assisted thoracoscopic surgery versus thoracotomy--a case-matched study. The Thoracic and cardiovascular surgeon. 2012;60(6):398–404. doi: 10.1055/s-0031-1295574. [DOI] [PubMed] [Google Scholar]
- 25.Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest. 2005;128(4):2696–2701. doi: 10.1378/chest.128.4.2696. [DOI] [PubMed] [Google Scholar]
- 26.Solaini L, Prusciano F, Bagioni P, Di Francesco F, Basilio Poddie D. Video-assisted thoracic surgery major pulmonary resections. Present experience. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2001;20(3):437–442. doi: 10.1016/s1010-7940(01)00850-8. [DOI] [PubMed] [Google Scholar]
- 27.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? The Annals of thoracic surgery. 2001;72(3):879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 28.He J, Shao W, Cao C, et al. Long-term outcome of hybrid surgical approach of video-assisted minithoracotomy sleeve lobectomy for non-small-cell lung cancer. Surg Endosc. 2011;25(8):2509–2515. doi: 10.1007/s00464-011-1576-6. [DOI] [PubMed] [Google Scholar]
- 29.d’Amato T, Santucci TS, Macherey RS, Bartley S, Maley RH, Jr, Landreneau RJ. Bilateral anterior minithoracotomy with video assistance for lung volume reduction surgery and pulmonary metastasectomy. Surg Endosc. 2002;16(2):364–366. doi: 10.1007/s00464-001-0056-9. [DOI] [PubMed] [Google Scholar]
- 30.Mineo TC, Pompeo E, Ambrogi V, Pistolese C. Video-assisted approach for transxiphoid bilateral lung metastasectomy. The Annals of thoracic surgery. 1999;67(6):1808–1810. doi: 10.1016/s0003-4975(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 31.Wright GM, Clarke CP, Paiva JM. Hand-assisted thoracoscopic surgery. The Annals of thoracic surgery. 2003;75(5):1665–1667. doi: 10.1016/s0003-4975(02)04626-x. [DOI] [PubMed] [Google Scholar]
- 32.Long H, Zheng Y, Situ D, Ma G, Lin Z, Wang J. Hand-assisted thoracoscopic surgery for bilateral lung metastasectomy through sternocostal triangle access. The Annals of thoracic surgery. 2011;91(3):852–858. doi: 10.1016/j.athoracsur.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 33.Ayarra Jarne J, Jimenez Merchan R, Congregado Loscertales M, et al. Resection of pulmonary metastases in 148 patients: analysis of prognostic factors. Archivos de bronconeumologia. 2008;44(10):525–530. [PubMed] [Google Scholar]
- 34.Pfannschmidt J, Muley T, Hoffmann H, Dienemann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. The Journal of thoracic and cardiovascular surgery. 2003;126(3):732–739. doi: 10.1016/s0022-5223(03)00587-7. [DOI] [PubMed] [Google Scholar]
- 35.Iizasa T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. The Annals of thoracic surgery. 2006;82(1):254–260. doi: 10.1016/j.athoracsur.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Tronc F, Conter C, Marec-Berard P, et al. Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2008;34(6):1240–1246. doi: 10.1016/j.ejcts.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 37.Long-term results of lung metastasectomy prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. The Journal of thoracic and cardiovascular surgery. 1997;113(1):37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 38.Hirosawa T, Itabashi M, Ohnuki T, Yamaguchi N, Sugihara K, Kameoka S. Prognostic factors in patients undergoing complete resection of pulmonary metastases of colorectal cancer: a multi-institutional cumulative follow-up study. Surgery today. 2013;43(5):494–499. doi: 10.1007/s00595-012-0373-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Hanaoka N, Sato K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World journal of surgery. 2009;33(3):505–511. doi: 10.1007/s00268-008-9875-3. [DOI] [PubMed] [Google Scholar]
- 40.Meimarakis G, Ruttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. The Annals of thoracic surgery. 2013;95(4):1170–1180. doi: 10.1016/j.athoracsur.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Choong PF, Pritchard DJ, Rock MG, Sim FH, Frassica FJ. Survival after pulmonary metastasectomy in soft tissue sarcoma. Prognostic factors in 214 patients. Acta orthopaedica Scandinavica. 1995;66(6):561–568. doi: 10.3109/17453679509002316. [DOI] [PubMed] [Google Scholar]
- 42.Meimarakis G, Angele M, Staehler M, et al. Evaluation of a new prognostic score (Munich score) to predict long-term survival after resection of pulmonary renal cell carcinoma metastases. American journal of surgery. 2011;202(2):158–167. doi: 10.1016/j.amjsurg.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Younes RN, Fares AL, Gross JL. Pulmonary metastasectomy: a multivariate analysis of 440 patients undergoing complete resection. Interactive cardiovascular and thoracic surgery. 2012;14(2):156–161. doi: 10.1093/icvts/ivr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandioler D, Kromer E, Tuchler H, et al. Long-term results after repeated surgical removal of pulmonary metastases. The Annals of thoracic surgery. 1998;65(4):909–912. doi: 10.1016/s0003-4975(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 45.Rasalkar DD, Chu WC, Lee V, Paunipagar BK, Cheng FW, Li CK. Pulmonary metastases in children with osteosarcoma: characteristics and impact on patient survival. Pediatric radiology. 2011;41(2):227–236. doi: 10.1007/s00247-010-1809-1. [DOI] [PubMed] [Google Scholar]