Abstract
Objectives
This work measured the amount of bound versus unbound water in completely-demineralized dentin.
Methods
Dentin beams prepared from extracted human teeth were completely demineralized, rinsed and dried to constant mass. They were rehydrated in 41% relative humidity (RH), while gravimetrically measuring their mass increase until the first plateau was reached at 0.064 (vacuum) or 0.116 g H2O/g dry mass (Drierite). The specimens were then exposed to 60% RH until attaining the second plateau at 0.220 (vacuum) or 0.191 g H2O/g dry mass (Drierite), and subsequently exposed to 99% RH until attaining the third plateau at 0.493 (vacuum) or 0.401 g H2O/g dry mass (Drierite).
Results
Exposure of the first layer of bound water to 0% RH for 5 min produced a −0.3% loss of bound water; in the second layer of bound water it caused a −3.3% loss of bound water; in the third layer it caused a −6% loss of bound water. Immersion in 100% ethanol or acetone for 5 min produced a 2.8 and 1.9% loss of bound water from the first layer, respectively; it caused a −4 and −7% loss of bound water in the second layer, respectively; and a −17 and −23% loss of bound water in the third layer.. Bound water represented 21–25% of total dentin water. Chemical dehydration of water-saturated dentin with ethanol/acetone for 1 min only removed between 25 to 35% of unbound water, respectively.
Significance
Attempts to remove bound water by evaporation were not very successful. Chemical dehydration with 100% acetone was more successful than 100% ethanol especially the third layer of bound water. Since unbound water represents between 75–79% of total matrix water, the more such water can be removed, the more resin can be infiltrated.
Keywords: Adhesive dentistry, Bound water, Bulk water, Collagen, dentin, Hydrogen bonding
1. Introduction
The elegant x-ray diffraction studies of Ramachandran [1], Bella et al. [2–4], Kramer et al. [5–8], and the nuclear magnetic resonance (NMR) and other studies by Fullerton et al. [9] and Cameron et al. [10] demonstrated that most proteins are surrounded by bound water. Water distributes electrostatic charges more uniformly [10] than would be possible in the absence of water. Nowhere is water more important than in collagen, the most common structural protein in the body [11].
The work of Cameron et al. [10] demonstrated that removal of bound water from tendon collagen is very difficult, requiring high vacuum and elevated temperature for days to weeks. However, the relative fraction of total dentin matrix water that is bound versus free has not yet been determined. In resin-dentin bonding, using the wet bonding approach, if too much free water is left in the matrix, application of BisGMA-containing adhesive blends can induce macroscopic phase separation [12] and even nanoscopic phase changes [13]. The use of solvated primers in dentin bonding is an attempt to remove as much free water as possible in a clinically relevant time (30–60 sec), to minimize phase changes and provide more volume for resin uptake into resin-dentin interfaces. Residual free water results in increased nanoleakage [14,15], water-tree formation [16,17], and degradation of matrix collagen [19–21].
In adhesive dentistry, dentists acid-etch mineralized dentin with 37% phosphoric acid for 15 s to solubilize apatite crystallites from collagen fibrils so that there is room for solvated adhesive monomers to flow around exposed collagen fibrils, to obtain micromechanical retention of resin composites to the underlying mineralized dentin. Acid-etching uncovers and also activates matrix-bound endogenous proteases of dentin (MMPs and cathepsins) [19,20] that add water across specific peptide bonds to slowly hydrolyze any collagen fibrils in the hybrid layer that remain uninfiltrated with adhesive resins.
X-ray diffraction studies of the structure of hydrated collagen in bovine tendon [22] revealed that as the water content decreases, the intermolecular spacing decreases to 13 Å and then remains constant even at subzero temperatures. These results indicate that the water that remains within the dehydrated tendon collagen is due to bound water. Increases in water content above 2 g H2O/g dry matrix is due to the accumulation of unbound water [22].
In mineralized dentin, the only free water is located in dentinal tubules and represents about 10 vol% [23]. There may be a low vol% of bound water in mineralized dentin. However, within seconds of acid-etching dentin with 32–37% phosphoric acid, all apatite crystallites are solubilized and extracted by phosphoric acid. The 48 vol% mineral volume [24] is instantly replaced by water that is distributed among tightly-bound, loosely-bound and free water compartments [25].
Most studies of water bound to collagen have been done on bovine tendon collagen because its collagen fibrils are all arranged parallel to each other [9]. Both Fullerton et al. [9] and Cameron et al. [10] have reported that almost all of the total water in tendon collagen is bound water. Dentin matrix has a very different organization than tendon. Most of the collagen fibrils in dentin are randomly organized. The matrix is penetrated by millions of micro-sized hollow tubules filled with free water [23]. The exact distribution of free versus bound water in demineralized dentin is unknown [25]. Those author [25] identified unfreezable bound water and freezable water in demineralized dentin by differential scanning calorimetry (DSC) and by proton nuclear magnetic resonance (1H-NMR). Before resin infiltration of demineralized dentin powder, large 1H-proton peaks at 4.69 ppm and smaller ones at 0.229 ppm were identified. The peak at 4.69 ppm was assigned to bound water. Although that peak fell to about half its original size after resin infiltration, its persistence indicates that adhesive monomers diffuse over bound water in demineralized dentin during the infiltration phase of dentin bonding [25]. This is very important new information on how resin monomers interact with collagen at the molecular level. Grégoire et al. [26] speculated that residual bound water is needed to prevent the collapse of collagen fibrils in dentin matrices during resin bonding procedures.
Bound water is regarded as structural water [2–9]. Bound water does not freeze when bulk water freezes [22,25]. It is unlikely that very much bound water can be removed by evaporation. If bound water represents a significant volume of the intermolecular space between adjacent collagen molecules, there may not be enough room for resin-infiltration [26]. However, Takahashi et al. [27] reported that demineralization of dentin increased the volume of collagen water in dentin, and that HEMA and TEGDMA could equilibrate with that increased volume of water, indicating that such water is not bound, and that there is sufficient room between collagen molecules for free water. Because the ratio of bound to free water has never been measured in dentin matrices, there is uncertainty regarding how much bound versus free water exists in acid-etched dentin.
The purpose of the present study was to quantitate the amount of bound versus free water in demineralized dentin matrices, and to determine how much of that water can be removed by clinically-related bonding procedures within 1–2 min. The test null hypotheses are that the relative amount of bound water does not exceed that of unbound water, that ethanol or acetone does not remove all bound water from demineralized dentin matrices within 1 min, and that ethanol or acetone does not removal all unbound water from demineralized dentin within 1 min.
2. Materials and methods
2.1 Preparation of dentin beams and their complete demineralization
Although our major interest is in the bound and free water content of demineralized dentin surfaces that are only 1–10 μm deep [27], there are few quantitative analytical methods that can be applied to characterize the bound water content of such microscopic structures. A convenient alternative approach is the macromodel of the hybrid layer [28].
Unerupted human third molars were collected under a protocol approved by the institutional review board of the Georgia Regents University with informed content. Dentin beams 2 × 1 × 6 mm of mid-coronal dentin were prepared from extracted, unerupted human third molars (18–21 yrs) using an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA). All beams were completely demineralized in 10% phosphoric acid for 16 hrs at 25 °C with constant tumbling to create macromodels of hybrid layers [28]. Decreases in the modulus of elasticity of dentin beams have been used to demonstrate their complete demineralization [28]. The stiffness of mineralized dentin is 18–20 GPa, while the stiffness of completely demineralized dentin is only 1–2 MPa [29]. After rinsing the completely demineralized beams in running water for 60 min, the beams were placed in water and their modulus of elasticity were determined by 3-point flexure [29].
2.2 Drying of demineralized dentin under vacuum and elevated temperature
Specimens of completely demineralized dentin beams were dehydrated using the method reported by Cameron et al. [10]. Briefly, the demineralized specimens were placed in a vacuum oven (VWR Scientific, Inc., Atlanta, GA, USA) and subjected to −762 torr of vacuum (25 °C) created by a vacuum pump until their water content fell to 0.10 g H2O/g dry mass. The temperature of the oven was then raised 10 °C/hr beginning at 25 °C until it reached 90 °C. The loss of dry mass was followed gravimetrically for 7 days using a Mettler/Toledo AG ultramicrobalance (Model XPS, Greifensee, Switzerland) capable of measuring to the nearest 0.01 mg. Thermal denaturation of the dentin matrix may be prevented if such matrices are partially dried [30]. Previously published differential scanning calorimetry (DSC) studies indicate that the denaturation temperature (Td) of dentin matrix is 65.5 °C in water-saturated matrices, but 176.1 °C in relatively dry dentin matrices [30]. When the specimens fell to a water content of 0.001 g H2O/g dry mass, they were regarded as being absolutely dry.
2.3 Drying of demineralized dentin without vacuum or elevated temperatures
Specimens of completely demineralized dentin beams (2 × 1 × 6 mm) were placed in sealed polyethylene containers half-filled with anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Co., Xenia, OH, USA) at 37 °C for 3–4 weeks until they reached a constant dry mass of 0.001 g H2O/g dry mass.
2.4 Sequential adsorbtion of bound water from water vapor
The final dry weight of the demineralized dentin beams was 0.001 ± 0.001 g H2O/g dry mass. These dry specimens were then slowly rehydrated in a sealed 41 ± 1% relative humidity RH [31] water vapor chamber (Fisher Scientific Traceable Hygrometer, Waltham, MA) [30] at 25 °C. The slow gain in dry mass was measured every 10 min on a Mettler XPS microbalance for 2 hrs. When the water content of the beams reached 0.06 g H2O/g dry mass, the rate of gain of dry mass abruptly slowed down and reached a plateau after 100–120 hrs. This was assumed to indicate that the first layer of bound water had been adsorbed. The specimens were then transferred to a sealed chamber containing a RH of 60% [31] to measure the next increment in bound water adsorption. After about 25 hrs, the specimen’s mass reached a second plateau of mass gain. Then the specimens were placed in a sealed chamber containing 99% RH [31] and weighed until they reached the third plateau of mass gain.
2.5 Use of low relative humidity to determine if adsorbed water can evaporate
New specimens of demineralized dry dentin were created as described above. When the dry specimens were allowed to reach their first plateau in mass gain, they were transferred to a sealed polyethylene container half-filled with fresh dry Drierite for 5 min at 25 °C and then immediately reweighed to determine if any adsorbed water evaporated into a zero % RH environment.
The experimental specimens were then allowed to adsorb more water until they reached the second plateau in mass gain. Those specimens were then sealed in fresh dry containers at 0% RH for 5 min to determine if any of the adsorbed water could evaporate in 5 min at 25 °C.
After allowing the specimens to reach the third plateau in mass of adsorbed water, they were again sealed into a container of fresh dry Drierite for 5 min to determine if any of the adsorbed water could evaporate from the specimens at 25 °C.
Finally, all specimens were immersed in liquid water at 25 °C for 1 hr, gently blot-dried on water-moist Kimwipes (Kimberly-Clark, Roswell, GA, USA) and placed into a sealed container of fresh, dry, Drierite for 5 min to see how much of their fully-hydrated mass could be lost at 25 °C in 5 min in a zero RH environment.
2.6 Use of water-free acetone or ethanol to remove bound water
New specimens of demineralized dry dentin were created as described in the previous section. When they fell to a water content of 0.001 g H2O/g dry mass, they were allowed to adsorb water vapor at 25 °C, 41% RH to the first plateau of dry mass. They were then immersed in 100% acetone for 5 min, then removed and the solvents allowed to evaporate for 1 min in 25 °C air to remove residual solvent. Similar experiments were performed for specimens immersed in 100% ethanol for 5 min. These solvents were stored in closed containers containing water-drying polymers (Molecular sieves, catalog number M515–500, Fisher Scientific, Atlanta, GA, USA) to insure that the solvents were dry.
Similar experiments were performed with other completely dry demineralized dentin specimens allowed to adsorb water up the second plateau of dry mass. They were then immersed in 100% acetone or ethanol for 5 min. After removal from the solvents, 1 min was allowed for evaporation of residual solvents before the final dry mass was measured.
The specimens were then exposed to 99% RH and allowed to reach their third plateau of dry mass. They were then immersed in fresh 100% ethanol or 100% acetone for 5 min to determine how much of the bound water could be removed by chemical dehydration. After removal from the solvents, the solvent in the demineralized dentin beams was allowed to evaporate for 1 min prior to remeasurement of their dry mass.
2.7 Statistical analyses
The distribution of bound and unbound water was listed with means ± SDs. The water content was expressed both as mass and as mass of water per mass dry matrix in Table 1. In Table 2, the % loss of bound and unbound water after 5 min of evaporation at 0% RH was not normally distributed, so the data were compared by the Kruskal-Wallis test for multiple groups and Dunn’s multiple comparisons test. The percent change in bound water (Table 3) after 5 min exposure to ethanol or acetone was compared using a Kruskal-Wallis test followed by Dunn’s multiple comparisons test. The percent loss of total water from fully hydrated dentin immersed in acetone or ethanol for varying times was compared using a two-way ANOVA (solvent vs. time) (Table 4). Although the data passed normality (p = 0.785) and equality of variances (p = 0.530), they showed significant interactions. Multiple comparisons were done using the Least Squares Means test at α = 0.05. The power of that test with an α = 0.05, was 1.00 for solvent, 1.00 for incubation time and 1.00 for incubation time x solvents. When the distributions of bound and unbound water obtained by vacuum/elevated temperature were compared to those obtained using Drierite, the data failed the normality test (p<0.05) and the equality of variance test (p<0.05). The interactions between bound water and method of drying were significantly (p<0.01). Thus, the data were analyzed by Least Square Means. The power of the analyses at a α = 0.5 was 1.0 for plateau, 0.797 for drying method and 1.0 for plateau x drying method.
Table 1.
Distribution of total water in demineralized dentin matrices
| Plateau | |||||||
|---|---|---|---|---|---|---|---|
| Specimens dried in vacuum oven | Initial H2O content | Dry | First | Second | Third | Final H2O content | % Recovery |
| Mass (mg) | 13.74 ± 0.49 | 4.65 ± 0.24 | 4.94 ± 0.29 | 5.67 ± 0.33 | 6.93 ± 0.42 | 10.13 ± 0.7 | -- |
| g H2O/g dry mass | 1.981 ± 0.103 | 0.001 ± 0.001 | 0.064 ± 0.01 | 0.220 ± 0.01 | 0.493 ± 0.04 | 1.171 ± 0.046 | 59.3% |
| Specimens dried in Drierite | Initial | Dry | First | Second | Third | Final | % Recovery |
| Mass (mg) | 13.37 ± 0.69 | 4.57 ± 0.31 | 5.10 ± 0.34 | 5.43 ± 0.36 | 6.39 ± 0.44 | 12.09 ± 0.98 | -- |
| g H2O/g dry mass | 1.931 ± 0.163 | 0.0008 ± 0.0012 | 0.116 ± 0.003 | 0.191 ± 0.004 | 0.401 ± 0.01 | 1.644 ± 0.094 | 85.0% |
| * MD simulation values | 0.000 | 0.0740 | 0.637 | ||||
Values are means ± SD (n = 10).
Molecular Dynamic Simulations – computer simulation of water binding to dry collagen.
Table 2.
Loss of bound water by 5 min evaporation in 0% RH sealed chamber
| Specimen dried in Drierite | % decrease in mass |
|---|---|
| First layer bound water | −0.295% ± 0.86%a |
| Second layer bound water | −3.27% ± 0.53%a,b |
| Third layer bound water | −6.17% ± 1.43%b,c |
Values are mean ± SD of % decreases in total water loss of Drierite dried specimens. Groups identified by different superscript letters are significantly different (p<0.05).
Table 3.
Loss of bound water by 5 min of chemical dehydration
| Specimens dried in Drierite | Ethanol | Acetone |
|---|---|---|
| First layer of bound water | 2.80 ± 2.49%a | 1.90 ± 0.06%a |
| Second layer of bound water | −3.65 ± 1.15%b | −7.41 ± 0.94%d |
| Third layer of bound water | −16.62 ± 2.02%c | −23.30 ± 2.60%e |
Values are percent gain or loss of bound water in mean ± SD (n = 6). Groups identified by different superscript letters are significantly different (p<0.05).
Table 4.
Percent loss of total water in wet, demineralized beams following immersion in neat acetone or ethanol
| Time (min) | Acetone | Ethanol |
|---|---|---|
| 1 | −34.40% ± 3.48%A | −24.65% ± 2.44%B |
| 2 | −43.29% ± 2.24%C | −30.08% ± 2.09%D |
| 5 | −58.22% ± 1.99%E | −34.99% ± 4.27%F |
| 10 | −71.76% ±2.95% G | −38.38% ± 3.78%F |
Values are mean ± 1 SD (n=5). Groups identified by different superscript letters are significantly different (p<0.05). Statistical analyses were done using a two-way ANOVA (solvent vs. time) that passed normality (p = 0.785) and equality of variance (p = 0.530) but had significant interactions. Multiple comparisons were done using the Least Squares Means test.
2.8 Molecular Dynamic Simulation of water-binding to collagen
To perform Molecular Dynamic (MD) simulations of molecular events occurring during resin-dentin bonding, a 3-D model of collagen was constructed using The BuScr. 1.07 [32], and two of such collagen models were integrated into a simulation box (15.6 Å × 27 Å × 153 Å) to have a hexagonal packing. We employed AMBER99SB-ILDN force field [33] which is known to be the most updated force field for proteins. Water molecules were described by the TIP3P water model [34]. After the energy minimization of the collagen model, MD simulations were performed using GROMACS 4.6.1 [35]. Starting with the initial configuration, the model was energy-minimized to prepare a reasonable starting structure in terms of geometry and solvent orientation, a NVT MD simulation was performed from 10K to 300K for 500ps to stabilize the temperature and pressure of the system, and subsequently, a NPT MD simulation was carried out for 15 ns to equilibrate the system. Then, another NPT MD simulation was performed for 2 ns to collect statistical data for analysis. The first layer of strongly bound water to carbonyl oxygen and amide hydrogens (Ramachandran water bridges) was defined as the waters within the distance of 0–2.25 Å from collagen, and the second layer of less strongly bound water was defined as the waters within the distance of 2.245–3.25 Å from collagen.
3. Results
3.1 Sequential adsorption of bound water
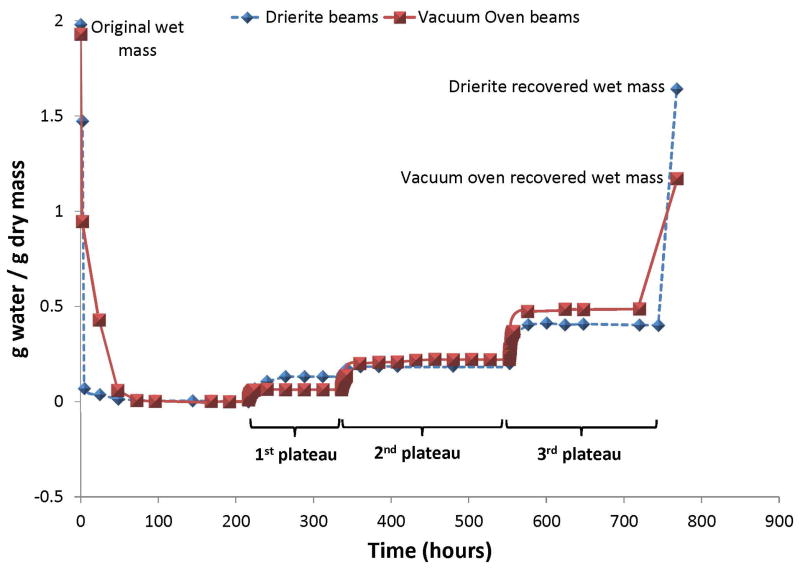
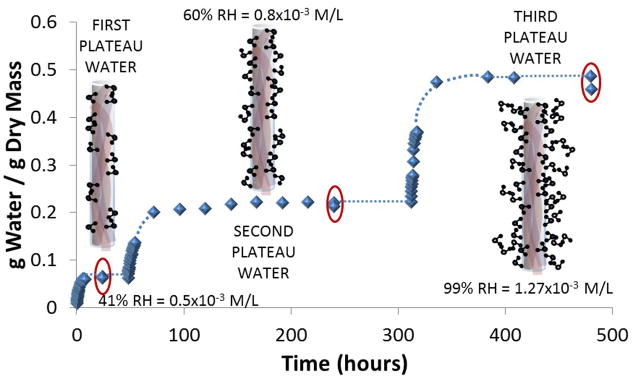
Prolonged drying in a vacuum oven at elevated temperature (90°C) for 8 days produced very dry dentin beams that had a water content of 0.001 ± 0.001 g H2O/g dry mass (Table 1, Fig. 1). This value was regarded as absolutely dry dentin. When these beams were allowed to hydrate at 41% RH at 25 °C, they rapidly gained dry mass over 4 hrs. At 4 hrs, the rate of rehydration of dentin matrices by water vapor suddenly slowed to a lower rate. The water content at the point where the water vapor adsorption reached its first plateau was 0.064 g H2O/g dry mass. The second water vapor adsorption reached a plateau at a water content of 0.220 g H2O/g dry mass. These values were assumed to be the mass of the matrix plus the first and second layers of adsorbed water, respectively. When the third layer of bound water reached a plateau, the water content reached a new level of 0.493 g H2O g/dry mass.
Fig. 1.
Adsorption of water vapor on dentin matrices that had been completely dried by high vacuum and elevated temperature. Water content is given in g H2O/g dry mass. Symbols indicate the means of 10 separate specimens. The standard deviations were smaller than the size of the symbols. The water content of dry matrices was 0.001 g H2O/g dry mass. Exposure of dry matrices to 41% RH produce absorption of bound water that plateaued at a water content of 0.064 g H2O/g dry mass. This first layer of bound water is thought to be due to Ramachandran single water bridges with carbonyl oxygens and amide hydrogens. Exposure of the first layer of bound water to 0% RH for 5 min produced very small loss of mass (see oblong symbol). Exposure of these specimens to 60% RH water vapor induce further water binding that reached a second, higher plateau at 0.220 g H2O/g dry mass. This layer of bound water is thought to be due to the formation of double water bridges. Exposure of the second layer of bound water to 0% RH for 5 min produced small loss of mass (see oblong symbol). When the specimens were exposed to 99% RH, they bound more water until they reached a third plateau at 0.493 g H2O/g dry mass. This third increment in bound water is thought to be due to water binding to hydrophilic side chains in collagen peptides that extend away from the central axis. Exposure of the third layer to 0% RH for 5 min produced a larger loss of mass (see oblong symbol). After 5 min, the specimens were replaced in 99% RH and they regained their original mass.
As will become apparent later in the present study, dentin specimens that were subjected to high vacuum and elevated temperatures were unable to rehydrate in liquid water back to their initial water content (Table 1). This led us to repeat specimen dehydration/rehydration studies at room temperature using prolonged exposure to 0% RH in sealed Drierite containers. In Drierite, the first plateau in mass occurred at a water content of 0.116 g H2O g/dry mass, instead of the water content seen in vacuum/heated specimens of 0.064 g H2O g/dry mass. In Drierite-treated specimens, the second plateau in mass occurred at a water content of 0.191 g H2O g/dry mass, instead of the 0.220 g H2O g/dry mass seen in vacuum/heated specimens. In Drierite-treated specimens, the third plateau in mass occurred at a water content of 0.401 g H2O g/dry mass, instead of the 0.493 g H2O g/dry mass seen in vacuum/heated specimens.
3.2 Loss of adsorbed water by evaporation for 5 min from specimens dried at elevated temperature
When specimens dried in high vacuum that had reached their first adsorption plateau were sealed in an air-tight container of fresh dry Drierite (relative humidity = 0%) for 5 min at 25 °C, and then immediately reweighed, they lost −0.295% of bound water (Table 2, Fig. 1 oblong symbol). When specimens dried in high vacuum that had reached their second plateau were sealed in containers of fresh Drierite for 5 min and then immediately reweighed, they lost 3.27% of bound water (Table 2, and oblong symbol).
When specimens dried in high vacuum that had reached their third plateau in mass were sealed in fresh Drierite for 5 min, and then immediately reweighed, they were found to have lost 6.17% of bound water (Table 2, and oblong symbol).
3.3 Removal of bound water by ethanol or acetone
When vacuum-dried dentin beams that had adsorbed the first layer of water vapor were immersed in 100% acetone or ethanol for 5 min, and the volatile solvents allowed to evaporate for 1 min, the percent decrease in total water was +2.80 ± 2.49% and +1.90 ± 0.069%, respectively (Table 3). That is, there was no loss of mass, but there was a slight gain in mass but it was not significantly different from zero. Thus, there was no loss of mass of the first layer of bound water, but they had gained mass due to the fact that 1 min was not enough to evaporate all residual volatile solvent from the specimens.
When specimens that had adsorbed the second layer of bound water were exposed to these solvents for 5 min, ethanol removed −3.65 ± 1.15% of the bound water, while acetone removed −7.41 ± 0.94%, indicating that these solvents had removed significant (p<0.05) amounts of bound water. When these procedures were repeated on specimens that had bound the third layer of water, ethanol removed −16.62 ± 2.02%, while acetone removed −22.30 ± 2.60% of the bound water (Table 3). That is, ethanol removed −16.62% (Table 3) of the third layer of bound water that comprised 13.72% of total water (Table 5). Thus, ethanol removed 0.1662% × 13.72% = 2.28% of the third layer of bound water. Each of these values of solvent removal of bound water were significantly difference from all others (p<0.05).
Table 5.
Percent distribution of total water in dentin matrix
| Fractional water | Vacuum oven | Drierite |
|---|---|---|
| 1st plateau | 3.21 ± 0.60%a | 6.03 ± 0.59%B |
| 2nd plateau | 7.97 ± 0.74% b | 3.71 ± 0.33%A |
| 3rd plateau | 13.72 ± 1.25%c | 11.22 ± 1.29%C |
| Total bound water | 24.90 ± 1.69%d | 20.96 ± 2.16%D |
| Total unbound water | 75.10 ± 1.69%e | 79.04 ± 2.16%E |
| Total water | 100% | 100% |
Values are mean ± SD (n=10). Groups identified by different lowercase letters in columns are significantly different (p<0.05). Groups identified by different uppercase letters in rows are significantly different (p<0.05). Statistical comparisons were done with a two-way ANOVA (drying method vs. time plateaus) using Holm-Sidek test for multiple comparisons at α = 0.05.
3.4 Removal of unbound water by ethanol or acetone
Another group of 10 beams that had never been dried were removed from liquid water, lightly blotted on water-moist Kimwipes and immediately weighed to determine their initial total mass. These specimens were immersed in 100% ethanol or acetone for 1, 2, 5 or 10 min. The volatile solvents were then allowed to evaporate for 1 min before reweighing to determine how much mass was removed by acetone or ethanol. The results are summarized in Table 4. When immersed in 100% ethanol, for 1, 2, 5 or 10 min, the specimens lost −24.7, −30.1, −35.0 and −38.4% of their total mass content, respectively. When the specimens were immersed in 100% acetone for the same time periods, they lost −34.4, −43.3, −58.2 and −71.8% of their total mass content, respectively (Table 4). We assume that these losses of total mass were due to the loss of unbound water.
3.5 Comparison of bound versus unbound water in demineralized dentin
To measure the relative amount of bound water compared to the unbound water, we measured the initial wet mass. We then substracted the absolutely dry mass from the initial wet mass to obtain the total mass of water. To measure the first plateau in bound water, we measured the first plateau mass of bound water minus the absolutely dry mass/total water content = % water content of first plateau. The same procedures were repeated for the second plateau of bound water. The second plateau bound water was measured by determining the second plateau mass minus the first plateau mass, divided by the total water content. For the third plateau of bound water, we measured the third plateau mass minus the second plateau mass divided by the total water content. Table 5 summarizes the results of this part of the study. Specimens dried at high vacuum and elevated temperatures exhibited bound water plateaus of 3.21, 7.97 and 13.72% of total water mass. Thus, bound water represented 24.9%, while unbound water represented 75.1% of the total water present in completed demineralized dentin matrices. For specimens dried in Drierite at 25 °C, the bound water plateaus were 6.03, 3.71 and 11.22%, giving a total bound water of 20.96%, while the unbound water represented 79.04% of the total dentin water (Table 5). Within the vacuum-treated group, each procedure produced statistically significant differences (p<0.05) compared to the previous state.
4. Discussion
As bound water in dentin only represents 21–25% of total dentin water (Table 5), the first null hypothesis that :there is no difference in the fraction of bound versus unbound water “ has to be rejected. Because ethanol and acetone did not remove more than −23% of bound water from demineralized dentin, we must reject the second null hypothesis “that ethanol and acetone cannot remove all bound water from dentin” (Table 3). Because only 24.7% of unbound water was removed from demineralized dentin in 1 min by ethanol and only 34.4% of unbound water was removed in 1 min by acetone (Table 4), the third null hypothesis that “ethanol and acetone does not remove all bound water from demineralized dentin matrices within 1 min” must be rejected.
Results from the present study confirm the work of previous authors [3,4,9] that collagen-bound water is resistant to removal by drying or the use of water-free polar solvents such as acetone or ethanol. Although bound water in completely demineralized dentin matrices only accounts for about 21–25% of the total water (Table 5), it is likely that solvated resin comonomers diffuse over the most tightly-bound water during resin infiltration [24] without removing it. Water bridging is used to span collagen peptides more than 5 Å apart [4]. Water bound to collagen has been characterized as a monomolecular layer [9], while others described the bound water as the first plateau, second plateau and third plateau of water [3,4].
The first water to bind to demineralized dentin occurred at a relatively low vapor pressure (41% RH) indicating that this water had highest affinity for the collagen matrix. In their famous review article on the structure of collagen. As early as 1961, investigators proposed that the triple helix of collagen was stabilized by one direct hydrogen bonds between every three amino acids in collagen peptides [36]. Later, others [1] extended that model by limiting direct hydrogen bonds to one such bond per three amino acid residues when adjacent peptides were within 3Å of each other, plus one and two water bridges when the peptides were separated by >5Å. Theoretical predictions of the bound water content of collagen occupied by Ramachadran water bridges (HRa) was made by assuming that the mean molecular weight of the average amino acid in collagen peptides is 91.2 kDa [37].
That is, as soon as the water content of dry demineralized dentin reached 0.0658 g H2O/g dry mass, all of the direct/indirect (e.g. water bridges) intramolecular hydrogen bonding had occurred. This is very close to the water content that was observed (0.064 g H2O/g dry mass) when demineralized dentin specimens had been dried in high vacuum at elevated temperatures (Table 1) and exposed to 41% RH. When we repeated the experiments at ambient pressure and temperature in Drierite, we obtained values that were twice the predicted values. We interpret that as being due to the fact that the Drierite dried dentin was not truly dry, and that it contained undetermined amounts of Ramachadran water, which is the last water to be driven off during drying, because it is the most tightly bound. When we modeled the hydration of water-free collagen peptides by Molecular Dynamic simulation, we obtained an initial water content of 0.076 g H2O/g collagen and a fully hydrated value of 0.637 (Table 1). The first simulation-predicted value was 17% above the measured value, while the predicted fully-hydrated collagen was 29% above the measured value for the first plateau of bound water (Table 1). Thus, the Molecular Dynamic (MD) simulations validated our measured values. Currently, MD simulations cannot predict how much water would be in dentinal tubules or their lateral branches, because it only simulates at the molecular level.
Fullerton and Rahal [37] pointed out that further water sorption produces Ramachadran water multiples of the first water adsorption. For instance, the second water sorption seems to occur into “cleft water” which is made up of a single water bridge plus double water bridges. Lim [38] proposed that water bound in the grooves of the collagen molecule, contains four water molecules per tri-peptide (i.e. 3 amino acids). This concept predicts that the second plateau in water adsorption should produce a hydration level (Hcleft) of 4 × 18/(3 × 91.2) = 0.2632 g H2O/g dry mass. Our second plateau in adsorbed water mass occurred at 0.220 g H2O/g dry mass in specimens dried in vacuum at elevated temperature and 0.191 g H2O/g dry mass in specimens dried in Drierite. We speculate that the lower value for the Drierite group may be due to the fact that (0.12 – 0.06 = 0.07) 0.07 g H2O/g dry mass was erroneously attributed to the first plateau. If we remove it from the first plateau and add it to the second (0.19 ± 0.07 = 0.260 g H2O/g dry mass), we obtain 0.260 that is very close to the predicted value of 0.263 g H2O/g dry mass. The third plateau in adsorbed water is thought to be due to water binding to side chains extending off the primary backbone of collagen. This can account for up to 0.54 g H2O/g dry mass [10] or 8 × 0.0658 g H2O/g dry mass.
This value is not a multiple of Ramachadran water. In the current study, the third plateau of bound water in the vacuum dried group was 0.493 g H2O/g dry mass, while it was 0.401 g H2O/g dry mass for the Drierite group, to give a final water content of 1.171 g H2O/g dry mass (Table 1) in the vacuum group and 1.644 g H2O/g dry mass in the Drierite group.
The differences in the above values may be due to differences in the orientation of dentin collagen fibrils, being randomly oriented in dentin, but parallel oriented in tendon. We believe that no free water accumulates in dentinal tubules or their lateral branches, until specimens are immersed in liquid water.
The similarities between the three plateaus in bound water mass in dentin vs. their theoretical predictions is reassuring because it suggests that our assumption is correct that initial water adsorption in dentin is following the results obtained by Cameron et al. [10] using tendon. Unlike tendon, which has hardly any free water, most of total water in demineralized dentin matrices is unbound (79–75%) compared to bound water that only occupies 21–25% of total water in dentin matrices (Table 5).
An alternative approach to studying bound versus unbound water in connective tissues is to use NMR. Fullerton et al. [9] measured the 1H-NMR spin-lattice relaxation of tendon collagen at different levels of hydration. They reported three different water relaxation times in addition to bulk water. Bound water has significantly slower relaxation times than free water. These authors found little bulk water in mammalian tendon; that is, most of the water in tendon is bound water. Traore et al. [39] measured transverse relaxation times of 1H-NMR in several water compartments of randomly-oriented connective tissue (epymysium). Their results showed that 55 ± 4% of the connective tissue contained tightly-bound water with low water diffusion coefficients, while 40 ± 3% contained loosely-bound water with moderate diffusion coefficients. Bulk water only occupied 5 ± 1% of the volume of the connective tissue. It had the lowest transverse relaxation time and the highest diffusion coefficient among the different categories of water associated with soft tissue collagen.
Cameron et al. [10] reported that tendon collagen contained three different bound water compartments: 1) a tightly-bound backbone water compartment consisting of 0.263 g H2O/g dry mass; 2) a side chain compartment (0.54 g H2O/g dry mass), which together with the backbone compartment, constituted the primary hydration compartment (0.8 g H2O/g dry mass) and 3) a less tightly-bound secondary hydration compartment (0.8 g H2O/g dry mass), giving a total hydration of 1.6 g H2O/g dry mass with little unbound water. The initial water content of demineralized dentin is significantly higher (p<0.01) than that of tendon, being 1.931–1.981 g H2O/g dry mass (Table 1).
Clearly, dentin matrix has a higher total water content than other connective tissues because dentin matrices are penetrated by dentinal tubules that are full of unbound water, and because the collagen fibrils are randomly-oriented instead of being parallel to each other. Due to the convergence of tubules toward the pulp chamber, there are fewer tubules per unit area in superficial dentin (i.e. about 1%) than in deep dentin (about 22%) [40]. Because the dentin specimens employed in the present study were derived from mid-coronal dentin, one may assume that their average tubule water content was about 10 vol%. However, branching off from each dentinal tubule are numerous arborising lateral branches of the main tubules that we speculate may occupy another 10% of the total dentin water. Thus, at least 20% and as much as 30% of the 75% unbound water is distributed within dentinal tubules and their branches. The random arrangement of collagen fibrils in dentin matrices increases the interstitial unbound water content. Both tendon and dentin contain proteoglycans between collagen fibrils [41,42]. Acid-etching of dentin with 35% phosphoric acid apparently denatures dentin proteoglycans after 15 s of etching [42]. The presence of proteoglycans in the interfibrillar spaces may act as molecular sieves and interfere with inward diffusion of dimethacrylates such as BisGMA [12,13,44].
Because about 90% of the dentin matrix is type I collagen [45], most of the bound water is bound to collagen. However, bound water must also bind to proteoglycans [41,42] and other non-collagenous proteins, including MMPs and cathepsins. It is unlikely that water bound to protease hydrolases can participate in hydrolysis of collagen. Rather, hydrolases probably activate free water molecules to cleave covalent bonds. More research needs to be done in that area.
Liquid water has a molar water concentration of 55.6 moles/L (1000 g/L / 18 g/mole = 55.6 moles moles/L). Water vapor has a much lower water concentration than liquid water. The mass of water vapor at 100% relative humidity (RH) at 25°C is 23.05 g H2O/m3 or 0.023 g H2O/L [31]. By dividing that water mass by the molar concentration of liquid water (55.6 moles/L), one obtains a water concentration in water vapor of only 4.14 × 10−4 moles/L. Thus, by using water vapor, one can expose dentin matrices to extremely low water concentrations, and slowly gravimetrically titrate that water onto the highest affinity binding sites, followed by the intermediate affinity binding sites, ending with the lowest affinity binding sites.
Chemical groups on collagen with the highest affinity for binding water, will become saturated with water vapor at the lowest relative humidity. Thus, the first plateau in the mass of adsorbed water would have the highest affinity for water. The second plateau in mass of adsorbed water would have the next highest affinity for water, while the third plateau in mass of adsorbed water would have the lowest affinity for water vapor, requiring a water vapor concentration of 99% RH to saturate those sites.
In the current experiment, small amounts of bound water could be evaporated from the first or second plateau of bound water when they were exposed to 0% RH, while a slightly larger fraction of bound water could be evaporated from bound water at the third plateau (Table 2). Similarly, exposure of dentin matrices that had reached the first plateau in bound water mass to ethanol or acetone removed little or no bound water, while a small fraction (3.7–7.4%) of the second plateau could be removed by ethanol or acetone (Table 3). However, when specimens that had adsorbed their third layer of bound water were exposed to ethanol for 5 min, the specimens lost −16.62% ± 2.02% of the 13.7% of bound water (compare Table 3 to Table 5), while acetone exposure caused a −23.30% loss of bound water from that same 13.7% of bound water. The gradual increase in the ability of water-free but water-miscible solvents to remove bound water when comparing the 1st, 2nd, and 3rd layers suggests that chemical dehydration can remove more of the least-firmly bound water (e.g. third layer) than more tightly bound water.. These results are consistent with the predicted affinities of bound water discussed above.
When water is adsorbed into hydrophilic resin matrices in water sorption/solubility studies, it becomes hydrogen-bonded to functional groups in the polymers that are capable of H-bonding (e.g hydroxyl groups of HEMA or BisGMA). This water is regarded as both tightly and loosely bound water [46]. It is not clear whether there is any free water (i.e. unbound water) in polymerized resins or is free water only found in water-filled diffusion channels called water-trees [47,48]. In cell membranes, bulk water passes across lipid boundaries via protein-lined water-filled membrane channels called “aquaporins” [49,50].
The model used in the present study to measure the rate and extent of bulk water removal from completely demineralized dentin is not clinically relevant. When using etch-and-rinse adhesives, acid-etching dentin with 37% phosphoric acid for 15 sec only removes the mineral matrix from the top 5–10 μm of dentin. Thus, the actual completely demineralized zone is only 5–10 μm thick, not 1 mm as it was in the completely demineralized dentin beams used in the current study. If one uses 1 min as the clinically relevant time for chemical dehydration of acid-etched dentin by primers containing acetone or ethanol, then the results show that when using acetone to remove water, only −34.4% of the free water is removed (Table 4). Even if clinicians are willing to wait for 5 min (an unrealistic time), acetone only removes −58.2% of unbound water. This leaves far too much residual water in bonded dentin [21].
The observation that the final water content of rehydrated demineralized dentin that had been completely dehydrated by elevated temperature (90°C) and high prolonged vacuum was only 1.171 ± 0.046 g H2O/g dry matrix compared to its original total water content of 1.981 ± 0.103 g H2O/g dry matrix (p<0.05) (Table 1, 59.3% recovery), indicates that creation of absolutely dry dentin matrices using heat and prolonged vacuum may alter their structure. When specimens were dried at room temperature in Drierite, the percent recovery was 85% (Table 1). Wang et al. [51] reported that the strength and stiffness of reconstituted tendon collagen could be improved by drying them at elevated temperature (110°C for 5 days). This is thought to remove some of the loosely-bound water (i.e. third plateau) that allows for more interpeptide hydrogen bonding between adjacent collagen peptides. More recently, Hayashi et al. [52] reported that heating mineralized dentin beams to 110–140°C could strengthen the beams. In a more recent study, Hayashi et al. [53] repeated those studies but then rehydrated the previously dehydrated specimens. These later studies indicate that some, but not all, of the increased flexural strength was lost on rehydration. More permanent changes in flexural strength were obtained by UV irradiation of dentin (5 min, 3200 mW/cm2). Thus, the inability of the dry demineralized dentin matrices, in the present study, to regain their original level of hydration may be due to structural changes in dry dentin resulting from the loss of bound water that was not replaced in exactly the same structures as it originally existed [54,55]. Thus, these gravimetric results should be confirmed by nondestructive 1H-NMR studies.
The results of the present work indicate that much of the bound water in demineralized dentin matrices cannot be removed in a clinically-relevant time (1–2 min). The fact that 5 min of spontaneous evaporation of free water at 0% RH only removed 3–6% of the bound water in the first two plateaus (Table 2), while it did not remove much of the bound water (Table 2), indicates that clinical attempts to remove free water should concentrate on evaporation of bulk water. However, when such procedures were tried by Fusayama [56], very low resin-dentin bond strengths were obtained (e.g. 2 MPa). This is because air-drying causes collapse of collagen fibrils upon themselves allowing adjacent collagen peptides to hydrogen bond with each other, which obliterates the interfibrillar spaces that serve as diffusion channels for resin comonomer infiltration [28]. When bond strengths fall below the forces of polymerization contraction (ca. 15 MPa), restorations debond from one or more walls of restorations during light-curing to form water-filled marginal defects that become colonized by bacteria. The collapse of the collagen network during air-drying is the result of its modulus of elasticity falling from 19–20 GPa in mineralized dentin to 1–2 MPa in completely demineralized dentin [29]. We speculate that if demineralized dentin can be stiffened by cross-linking agents [57,58], they may become stiff enough to prevent the collagen fibrils from collapsing when water is evaporated.
The time to evaporate water from acid-etched dentin is immediately after acid-etching and rinsing. At tooth temperature (34 °C), pure water has a vapor pressure of 40 mm Hg (40 Torr). After addition of adhesive monomers (containing up to 3–4 moles/L), the vapor pressure of water is reduced according to Raoult’s Law (molar masses of non-volatile, non-electrolytes lower the vapor pressure of their solvent). An additive problem is that as water is evaporated from the mixture, it increases the molar concentration of adhesive monomers, further reducing the vapor pressure of water [59,60]. Clearly, the current approaches to resin-dentin bonding need to be modified to facilitate removal of unbound water so that more adhesive monomers can envelope collagen fibrils.
The results of the present study indicate that any concerns investigators had about inadvertent removal of bound water from collagen fibrils during resin-dentin bonding are of minor importance compared to the excess of residual unbound water.
5. Conclusion
Because bound water only represents 21–25% of the total water in demineralized dentin, there is the opportunity to remove up to 75–79% of the unbound water in dentin and replace it with 75 wt% adhesive resins during adhesive bonding procedures. The bound water in dentin matrices that cannot be evaporated or removed by organic solvents, may hold the interfibrillar spaces between collagen fibrils open to serve as diffusion channels for resin infiltration. The unbound water must be removed prior to application of adhesive monomers because addition of nonvolatile monomers lowers the vapor pressure of water [59,60], making it difficult to remove water by evaporation.
Fig. 2.
Change in water content (g H2O/g dry mass) of absolutely dry demineralized dentin beams over time. The specimens that were dried for 2 hrs in the vacuum oven had a water content of 0.946 g H2O/g dry mass (squares). This fell to 0.429 and then to 0.059 g H2O/g dry mass in the vacuum oven at 37°C over the next 50 hrs. Then the oven temperature was increased 10°/hr until the water content fell to 0.001 g H2O/g dry mass. Specimens dried in Drierite for 2 hrs had a water content of 1.474 g H2O/g dry mass that fell to 0.001 g H2O/g dry mass after 200 hrs in 0% RH Drierite. Then both groups (n = 10) were exposed to 41% RH and weighed until they reached their first plateau in mass gain, at a water content of 0.064 g H2O/g dry mass (vacuum oven group, squares) or 1.116 g H2O/g dry mass (Drierite group, diamonds). The beams were then exposed to 60% RH water vapor and reweighed until they reached their second plateau in mass at 0.220 g H2O/g dry mass (squares) or 0.191 g H2O/g dry mass. They were then placed in water vapor of 99% RH until they reached their third plateau in mass at 0.401 g H2O/g dry mass for the Drierite group (diamonds) or 0.493 g H2O/g dry mass for the vacuum oven group (squares). Both groups were then immersed in liquid water at 25°C for 1 hr to fully hydrate. The final water content was 1.71 g H2O/g dry mass for the Drierite group (diamonds) or 1.71 g H2O/g dry mass for the vacuum oven group (squares), showing that the vacuum group did not fully recover their initial hydration.
Acknowledgments
This work was supported, in part, by grant R01 DE015306 from the NIDCR and a GRU/GT Seed Grant to DP (PI) and by the King Abdulaziz University that supports DP as a Highly Cited Scholar. The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramachandran GN, Chandrasekharan R. Interchain hydrogen bonds via bound water molecules in the collagen triple helix. Biopolymers. 1968;6:1649–1658. doi: 10.1002/bip.1968.360061109. [DOI] [PubMed] [Google Scholar]
- 2.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1. 9 Å resolution. Science. 1994;226:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 3.Bella J, Brodsky B, Berman HM. Hydration structure of a collagen peptide. Structure. 1995;3:893–906. doi: 10.1016/S0969-2126(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 4.Bella J, Bermam HM. Crystallographic evidence for C alpha-H—O hydrogen bonds in the collagen triple helix. J Mol Biol. 1996;264:734–742. doi: 10.1006/jmbi.1996.0673. [DOI] [PubMed] [Google Scholar]
- 5.Kramer RZ, Vitagliano L, Bella J, Berisio B, Mazzarella L, Brodsky B, Zagari A, Berman HM. X-ray crystallographic determination of a collagen-like peptide with the repeating sequence (Pro-Pro-Gly) J Mol Biol. 1998;280:623–638. doi: 10.1006/jmbi.1998.1881. [DOI] [PubMed] [Google Scholar]
- 6.Kramer RZ, Bella J, Mayville P, Brodsky B, Berman HM. Sequence dependent conformational variations of collagen triple-helical structure. Nat Struct Biol. 1999;6:454–457. doi: 10.1038/8259. [DOI] [PubMed] [Google Scholar]
- 7.Kramer RZ, Venugopal MG, Bella J, Mayville P, Brodsky B, Berman HM. Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J Mol Biol. 2000;301:1191–1205. doi: 10.1006/jmbi.2000.4017. [DOI] [PubMed] [Google Scholar]
- 8.Kramer RZ, Bella J, Brodsky B, Berman HM. The crystal and molecular structure of a collagen-like peptide with a biologically-relevant sequence. J Mol Biol. 2001;311:131–147. doi: 10.1006/jmbi.2001.4849. [DOI] [PubMed] [Google Scholar]
- 9.Fullerton GD, Amurao MR. Evidence that collagen and tendon have monolayer water coverage in the native state. Cell Biol Int. 2006;30:56–65. doi: 10.1016/j.cellbi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Cameron IL, Short N, Fullerton GD. Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type I collagen. Cell Biol Int. 2007;31:531–539. doi: 10.1016/j.cellbi.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Li ST. Biologic biomaterials: Tissue-derived biomaterials (collagen) In: Bronzino JD, editor. The Biomedical Engineering Handbook. 2. Boca Raton: CRC Press LLC; 2000. pp. 42.1–42.4. [Google Scholar]
- 12.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res Part B: Appl Biomater. 2009;88B:339–348. doi: 10.1002/jbm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: Leakage with the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 15.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakge expression in single-step adhesives. J Dent Res. 2002;81:472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 16.Tay FR, Pashley DH. Water treeing – a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16:6–12. [PubMed] [Google Scholar]
- 17.Tay FR, Pashley DH, Yiu C, Cheung C, Hashimoto M, Itou K, Yoshiyama M, King NM. Nanoleakage types and potential implications: Evidence from unfilled and filled adhesives with the same resin composition. Am J Dent. 2004;17:182–190. [PubMed] [Google Scholar]
- 18.Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CKY. Water treeing in simplified dentin adhesives – Déjà vu? Buonocore Memorial Lecture Oper Dent. 2005;30:561–579. [PubMed] [Google Scholar]
- 19.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol ILS, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Review: Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013a;29:116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol ILS, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer: A review. Dent Mater. 2013b;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brackett MG, Brackett WW, Sword RJ, Qi YP, Yiu LN, Pucci CR, Dib A, Pashley DH, Tay FR. The critical barrier to progress in dentin bonding with etch-and-rinse technique. J Dent. 2011;39:238–248. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki N, Shiwa S, Yagihara S, Hikichi K. X-ray diffraction studies on the structure of hydrated collagen. Biopolymers. 1983;22:2539–2547. doi: 10.1002/bip.360221208. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GW, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 24.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Quintessence Publishing Co., Inc; Chicago: 1998. pp. 37–56. [Google Scholar]
- 25.Grégoire G, Sharrock P, Delanné M, Delisle M-B. Depletion of water molecules during ethanol wet-bonding with etch-and-rinse dental adhesives. Mater Sci Eng. 2013:C33-21–27. doi: 10.1016/j.msec.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic-matrix-limitations of restorative dentistry hidden on the nanoscale. Acta Biomater. 2012;8:2419–2433. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi M, Nakajima M, Tagami J, Scheffel D, Carvalho R, Mazzoni A, Carrilho M, Cadenaro M, Tezvergil-Mutluay A, Breschi L, Tjäderhane L, Jang SS, Tay FR, Agee K, Pashley DH. The importance of size-exclusion characteristics of type I collagen in bonding to dentin matrices. Acta Biomaterialia. 2013;9:9522–9528. doi: 10.1016/j.actbio.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrices and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 29.Scheffel DLS, Hebling J, Scheffel RH, Agee KA, Turco G, De Souza Costa CA, Pashley DH. Inactivation of matrix-bound MMPs by cross-linking agents in acid-etched dentin. Oper Dent. 2014;39:152–158. doi: 10.2341/12-425-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong SR, Jessop JLP, Winn E, Tay FR, Pashley DH. Denaturation temperatures of dentin matrices. I. Effect of demineralization and dehydration. J Endod. 2006;32:638–641. doi: 10.1016/j.joen.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 31.Weast RC, Lide DR, editors. Handbook of Chemistry and Physics. 51. Chemical Rubber Co; Cleveland: 1970. pp. 15–25.pp. E-35 Constant humidity solutions, Table on. [Google Scholar]
- 32.Rainey JK, Goh MC. An interactive triple-helical collagen builder. Bioinformatics. 2004;20:2458–2459. doi: 10.1093/bioinformatics/bth247. [DOI] [PubMed] [Google Scholar]
- 33.Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 35.Hess B, Kutzner C, van der Spoel D, Lindahl E. Algorithms for highly efficient load-balanced, and sealable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 36.Rich A, Crick F. The molecular structure of collagen. J Mol Biol. 1961;3:483. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- 37.Fullerton GD, Rahal A. Collagen structure: The molecular source of the tendon magic angle effect. J Mag Res Imag. 2007;25:345–361. doi: 10.1002/jmri.20808. [DOI] [PubMed] [Google Scholar]
- 38.Lim VI. A novel structural model for collagen: Water-carbonyl helix. FEBS Letters. 1981;132:1–5. [Google Scholar]
- 39.Traore A, Foucat L, Renou JP. 1N-NMR study of water dynamics in hydrated collagen: transverse relaxation-time and diffusion analysis. Biopolymers. 2000;53:476–483. doi: 10.1002/(SICI)1097-0282(200005)53:6<476::AID-BIP4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Pashley DH. Dynamics of the pulpodentin complex. Crit Rev Oral Biol Med. 1996;7:104–133. doi: 10.1177/10454411960070020101. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg M, Takagi M. Dentin proteoglycans: composition ultrastructure and functions. Histochem J. 1993;251:781–806. [PubMed] [Google Scholar]
- 42.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (“shape modules”) in extracellular matrix of fibrous connective tissues and their stability of various chemical environments. J Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breschi L, Gobbi P, Lopes M, Prati C, Falconi M, Teti G, Mazzoni A. Immunocytochemical analysis of dentin: A double-labeling technique. J Biomed Mater Res. 2003;67A:11–17. doi: 10.1002/jbm.a.10048. [DOI] [PubMed] [Google Scholar]
- 44.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent Mater. 2009;25:1050–1057. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linde A. Noncollagenous proteins and proteoglycans in dentinogenesis. In: Linde A, editor. Dentin and Dentinogenesis. II. CRC Press; Boca Raton: 1984. p. 56. Chapter 9. [Google Scholar]
- 46.Ping ZH, Nguyen QT, Chen SM, Zhou JQ, Ding YD. States of water in different hydrophilic polymers – DSC and FTIR studies. Polymers. 2001;42:8461–8467. [Google Scholar]
- 47.Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CKY. Water treeing in simplified dentin adhesives – Déjà vu? 2005 Buonocore Memorial Lecture. Oper Dent. 2005;30:561–579. [PubMed] [Google Scholar]
- 48.Durate S, Jr, Phark J-H, Varjão FM, Sadan A. Nanoleakage, ultramorphological characteristics, and microtensile bond strengths of a new low-shrinkage composite to dentin after artificial aging. Dent Mater. 2009;25:589–600. doi: 10.1016/j.dental.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 49.de Groot BL, Grubmüller H. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- 50.Kozono D, Yasui M, King LS, Agre P. Aquaporin water channels: atomic structure and molecular dynamics meet clinical medicine. J Clin Invest. 2002;109:1395–1399. doi: 10.1172/JCI15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang MC, Pins GD, Silver GH. Collagen fibers with improved strength for repair of soft tissue injuries. Biomaterials. 1994;15:507–512. doi: 10.1016/0142-9612(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M, Koychev EV, Okamura K, Sugeta A, Hongo C, Okumura K, Ebisu S. Heat treatment strengthens human dentin. J Dent Res. 2008;87:762–766. doi: 10.1177/154405910808700807. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi M, Okamura K, Koychev EV, Furuya Y, Sugeta A, Ota J, Ebisu S. Effects of rehydration on dentin strengthened by heating or UV radiation. J Dent Res. 2010;89:154–158. doi: 10.1177/0022034509354564. [DOI] [PubMed] [Google Scholar]
- 54.Fratzl P, Daxer A. Structural transformation of collagen fibrils in corneal stroma during drying: An x-ray scattering study. Biophys J. 1993;64:1210–1214. doi: 10.1016/S0006-3495(93)81487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Feninat F, Ellis TA, Sacher E, Stangel I. Moisture-dependent renaturation of collagen in phosphoric acid etched human dentin. J Biomed Mater Res. 1998;42:549–553. doi: 10.1002/(sici)1097-4636(19981215)42:4<549::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Fusayama T. New Concepts in Operative Dentistry. Quintessence Publishing Co., Inc; Chicago: 1980. pp. 68–69.pp. 118–119. [Google Scholar]
- 57.Liu Y, Dusevich V, Wang Y. Proanthocyanidins rapidly stabilize the demineralized dentin layer. J Dent Res. 2013;92:746–752. doi: 10.1177/0022034513492769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Wang Y. Proanthocyanidins efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analysis. J Dent. 2013;41:535–542. doi: 10.1016/j.jdent.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pashley EL, Zhang Y, Lockwood P, Rueggeberg F, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998;14:6–10. doi: 10.1016/s0109-5641(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 60.Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Di Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009;25:621–628. doi: 10.1016/j.dental.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]