Abstract
Hypoxia plays an important role in placental trophoblast differentiation and function during early pregnancy. Hypoxia-inducible factor 1 alpha (HIF1a) is known to regulate cellular adaption to hypoxic conditions. However, our current understanding of the role of HIF1a in trophoblast physiology is far from complete. Metastasis Associated Protein 1 and 3 (MTA1 and MTA3) are components of the Nucleosome Remodeling and Deacetylase (NuRD) complex, a chromatin remodeling complex, and are highly expressed in term placental trophoblasts. However, the role of MTA1 and MTA3 in the hypoxic placental environment of early pregnancy is unknown. In the present study, we examined the association among MTA1, MTA3 and HIF1a expression under hypoxic conditions in trophoblasts both in vivo and in vitro. We first investigated the localization of MTA1 and MTA3 with HIF1a expression in the placental trophoblast of 1st trimester placenta via immunohistochemistry. Our data reveals that under physiologically hypoxic environment, MTA1 and MTA3 along with HIF1a are highly expressed by villous trophoblasts. Next, we investigated the effect of hypoxia on these genes in vitro using the first trimester-derived HTR8/SVneo cell line and observed up-regulation of MTA1 and MTA3 as well as HIF1a protein following hypoxia treatment. To investigate the direct effect of MTA1 and MTA3 upon HIF1a, we over-expressed MTA1 and MTA3 genes in HTR8/SVneo cells respectively and examined protein levels of HIF1a via Western blot as well as HIF1a target gene expression using a luciferase assay driven by a hypoxia-response element promoter (HRE-luciferase). We found that over-expressions of MTA1 and MTA3 up-regulate both HIF1a protein level and HRE-luciferase activity under hypoxic condition. In summary, both MTA1 and MTA3 are induced by hypoxia and up-regulate HIF1a expression and HIF1a target gene expression in trophoblasts. These data suggest that MTA1 and MTA3 play critical roles in trophoblast function and differentiation during early pregnancy.
Keywords: Chromatin remodeling, Trophoblast, Hypoxia
INTRODUCTION
Trophoblast is a unique cell type initially formed within the highly hypoxic environment of early pregnancy which gradually becomes normoxic as placentation progresses. During the first trimester of human pregnancy (8-week) and prior to extravillous trophoblast invasion and spiral artery remodeling, trophoblasts exist in a physiologically hypoxic environment (1 to 2% O2) [1,2]. However, by 12-week of pregnancy and following completion of spiral artery remodeling, the O2 partial pressure has increased significantly (5 to 8%, the “normoxic condition”) [3]. Hence, the hypoxia environment is important for proper trophoblast differentiation and development during the early stages of pregnancy.
Beside its role in normal trophoblast development in the early pregnancy, hypoxia also represents a significant pathological factor contributing to the placental abnormalities seen in trophoblast-related diseases, such as preeclampsia. Hypoxia has been shown to directly induce the trophoblast apoptosis and has been linked to the insufficient placentation seen in preeclamptic placenta [4]. Due to the importance of hypoxia on trophoblast physiology and pathology understanding the response and adaption of trophoblasts to hypoxia is critical for the trophoblast survival, differentiation and function. These mechanisms are also beneficial for the inference and medicine of preeclampsia, the most prevalent of pregnancy diseases.
Hypoxia –inducible factor1 (HIF1) is a hypoxia response transcription factor that regulates fundamental cellular processes including glycolysis, erythropoiesis, angiogenesis and the prevention of apoptosis in response to the low oxygen pressure [5,6]. Its major function is to enable cellular adaption to and survival in hypoxic environments. Previous studies suggest that intracellular level of HIF1a are tightly regulated, as abnormally high level of HIF1a have been documented within preeclamptic placenta, and overexpression of HIF1a under normoxic condition has been shown to induce trophoblast apoptosis [7]. HIF1 is a heterodimeric basic helix-loop-helix structure, composed by two subunits, HIF1a and HIF1b. HIF1a is regulated by O2 partial pressure and rapidly degraded under the normoxic condition with a half-life of less than 5 min. Hence, HIF1 activity is dependent mainly upon available amounts of HIF1a.
It is known that HIF1a regulation occurs predominantly at the post-translation level via protein stabilization in response to O2 partial pressure. However, recent studies in cancer cells have shown that HIF1a is also responsive to post-translational modification, such as acetylation [8]. However, studies examining the regulatory mechanism of HIF1a protein stability within trophoblasts are limited.
MTA1 and MTA3 are components of the Nucleosome Remodeling and Deacetylation complex (NuRD) which regulate protein acetylation (e.g. histone) via its de-acetylation activity. MTA1 and MTA3 are expressed in full term placenta [9], and have been previously shown to regulate genes implicated in trophoblast fusion and invasion [10]. However, the expression of MTA1 and MTA3 in the hypoxic placenta of early pregnancy and an examination into their potential role in hypoxia response and HIF1a regulation within trophoblasts has not been reported. Previous report has shown that, in cancer cells, overexpression of MTA1 up-regulates HIF1a protein level via adjusting its acetylation level [11]. Hence in this study, we investigated whether MTA1 and MTA3 regulate HIF1a in the placental trophoblasts of early pregnancy. Our results show that MTA1 and MTA3 are involved in the hypoxia response cascade through regulation of HIF1a protein level in trophoblasts.
MATERIALS AND METHODS
Placental samples Immunohistochemistry (IHC )
De-identified formalin-fixed and parrafin wax embedded blocks of 9-week human placenta sections were obtained from Michigan State University’s Center for Women’s Health Research, Human Female Reproductive Tract Biorepository in accordance with appropriate institutional review. 4μM sections were dewaxed in xylene, rehydrated in a graded ethanol series and subjected to antigen unmasking with a high PH 9.0 buffer (Vector). Primary immunostaining with antibodies specific to MTA3 (Abcam 87275), MTA1 (Cell signaling 5647) and HIF1a (R&D MAB1935) was followed by exposure to biotin-conjugated secondary antibodies, and then horseradish peroxidase conjugated Streptavidin (Vector). Positive immunostaining was detected with following exposure to a diaminobenzidene (DAB) substrate (brown precipitate) and nuclei counterstained with hematoxylin.
Cell culture, cytoplasm and nuclear protein extraction
The trophoblast cell line HTR8/SVneo (gift from Dr. C. Graham) was cultured in DMEM/F12 supplemented with 10% FBS, 2 mmol/L L-glutamine and 1% Pen/Strep. The cytoplasm and nuclear protein were extracted from the 95% confluent cells using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo scientific).
Lentivirus mediated over-expression
cDNA clones of MTA1 and MTA3 were purchased from Biosystem (MHS1010-9205621 and EHS1001-35695). The open reading frames of MTA1 and MTA3 were amplified by PCR using primers (MTA1 Forward: 5′-ATGGCCGCCAACATGTACAGG-3′; MTA1 Reverse: 5′-GTCCTCGATGACGATGG-3′; MTA3 Forward: 5′-ATGGCGGCCAACATGTACCGGGT-3′; and MTA3 Reverse: 5′-AGAATTTAAAAGCATCTTACA-3′) and then inserted into Lentivirus vector pLenti6-V5, downstream of CMV promoter (Invitrogen). After transfection of MTA1V5 and MTA3V5 vectors with packaging plasmids (Invitrogen) into 293 cells, according to manufacturer’s instructions. Lentivirus from the supernatant of culture media of 293 cells was collected and stored at −70° until future use. HTR8/SVneo cells were infected by adding 100 μl lentivirus containing media per well to the cultured trophoblasts and passaged at least 5 times in the presence of 5ng/ml Blasticidin (approximately 1 month). HTR8/SVneo cells similarly infected with empty vector (pLenti-V5) were used as controls. Transgenic along with control cells were used for functional and biochemical analysis.
Hypoxia treatment
The hypoxia control system (Plas Labs Inc, MI, US) was used for hypoxia treatments with 1% O2 considered hypoxic condition. Post treatment, proteins were extracted within 1 minute of chamber removal to prevent HIF1a protein degradation.
Western blot
For Western blot analysis, proteins were isolated in RIPA buffer with protease inhibitor cocktail (Invitrogen) and quantified using bichinchoninic acid (BCA-Thermo Scientific). 10 μg proteins were loaded per well on 10% reducing gel, transferred to PVDF membrane and the membrane immunoblotted with antibodies specific to HIF1a (Cell Signaling, 3716), Actin (Cell signaling, 4967), MTA1 (Cell signaling 5647), MTA3 (Abcam 87275) and V5 (Invitrogen, R96025).
HRE -Luciferase reporter assay
The HRE-luciferase plasmid encoding the firefly luciferase reporter gene under the control of a minimal (m)CMV promoter and five repeats of the hypoxia transcriptional Response Element (HRE) was previously reported [12] and was purchased from Addgene. The relative activity level of HRE-promoter as determined using the Dual-Luciferase Reporter Assay System (Promega) following our previous publication [13] based on co-transfection of HRE-luciferase plasmid and pRL-TK plasmid. Briefly, both plasmids are transfected into MTA1-overexpressing, MTA3-overexpressing and control cells. Post-transfection 20hrs, cells were exposed to hypoxic conditions (1% O2) for 24 hrs. Then cells were lysed in 1× passive lysis buffer and TRE-luciferase activity compared with the activity of pRL-TK.
Statistical analysis
The student’ T-Test was used for the statistical analysis of luciferase reporter assay. A p-value of less than 0.05 was considered statistically significant.
Results
Co-localization of MTA 1, MTA 3 and HIF1a in 1st trimester placenta
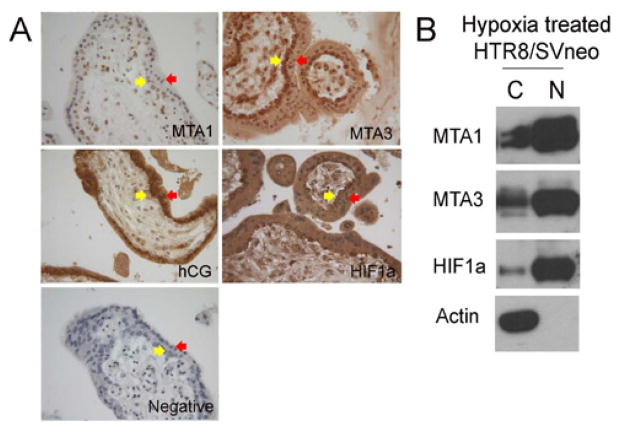
To investigate the expression of MTA1, MTA3 and HIF1a within the physiologically hypoxic placental environment which exists prior to the onset of spiral artery remodeling, we performed immunohistochemistry (IHC) on formalin-fixed paraffin wax embedded sections of 9-week human placenta (Figure 1a). The staining of hCG demonstrates the cytotrophoblasts within the placenta. We found that MTA1, MTA3 and HIF1a were expressed in the villous trophoblasts (both cytotrophoblasts and syncytiotrophoblasts), higher expression in cytotrophoblasts as compared to syncytiotrophoblasts. To further verify these results, we treated the 1st trimester placenta-derived trophoblast cell line HTR8/SVneo with hypoxia condition for 24 hours and examined the nuclear and cytoplasmic expressions of MTA1, MTA3 and HIF1a by Western blot. We used Actin, a typical cytoplasm protein, as the method control of cytoplasmic-nuclear protein extraction. We found that MTA1, MTA3 and HIF1a were highly expressed in nuclei and low expressed in cytoplasm (Figure 1b), which confirms that MTA1, MTA3 and HIF1a in trophoblasts under hypoxic condition.
Figure 1.
Co-localization of MTA1, MTA3 and HIF1a in 1st trimester placenta and hypoxia treated HTR8/SVneo cells. A. Expressions of MTA1, MTA3, hCG and HIF1a in villous trophoblasts of 9-week placenta detected by IHC. Yellow arrows indicate cytotrophoblasts and red arrows indicate syncytiotrophoblasts. B. Expressions of MTA1, MTA3, HIF1a and Actin (a typical cytoplasmic marker protein) in the cytoplasm (C) and nuclei (N) of HTR8/SVneo following hypoxia treatment (1%O2) as detected by Western blot.
Hypoxia induction of MTA 1, MTA 3 and HIF1a in HTR 8/SVneo cells
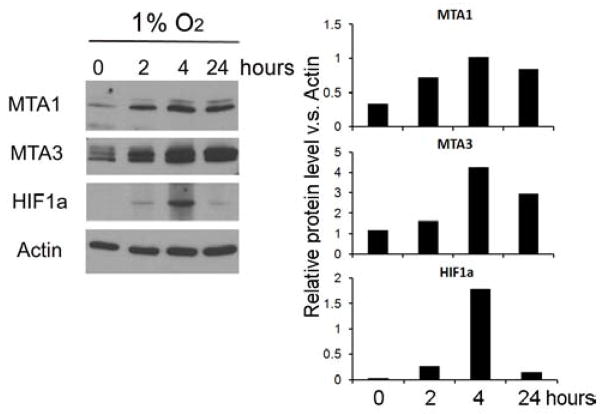
To investigate the direct effect of hypoxia on MTA1, MTA3 and HIF1a during early pregnancy, we exposed the 1st trimester placenta-derived trophoblast cell line HTR8/SVneo, to hypoxic conditions (1% O2) for 0, 2, 4 and 24 hours. Quantified Western blot analysis (normalized by Actin level) reveals that all three proteins were induced by hypoxia treatment, with expression peaking at 4 hours post treatment (Figure 2).
Figure 2.
Hypoxia induces expressions of MTA1, MTA3 and HIF1a in HTR8/SVneo cells as detected by western blot. The representative western blot results are shown in left panel, and their quantified results (normalized by Actin) are demonstrated in right panel.
Up-regulation of HIF1a expression by MTA 1 and MTA 3 in HTR 8/SVneo cells
To further investigate the effects of MTA1 and MTA3 on HIF1a in HTR8/SVneo cells, we over-expressed V5 tagged MTA1 and MTA3 protein (MTA1V5 and MTA3V5) in HTR8/SVneo cells by infecting lentivirus containing MTA1 or MTA3 cDNA downstream of CMV promoter. The cells infected with empty-vector lentivirus were used as control. Over-expressions of MTA1V5 and MTA3V5 were verified via immunofluorescence (IF) (Suppliment Figure). Transfected cells then exposed to hypoxia (normoxia for controls), and MTA1V5, MTA3V5, HIF1a and Actin levels were analyzed via western blot (Figure 3a). HIF1a expression was undetectable in both MTA1V5- and MTA3V5-overexpressing cells under normoxic condition. However, upon hypoxia treatment, HIF1a expression was highly induced in both MTA1V5- and MTA3V5- overexpressing cells as compared to empty-vector controls (Figure 3a).
Figure 3.
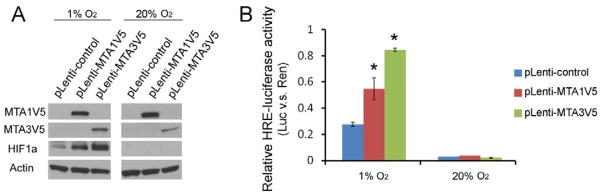
Up-regulation of HIF1a in protein levels and HRE-luciferase activity levels by MTA1 and MTA3 in HTR8/SVneo cells as detected by Western blot and HRE-luciferase assay, respectively. A. Expressions of V5 tagged MTA1 and MTA3 (MTA1V5 and MTA3V5), HIF1a and Actin were detected in MTA1V5-, MTA3V5- overexpressing and control HTR8/SVneo cells under hypoxic (1% O2) and normoxic (20% O2) conditions. MTA1V5 and MTA3V5 proteins were detected by V5 antibody. B. Relative HRE-luciferase activity levels (normalized using the internal control pRen-TK). Asterisk represents the statistical significance (p < 0.05) compared with the control. Results are representative of 3 biological repeats.
Up-regulation of hypoxia response genes by MTA 1 and MTA 3 in HTR 8/SVneo cells
To investigate the effects of MTA1 and MTA3 on HIF1a mediated gene expression (on genes which genes typically contain the Hypoxia transcriptional Response Element (HRE) in their regulatory regions [14]), we examined the HRE-luciferase reporter activity using a plasmid, previously reported to demonstrate HIF1a mediated gene expression [12]. We transfected this plasmid into both MTA1-, MTA3-overexpressing and control cells after 24 hrs hypoxia treatment. We found that the activities of HRE-luciferase reporter were significantly higher in MTA1- and MTA3- overexpressing cells compared to the control cells (Figure 3b).
DISCUSSION
Hypoxia is critical for the trophoblast differentiation, placental development and has been linked to the development of trophoblast-associated diseases [1,2,15]. In the present study, we observed that in the hypoxic environment of early pregnancy (the 9-week placenta), MTA1 and MTA3 along with HIF1a are highly expressed in villous trophoblast, with higher expression in cytotrophoblasts as compared to syncytiotrophoblasts (Figure 1). These results suggest that MTA1and MTA3 have a potential role in the trophoblast hypoxia response. Indeed, we found that hypoxia induces MTA1 and MTA3 expression, similar to its induction of HIF1a (Figure 2). These results further suggest that regulation of MTA1 and MTA3 are important for hypoxic response in trophoblasts during early pregnancy.
HIF1 and HIF2 are the predominant members of the hypoxia-inducible factors (HIFs) family, which regulate the cellular response to hypoxia environment. Previous studies suggest that it is HIF1 (and not HIF2) which is critical for proper placental development. HIF1 knock-out mice show a reduced-size placenta and disrupted trophoblast differentiation [16], whereas, HIF2 knock-out mice have no placental abnormalities [17]. Therefore, in this study, we focused on the regulation of HIF1a. It has previously been reported that overexpression of MTA1 stabilized HIF1a protein under both normoxic and hypoxic conditions in breast cancer cells [8]. In this study, we found, under normoxic condition, over-expression of MTA1 and MTA3 in trophoblasts did up-regulate HIF1a expression and HIF1a mediated gene expression as determined by HRE-luciferase activity levels (Figure 3). However, under normoxic condition, MTA1 and MTA3 had no effect on the levels of HIF1a and HRE-luciferase (Figure 3). This difference may be due, in part, to the unique molecular regulation present in cancer cells as compared to trophoblasts. Further studies examining the mechanism by which MTA1 and MTA3 regulate/stabilize HIF1a are needed to address this discrepancy.
In conclusion
Induced by hypoxia, MTA1and MTA3 up-regulate HIF1a expression, suggesting that MTA1 and MTA3 are critical in the hypoxia response cascade in the placental trophoblasts of early pregnancy.
Acknowledgments
We acknowledge Dr. Trixie Smith for critically reading the manuscript and providing constructive criticisms.
Funding
This work was supported by the National Institute of Child Health and Human Development (Grant number: U54HD040093). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta--myth or reality? Am J Obstet Gynecol. 1997;176:695–705. doi: 10.1016/s0002-9378(97)70572-6. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai TC, Wong-Faull DC, Claycomb R, Wong DL. Hypoxic stress-induced changes in adrenergic function: role of HIF1 alpha. J Neurochem. 2009;109:513–524. doi: 10.1111/j.1471-4159.2009.05978.x. [DOI] [PubMed] [Google Scholar]
- 6.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 7.Jeon SY, Lee HJ, Na KH, Cha DH, Kim JK, Park JW, et al. Hypoxia-induced downregulation of XIAP in trophoblasts mediates apoptosis via interaction with IMUP-2: implications for placental development during pre-eclampsia. J Cell Biochem. 2013;114:89–98. doi: 10.1002/jcb.24304. [DOI] [PubMed] [Google Scholar]
- 8.Yoo YG, Kong G, Lee MO. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO J. 2006;25:1231–1241. doi: 10.1038/sj.emboj.7601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brüning A, Makovitzky J, Gingelmaier A, Friese K, Mylonas I. The metastasis-associated genes MTA1 and MTA3 are abundantly expressed in human placenta and chorionic carcinoma cells. Histochem Cell Biol. 2009;132:33–38. doi: 10.1007/s00418-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Miyazaki J, Nishizawa H, Kurahashi H, Leach R, Wang K. MTA3 regulates CGB5 and Snail genes in trophoblast. Biochem Biophys Res Commun. 2013;433:379–384. doi: 10.1016/j.bbrc.2013.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon HE, Cheon H, Chun KH, Lee SK, Kim YS, Jung BK, et al. Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncol Rep. 2006;16:929–935. [PubMed] [Google Scholar]
- 12.Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Wang K, Leach R. 5-Aza-dC treatment induces mesenchymal-to-epithelial transition in 1st trimester trophoblast cell line HTR8/SVneo. Biochem Biophys Res Commun. 2013;432:116–122. doi: 10.1016/j.bbrc.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 16.Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 17.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]