Abstract
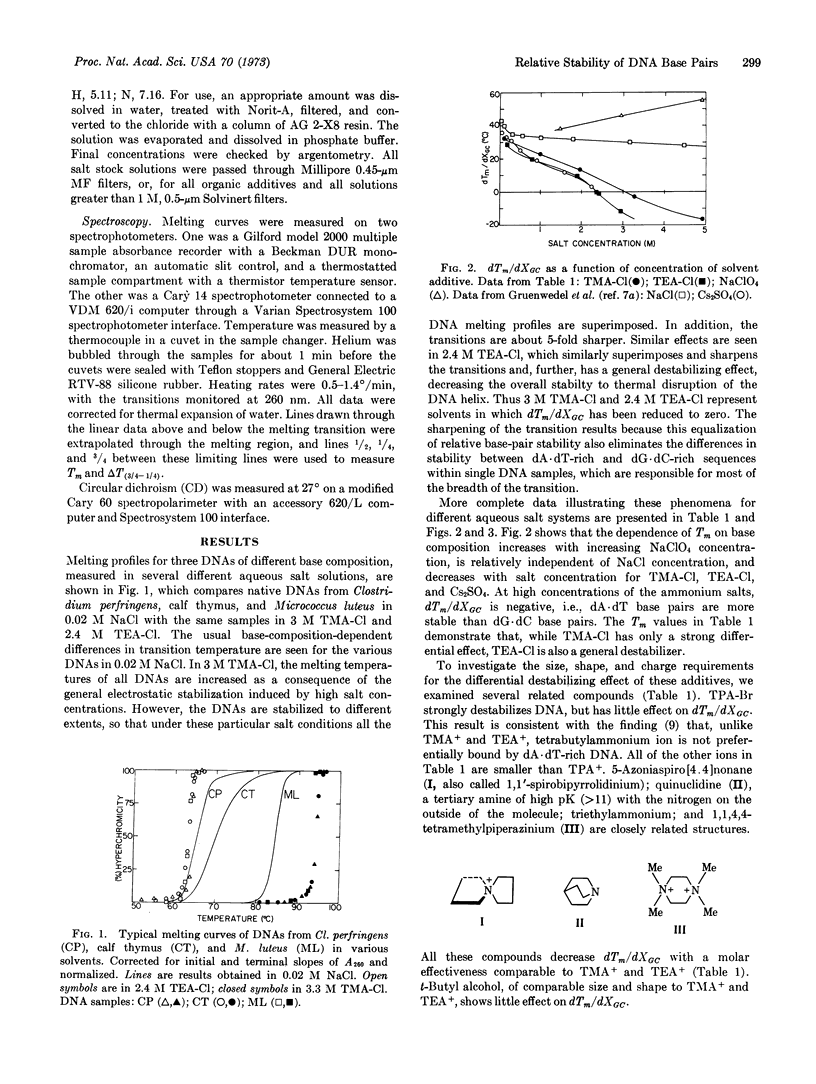
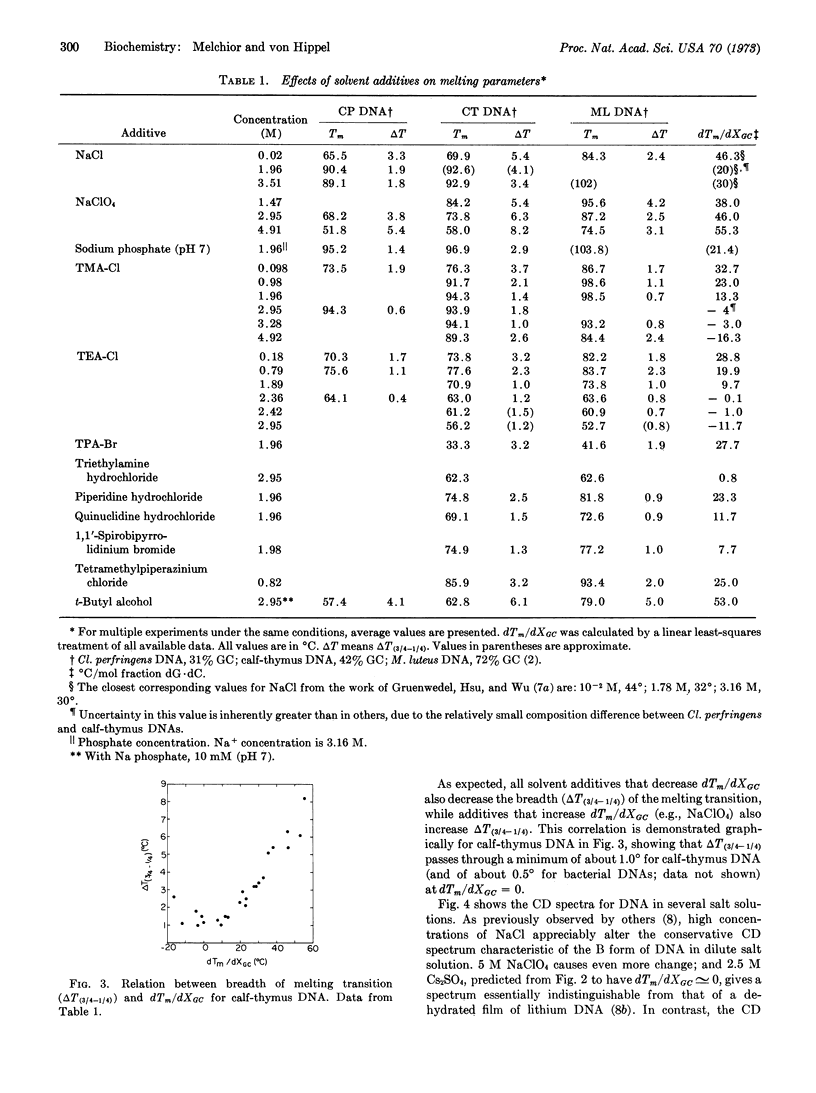
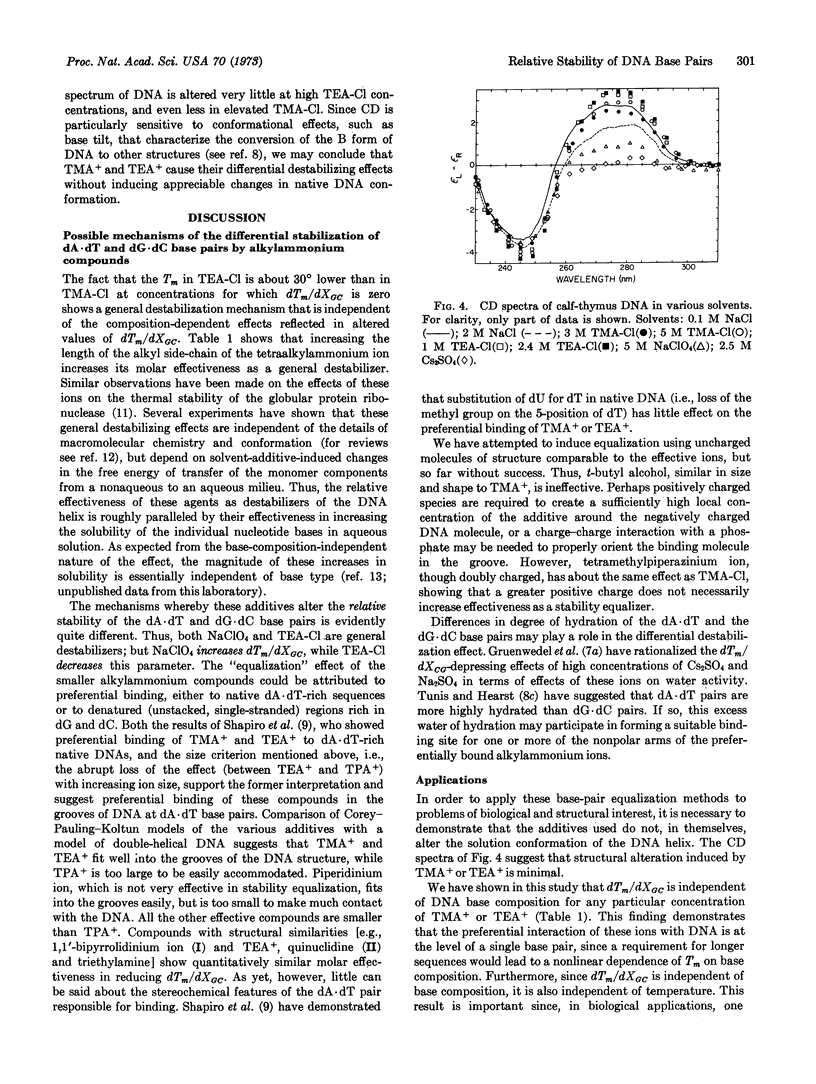
Several small alkylammonium ions can eliminate, or even reverse, the usual dependence of the DNA transition temperature on base composition. For example, in 3 M tetramethylammonium chloride, or 2.4 M tetraethylammonium chloride, DNAs of different base compositions all melt at a common temperature, and with a greatly decreased breadth of transition reflecting only the sequence-independent components of melting cooperativity. At still higher concentrations of such additives, dG·dC-rich DNAs melt at lower temperatures than dA·dT-rich molecules. Circular dichroism spectra show that these additives alter the structure of the DNA double helix very little at room temperature. This differential (base-specific) effect on helix stability is investigated with several small additives related to the tetraalkylammonium ions. Additives larger than tetraethylammonium ion have little differential effect on helix stability. Preferential binding of ions to dA·dT base pairs, requiring fit into a “groove” of DNA, is consistent with these data and with equilibrium binding studies. These differential effects can be distinguished from general destabilizing effects, which are independent of specific features of macromolecular conformation or chemistry. Possible experimental uses of this ability to alter the base-composition-dependent components of the stability of the DNA helix are discussed, as well as the insight this phenomenon provides into the molecular basis for the differential stability of dA·dT and dG·dC base pairs.
Keywords: solvent additives, tetraalkylammonium ions, melting transitions, circular dichroism, cooperativity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROTHERS D. M., KALLENBACH N. R., ZIMM B. H. THE MELTING TRANSITION OF LOW-MOLECULAR-WEIGHT DNA: THEORY AND EXPERIMENT. J Mol Biol. 1965 Apr;11:802–820. doi: 10.1016/s0022-2836(65)80037-7. [DOI] [PubMed] [Google Scholar]
- Eiss H., McQuarrie D. A., MTague J. P., Cohen E. R. On the melting of copolymeric DNA. J Chem Phys. 1966 Jun 15;44(12):4567–4581. doi: 10.1063/1.1726675. [DOI] [PubMed] [Google Scholar]
- Fuke M., Wada A., Tomizawa J. I. Denaturation at the right-hand end of the DNA molecule of lambda bacteriophage. J Mol Biol. 1970 Jul 28;51(2):255–266. doi: 10.1016/0022-2836(70)90141-5. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., HERSKOVITS T. T. Nonaqueous solutions of DNA. Reversible and irreversible denaturation in methanol. Arch Biochem Biophys. 1961 Oct;95:114–129. doi: 10.1016/0003-9861(61)90116-3. [DOI] [PubMed] [Google Scholar]
- Gruenwedel D. W., Hsu C. H., Lu D. S. The effects of aqueous neutral-salt solutions on the melting temperatures of deoxyribonucleic acids. Biopolymers. 1971;10(1):47–68. doi: 10.1002/bip.360100106. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Grant M. E. The effects of aqueous salt solutions on the activity coefficients of purine and pyrimidine bases and their relation to the denaturation of deoxyribonucleic acid by salts. J Biol Chem. 1966 Sep 10;241(17):4030–4042. [PubMed] [Google Scholar]
- Roufogalis B. D., Thomas J. Potentiation of acetylcholinesterase by a series of quaternary ammonium compounds. J Pharm Pharmacol. 1968 Feb;20(2):135–145. doi: 10.1111/j.2042-7158.1968.tb09703.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J. T., Stannard B. S., Felsenfeld G. The binding of small cations to deoxyribonucleic acid. Nucleotide specificity. Biochemistry. 1969 Aug;8(8):3233–3241. doi: 10.1021/bi00836a015. [DOI] [PubMed] [Google Scholar]
- Studdert D. S., Patroni M., Davis R. C. Circular dichroism of DNA: temperature and salt dependence. Biopolymers. 1972;11(4):761–779. doi: 10.1002/bip.1972.360110404. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. Optical rotatory dispersion of DNA in concentrated salt solutions. Biopolymers. 1968;6(8):1218–1223. doi: 10.1002/bip.1968.360060816. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., McGhee J. D. DNA-protein interactions. Annu Rev Biochem. 1972;41(10):231–300. doi: 10.1146/annurev.bi.41.070172.001311. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J Biol Chem. 1965 Oct;240(10):3909–3923. [PubMed] [Google Scholar]



