Abstract
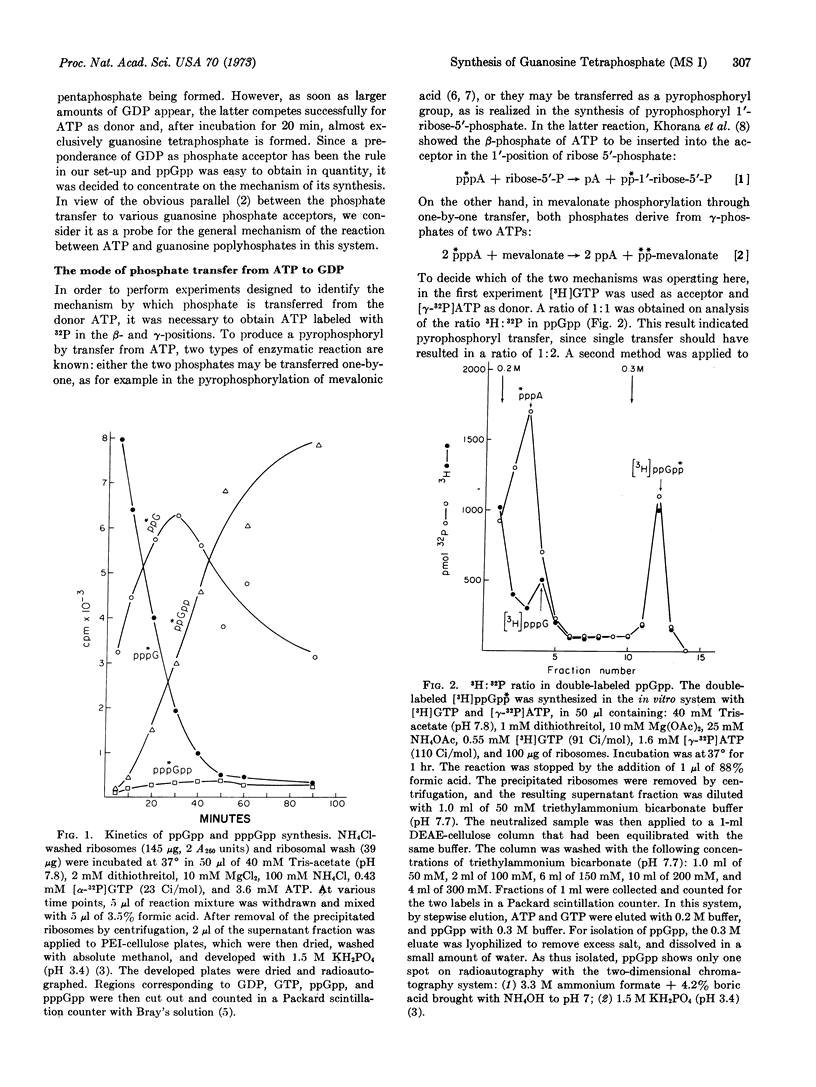
The phosphate transfer system of Haseltine et al., consisting of a ribosomal wash obtained from a stringent strain of Escherichia coli, washed ribosomes, GTP, and ATP, was used to prepare large quantities of guanosine tetra- and pentaphosphates, the magic spot compounds MS I and MS II of Cashel and Gallant. In our hands, the Haseltine et al. system yielded predominantly guanosine tetraphosphate, ppGpp. This system was used exclusively in the described experiments, with ATP labeled with 32P in the β- and γ-positions as donor. The β-label was found to produce a ppGp⃰p and the γ-label a ppGpp⃰. Furthermore, [3H]GDP + [γ-32P]ATP yielded ppGpp in a 3H:32P ratio of 1:1. The results indicate a transfer of the terminal pyrophosphoryl group of ATP as a unit.
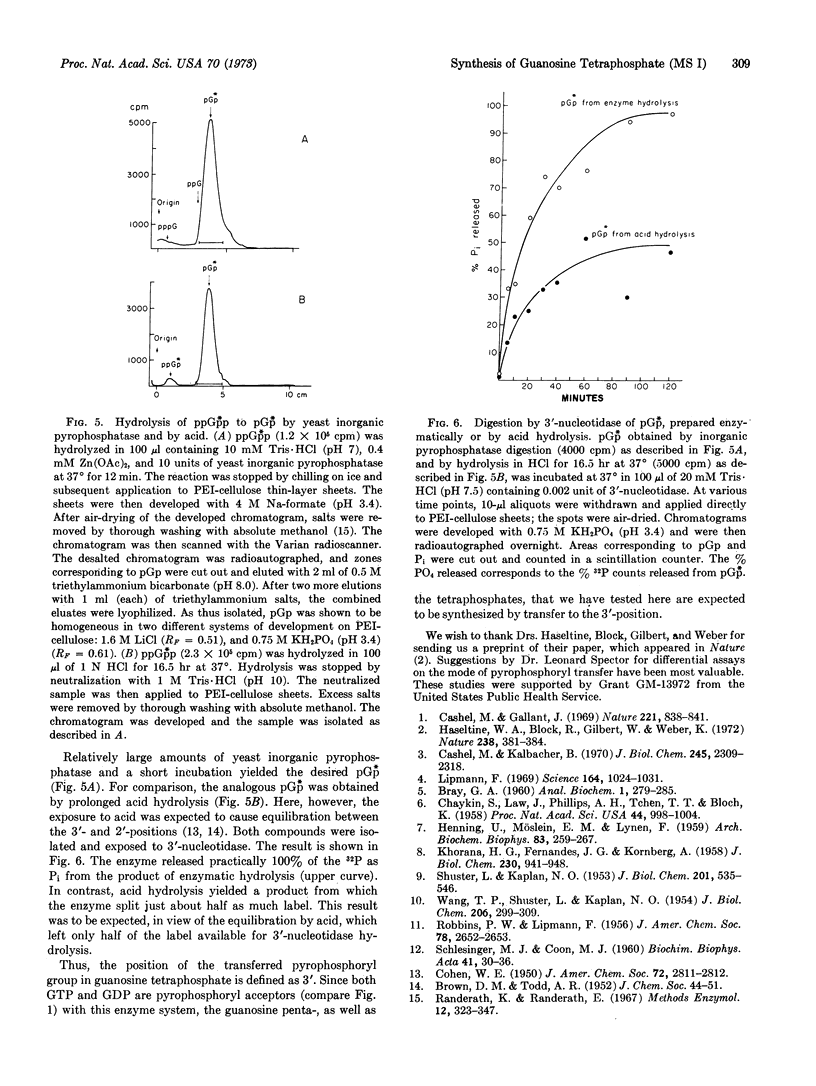
The position of the transferred pyrophosphoryl was assayed for by preparation of pGp⃰ from ppGp⃰p with Zn++-activated inorganic pyrophosphatase from yeast. The pGp⃰ was then assayed with 3′-nucleotidase, which liberated practically all the labeled phosphate. This result indicate that the phosphate transfer from ATP to GDP yields guanosine 5′-diphosphate-3′-diphosphate.
Keywords: E. coli, stringent, relaxed control, phosphate transfer
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Chaykin S., Law J., Phillips A. H., Tchen T. T., Bloch K. PHOSPHORYLATED INTERMEDIATES IN THE SYNTHESIS OF SQUALENE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):998–1004. doi: 10.1073/pnas.44.10.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNING U., MOSLEIN E. M., LYNEN F. Biosynthesis of terpenes. V. Formation of 5-pyrophosphomevalonic acid by phosphomevalonic kinase. Arch Biochem Biophys. 1959 Jul;83(1):259–267. doi: 10.1016/0003-9861(59)90031-1. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- KHORANA H. G., FERNANDES J. F., KORNBERG A. Pyrophosphorylation of ribose 5-phosphate in the enzymatic synthesis of 5-phosphorylribose 1-pyrophosphate. J Biol Chem. 1958 Feb;230(2):941–948. [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. J., COON M. J. Hydrolysis of nucleoside diand triphosphates by crystalline preparations of yeast inorganic pyrophosphatase. Biochim Biophys Acta. 1960 Jun 17;41:30–36. doi: 10.1016/0006-3002(60)90365-6. [DOI] [PubMed] [Google Scholar]
- SHUSTER L., KAPLAN N. O. A specific b nucleotidase. J Biol Chem. 1953 Apr;201(2):535–546. [PubMed] [Google Scholar]
- WANG T. P., SHUSTER L., KAPLAN N. O. The monoester phosphate grouping of coenzyme A. J Biol Chem. 1954 Jan;206(1):299–309. [PubMed] [Google Scholar]



