Abstract
The new methods of different administration times for the treatment of primary dysmenorrhea are more widely used clinically; however, no obvious mechanism has been reported. Therefore, an animal model which is closer to clinical evaluation is indispensable. A novel animal experiment with different administration times, based on the mice oestrous cycle, for primary dysmenorrhoea treatment was explored in this study. Mice were randomly divided into two parts (one-cycle and three-cycle part) and each part includes five groups (12 mice per group), namely, Jingqian Zhitong Fang (JQF) 6-day group, JQF last 3-day group, Yuanhu Zhitong tablet group, model control group, and normal control group. According to the one-way ANOVAs, results (writhing reaction, and PGF2α, PGE2, NO, and calcium ions analysis by ELISA) of the JQF cycle group were in accordance with those of JQF last 3-day group. Similarly, results of three-cycle continuous administration were consistent with those of one-cycle treatment. In conclusion, the consistency of the experimental results illustrated that the novel animal model based on mice oestrous cycle with different administration times is more reasonable and feasible and can be used to explore in-depth mechanism of drugs for the treatment of primary dysmenorrhoea in future.
1. Introduction
Dysmenorrhoea, which is caused by female endocrine disorders, is one of the unresolved problems in medical science. Approximately 80% of women globally had dysmenorrhoea in different degrees according to a report from the British medical authority [1]. This condition is classified into two categories: primary and secondary dysmenorrhoea. The incidence of primary dysmenorrhoea is higher. In an epidemiological study, 53.2% of women in China have dysmenorrhoea during their menstrual period, and the incidence rate is about 43% to 70% overseas [2]. Primary dysmenorrhoea can be mostly experienced by young women, which occurs without an identifiable pelvic pathology. Such condition is mainly characterised by cramping pain in the lower abdomen immediately before or during menstruation, which does affect the quality of their life and work [3].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used drugs for primary dysmenorrhoea treatment [4], particularly for cases in which subjective symptoms are moderate. However, NSAIDs have side effects, such as damage of liver and kidney after discontinuing medication [5].
Chinese medical treatment of primary dysmenorrhoea has a long history. Based on the rich clinical experience and syndrome differentiation theory, primary dysmenorrhoea was recently divided into five types, including the qi stagnation and blood stasis type, cold-damp stagnation type, qi and blood deficiency type, and liver and kidney deficiency type [6]. Traditional Chinese medicine (TCM) treats primary dysmenorrhoea by fundamentally regulating the female physique and body balance of Yin and Yang, and TCM mostly has no obvious side effects. Therefore, such treatment has elicited considerable attention worldwide.
Jingqian Zhitong Fang (JQF) prescription is a typical and relatively simple TCM formula. Such prescription originally came from Fo Shou San and has been proven effective for treating primary dysmenorrhoea. Radix Angelicae Sinensis (Angelica sinensis Diels) is the main drug in JQF and the main constituent that affects blood [7]. It could enrich the blood and alleviate menstrual pain [8]. Rhizoma Chuan Xiong (Ligusticum chuanxiong Hort.) can treat menstruation, amenorrhea, dysmenorrhoea, and headache [9]. Herba Leonuri Motherwort (Leonurus japonicus Houtt.) can also regulate menstruation [10]. Radix Paeoniae Rubra (Paeonia lactiflora Pall.) is used in clinical treatment of heat and cold blood, as well as for healing bruises and relieving pain [11]. We developed an ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) method to construct the chromatographic fingerprint and to identify the constituents of JQF. Based on experimental pharmacological studies [12–14], JQF has a wide variety of actions, including antinociceptive, anti-inflammatory spasmolysis and vasorelaxation, all of which may contribute to its therapeutic action.
Some restricted problems such as slow onset, long treatment cycle (the period of TCM treatment is three months per course), and poor taste of drugs hinder the modernization and internationalization of TCM [15, 16]. Such problems need to be resolved. Recently, through continuous exploration, short-term medication and effective treatment method at different administration times for primary dysmenorrhoea are widely used clinically [17–21]. The administration time is 3 days (d) to 7 d before menstruation instead of the entire physiological cycle during medication. This treatment was only reported in clinical trials and never occurred in animal experiments. An animal experiment for primary dysmenorrhoea could facilitate not only observational changes of induced pain factor content but also an in-depth study of the pathway, receptor cells, and uterine tissues. After many years of discussion and research, animal experiments for primary dysmenorrhoea have already found a certain research foundation [22–24]. Animal experiments are important means for investigating the mechanisms of action underlying primary dysmenorrhoea.
Recently, animal studies have been conducted to explore the basis and mechanism of primary dysmenorrhoea treatment and developed a general animal model of this condition. Therefore, the current experiment selected the point of different administration times to treat primary dysmenorrhoea on the basis of the formal clinical studies [25–27]. The modelling time was selected in line with oestrous cycle by observing the mice vaginal smears and provided medicine in groups combined with clinical treatment. The scientific connotation of primary dysmenorrhoea treatment can then be explored at different administration times by animal experimental study. This treatment provides basic ideas for further research and also facilitates new information and methods for animal studies of primary dysmenorrhoea, which have explored the mechanism of action and dosage of drugs in recent years. Such treatment also provides further experimental data for TCM treatment of primary dysmenorrhoea and promotes modernisation and globalisation of TCM application.
In this study, we aimed to explore and establish an animal model by evaluating primary dysmenorrhoea treatment at different administration times, in which the primary dysmenorrhoea model was based on the proestrus stage of mice. Furthermore, the present study was a preliminary study on the mechanism of the treatment and optimized the animal primary dysmenorrhoea model.
2. Material and Methods
2.1. Experimental Animal
A total of 140 female Kunming mice (weighing 18 g to 22 g) without mating were purchased from the Beijing Hua Fukang Experimental Animal Technology Co., Ltd. (approval number: 2013-0004). The animal care complied with the United States National Institutes of Health Advocacy. The mice were housed in individual cages with food and water ad libitum in a 12 h : 12 h light/dark cycle with indoor temperature of 25°C and 45% humidity.
2.2. Experimental Equipment and Reagents
For intervention, oestradiol benzoate for injection (Ningbo Sansheng Pharmaceutical Co., Ltd., batch number: 120904), oxytocin for injection (Shanghai Hefeng Factory, batch number: 130115), and Yuanhu Zhitong tablets (Tianjin Tongrentang Group Limited by Share Ltd., batch number: c91017) were used.
For outcome, prostaglandin E2 assay kit, prostaglandin F2α assay kit, nitric oxide assay kit (nitrate reductase method), and calcium colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute); YXJ-type high speed centrifuge (Changzhou Jiancheng Teaching Instrument Factory); OLYMPUS U-CMAD3 optical microscope; Japanese OLYMPUS, OLYMPUS C5060-ADU optical camera; Japanese OLYMPUS; gas bath thermostats oscillator (Jintan Ronghua Instrument Manufacturing Co., Ltd.); and Infinite 200 PRO automatic enzyme mark instrument (Tecan Group, Ltd.) were applied.
2.3. Determination of Oestrous Cycle and Experimental Groups
All 140 mice were fasted for 2 d and were randomly divided into groups before starting the experiments. At 8:00 AM, the mice vaginal mucus were collected on a glass slide fixed with methanol and stained with HE to determine the oestrous cycle time and to divide the same oestrous cycle mice in a group [28]. As a result of individual differences of the oestrous cycle of mice, 128 mice were confirmed with an oestrous cycle of 6 d, among which 120 mice were selected for experiment.
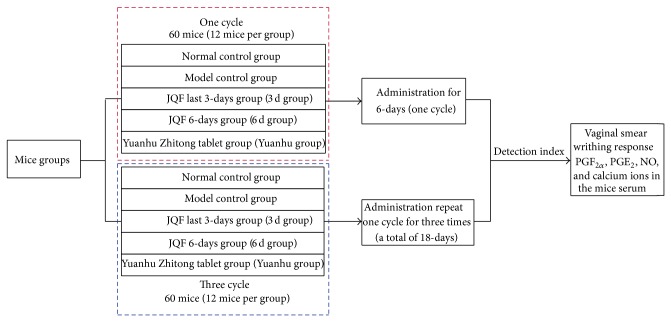
The entire experiments were departed into two parts to investigate the efficiency of treatment for one and three cycles. In each treatment cycle, mice were randomized into five groups, namely, JQF cycle group (6 d group), JQF last 3 d group (3 d group), Yuanhu Zhitong tablet group (Yuanhu group), model control group, and normal control group (Figure 1).
Figure 1.
The experimental scheme. The entire experiments were departed into two parts to investigate the efficiency of treatment for one and three cycles. In each treatment cycle, mice were randomized into five groups, namely, JQF cycle group (6 d group), JQF last 3 d group (3 d group), Yuanhu Zhitong tablet group (Yuanhu group), model control group, and normal control group.
2.4. Primary Dysmenorrhoea Mice Model and Administration
The primary dysmenorrhoea mice model is improved from reference [29] of building the dysmenorrhoea model and assessment methods. Firstly, the model should determine the proestrus stage of the mice via vaginal smear test, and then dysmenorrhoea mice were modelled. In detail, oestradiol benzoate injection was subcutaneous injected to mice except those in the normal control group to establish the animal model of primary dysmenorrhoea. For the JQF last 3 d group (in one cycle, mice were given saline for the first 3 days, and JQF was replaced for the remaining last 3 days) and 6 d group, mice were intragastrically given JQF (0.218 g/kg). As a positive control, Yuanhu Zhitong tablet group (0.1 g/kg) were intragastrically administered (0.02 mL/g). Simultaneously, saline at the same dose was given to mice in the normal control group (Figure 1 and Table 1).
Table 1.
Primary dysmenorrhoea mice model and administration.
| Normal | Model | 3 d | 6 d | Yuanhu | |
|---|---|---|---|---|---|
| D1 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Saline | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
|
| |||||
| D2 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Saline | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
|
| |||||
| D3 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Saline | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
|
| |||||
| D4 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Jingqian Zhitong Fang (JQF) | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
|
| |||||
| D5 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Jingqian Zhitong Fang (JQF) | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
|
| |||||
| D6 | Saline | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate | Oestradiol benzoate |
| Saline | Saline | Jingqian Zhitong Fang (JQF) | Jingqian Zhitong Fang (JQF) | Yuanhu Zhitong tablet | |
Note: the dosage of each drug was subcutaneous injection of oestradiol benzoate or saline (10 mg/kg); JQF (0.218 g/kg) and Yuanhu Zhitong tablet (0.218 g/kg) were intragastrically administered (0.02 mL/g).
For each group, moulding (saline or oestradiol benzoate) and drugs intervention (saline, JQF, or Yuanhu Zhitong tablet) were almost simultaneously performed in each day.
2.5. Writhing Response and Preparation for Determination
The drug effect on the behavioural pattern of mice was investigated. Writhing response complied with the Schmauss and Yaksh's [30] standard. The mice were fasted with water access for 12 h to 16 h prior to initiation of the study. On the sixth day, after the injection of oestradiol benzoate moulding for 40 min, the mice were administered oxytocin 2 U/mice (0.01 mL/g) by intraperitoneal injection. After 5 min, the writhing reaction times were recorded for 30 min. Blood was then extracted from the retroorbit sinus. Plasma specimens were separated by centrifugation at 3500 r·min−1 for 15 min at −4°C and then stored at −20°C until analysis. The specific method for sample detection strictly complied with the kit operation. The experiment complied with the Guide for the Care and Use of Laboratory Animals (National Research Council of the USA, 1996) and related ethical regulations of the China Academy of Chinese Medical Sciences.
2.6. Statistical Management
SPSS 13.0 software was used for statistical analysis. One-way ANOVA was used to analyse the difference between the two groups, and data were expressed as mean ± standard error of mean. A P value less than 0.05 or 0.01 was considered as significance.
3. Results
3.1. Stage of Oestrous Cycle Typical Vaginal Smear
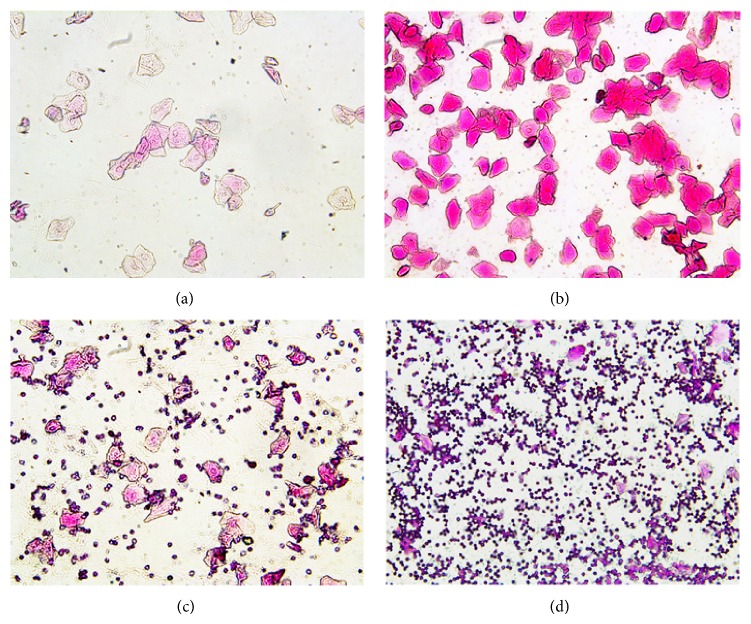
The characteristic of proestrus smear was almost nucleated epithelial and occasionally had few keratinocytes (Figure 2(a)). The smear was almost entirely covered with nucleus-free keratinocytes in the estrus (Figure 2(b)). White blood cells, keratinocytes, and nucleated epithelial cells simultaneously existed in the metestrus (Figure 2(c)). The diestrus mainly had white blood cells (Figure 2(d)).
Figure 2.
Mice typical vaginal smear of different stages of the oestrous cycle (200x). (a) Proestrus smear; (b) estrus smear; (c) metestrus smear; (d) diestrus smear.
3.2. Effects of Different Administration Times on Oxytocin-Induced Dysmenorrhoea Mice Model
Compared with the normal control group, the writhing times of mice in the model control group were significantly increased (P < 0.01) (Figure 3). Compared with the model control group, the writhing times of the mice were significantly decreased in the one-cycle parts (6 d and the last 3 d group, P < 0.01). Results of the three-cycle continuous moulding administration experiment were similar to those of the one-cycle treatment, but without significance between one- and three-cycle parts. Compared with the model control group, moreover, Yuanhu group decreased the writhing times of the mice (P < 0.05 or P < 0.01), not only in the one-cycle part but also in the three-cycle part.
Figure 3.
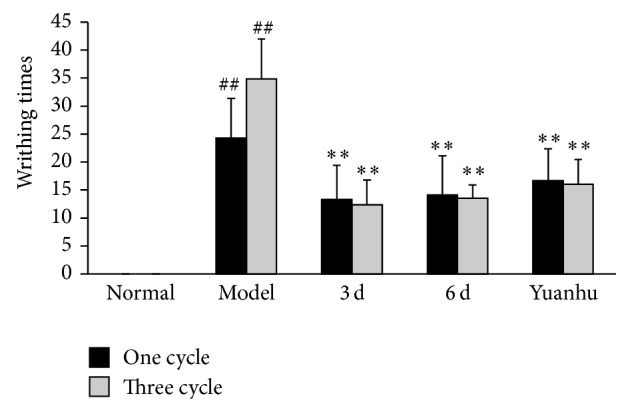
Effects of different administration times on oxytocin-induced dysmenorrhoea mice model. Values are means ± SD (n = 12) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups; Yuanhu group versus model control group.
3.3. Effects of PGF2α and PGE2 at Different Administration Times on Dysmenorrhoea Mice
Compared with the normal control group, PGF2α of the model control group was significantly increased, and PGE2 was significantly decreased, whereas the serum of PGF2α and PGE2 ratio was significantly increased (P < 0.01). Compared with the model control group, PGF2α was significantly decreased, and PGE2 was significantly increased; the serum of PGF2α and PGE2 ratio tended to be normal in the 6 d group and the last 3 d group (P < 0.01). The results of the three-cycle continuous moulding administration experiment were similar to those of the one-cycle treatment. Compared with the model control group, the one-cycle experiment of the Yuanhu group decreased the PGF2α, whereas PGE2 increased (P < 0.05). However, no obvious difference was found in the serum of PGF2α and PGE2 ratio. After three cycles of continuous administered treatment, the effects of all indexes were significantly modulated (P < 0.01) (Figures 4, 5, and 6).
Figure 4.
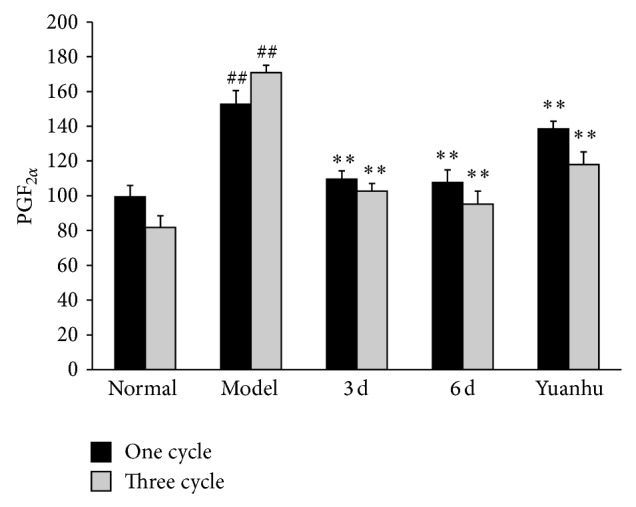
Effects of PGF2α at different administration times on dysmenorrhoea mice. Values are means ± SD (n = 6) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups; Yuanhu group versus model control group.
Figure 5.
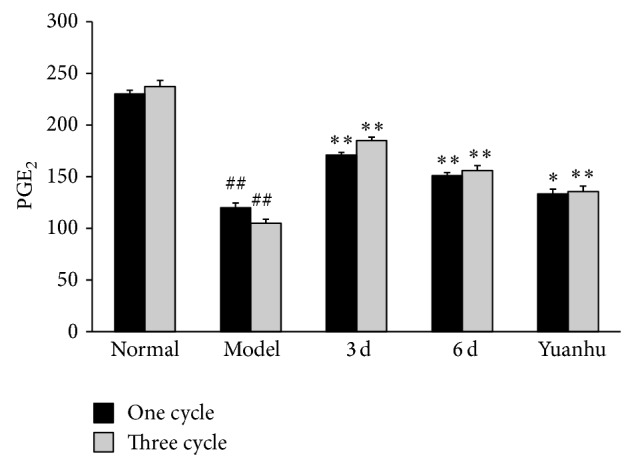
Effects of PGE2 at different administration times on dysmenorrhoea mice. Values are means ± SD (n = 6) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups versus model control group; Yuanhu group versus model control group in one cycle * P < 0.05 and three cycles ** P < 0.01.
Figure 6.
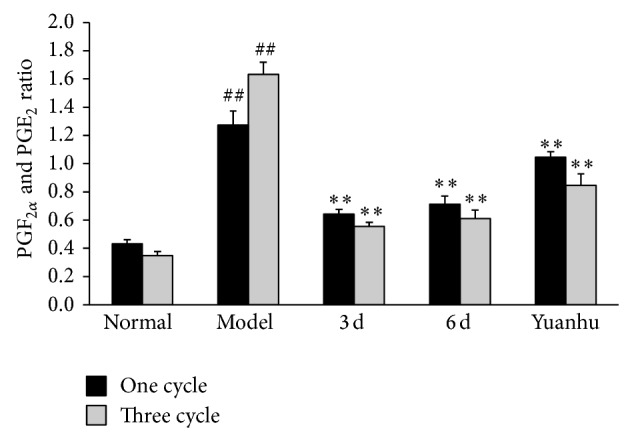
Effects of PGF2α and PGE2 ratio at different administration times on dysmenorrhoea mice. Values are means ± SD (n = 6) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups versus model control group; Yuanhu Zhitong tablet group versus model control group only in three cycles ** P < 0.01.
3.4. Effects of NO and Calcium Ions at Different Administration Times on Dysmenorrhoea Mice
Compared with the normal control group, NO contents in the serum of the model control group were significantly decreased, whereas calcium ions were significantly increased (P < 0.01). Compared with the model control group, NO contents in the serum were significantly increased, whereas calcium ions were significantly decreased in the 6 d and the last 3 d group (P < 0.01). The results of the three-cycle continuous moulding administration experiment were similar to those of the one-cycle treatment. Compared with the model control group, the Yuanhu group significantly decreased the calcium ions in both one-cycle and three-cycle experiments (P < 0.01). However, no obvious difference was found in the NO contents of the serum in the one-cycle experiment. The NO contents were significantly increased after three cycles of continuous administered treatment (P < 0.05) (Figures 7 and 8).
Figure 7.
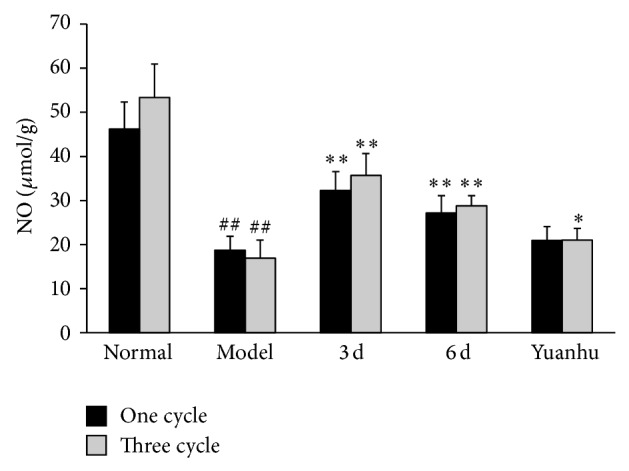
Effects of NO at different administration times on dysmenorrhoea mice. Values are means ± SD (n = 6) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups versus model control group; Yuanhu group versus model control group only in three cycles * P < 0.05.
Figure 8.
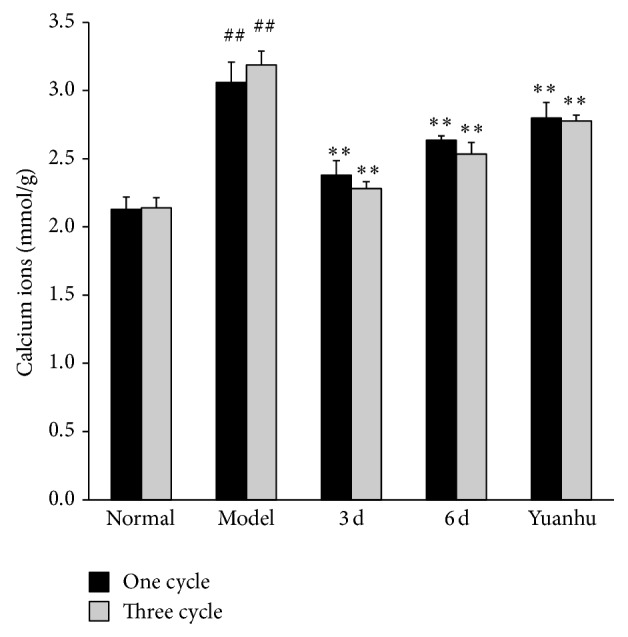
Effects of calcium ions at different administration times on dysmenorrhoea mice. Values are means ± SD (n = 6) and significant difference compared with control group; ## P < 0.01, model control group versus normal control group; ** P < 0.01, 6 d and 3 d groups; Yuanhu group versus model control group.
4. Discussion
4.1. Screening of Experimental Animal and Basis of Another Administration Time
In this study, experimental animal selection was defined based on our previous study on vaginal smear oestrous cycle of female rats and mice. The oestrous cycle of mice was generally from 5 d to 6 d, whereas that of rats was from 4 d to 5 d [31]. Owing to the fact that each stage of the oestrous cycle of rats was shorter and almost the same, the drug cannot completely play its role, and the results may be affected. On the basis of the time of primary dysmenorrhoea in animal model, the mice need only 6 d, and the rat usually takes about 10 d [32], which is not conducive to perform three cycles of continuous administration experiment. Finally, the female mice were selected as the experimental animals.
The female physiology cycle is divided into four phases [33]: menstrual period (1 d to 4 d of the menstrual cycles); estrin phase (5 d to 14 d of the menstrual cycles, follicular maturation stage); progestational stage (15 d to 24 d of the menstrual cycles, corpus luteum maturation stage); and premenstrual period (25 d to 28 d of the menstrual cycles, degeneration of the corpus luteum period). In this study, on the basis of this theory and the result of JQF in previous clinical studies, the medication in luteal regression period of patients was effective, and the diestrus of mice was at the last 3 d of the cycle through vaginal smear. Therefore, the last 3 d in the cycle group (the last 3 d group) that equals administration in the corpus luteum period [34] was finally defined as another time of administration. To explore the different administration times of primary dysmenorrhoea, the results of the experiment cycle group (6 d group) were compared with those of the animal experiment.
4.2. Relationship between Related Pain Factors and Primary Dysmenorrhoea
This research determined four kinds of wide pain factors from the existing primary dysmenorrhoea animal experiment references to explore the scientific connotation of different administration times for primary dysmenorrhoea treatment from the mechanism perspective. Prostaglandin was the most relevant pain factor to primary dysmenorrhoea [35]. PGF2α combined with PGF2α receptor on the spiral arterioles increased uterine contractility, resulting in ischemia pain [36]; PGE2 could inhibit uterine contraction and induce uterine relaxation. The content of PGF2α/PGE2 in endometrium of dysmenorrhoea patients is higher than that of healthy women [37]. NO influenced the primary dysmenorrhoea from many aspects. NO could regulate uterine contraction and execute pain modulation of peripheral and central levels [38]. Through the NO-CGMP pathway, NO can cause pain and analgesia. The decrease in NO could promote nociceptive transmission and pain, whereas the increase in NO could inhibit analgesia [39]. Calcium ions also play an important role in primary dysmenorrhoea. Intracellular calcium overload induced cell membrane damage and cell energy metabolism; it also contributed to the ischemia-reperfusion injury, causing vasoconstriction of uterine blood vessels (uterine ischemia), increasing uterine smooth muscle contraction, and reducing the endometrial blood supply, which lead to the occurrence of dysmenorrhoea [40].
4.3. Open Problems
The following problems need further investigation: whether only the selected pain factors could reflect the results which were consistent with the clinical treatment; whether the association with drug resistance resulted in no difference between the one-cycle group and the last 3 d of cycle group of primary dysmenorrhoea model mice; the main reason and whether it is related to the mechanism of possible hormone regulation. Given the preliminary results of this experimental study, further clinical and animal experimental studies will be conducted.
5. Conclusion
Both in one-cycle and three-cycle experiments, comparing with the model control group, the writhing times and contents of PGF2α and calcium ion in serum were significantly decreased by administration of JQF (last 3 d group and 6 d group) or Yuanhu Zhitong tablet. Similarly, levels of PGE2, NO as well as PGF2α and PGE2 ratio were normalized in the JQF last 3 d group and 6 d group. Moreover, results in the three-cycle part were the same as that of one cycle, which proved that the cycles and the different times in the same cycle (the last 3 d group and 6 d group) of administration with JQF had the same treatment effect and all remained valid. What we found confirmed the clinical effective treatment of selecting 3 d to 7 d before menstruation instead of the entire physiological cycle therapy in primary dysmenorrhoea treatment. The animal model designed according to the mice oestrous cycle, in this study, may be more feasible and closer to the evaluation of clinical curative effect. It supplies a good foundation for further exploration of in-depth mechanisms of primary dysmenorrhoea treatment.
Acknowledgment
This project was supported by the Research Programs of Application of Basic and Frontier Technology in Tianjin (13JCYBJC23900).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Proctor M., Farquhar C. Diagnosis and management of dysmenorrhoea. British Medical Journal. 2006;332(7550):1134–1138. doi: 10.1136/bmj.332.7550.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis A. R., Westhoff C. L. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. Journal of Pediatric and Adolescent Gynecology. 2001;14(1):3–8. doi: 10.1016/S1083-3188(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 3.Davis A. R. D., Westhoff C. L. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. Journal of Pediatric and Adolescent Gynecology. 2001;14(1):3–8. doi: 10.1016/s1083-3188(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan Ö., Nazıroğlu M., Güney M., Aykur M. Non-steroidal anti-inflammatory drug modulates oxidative stress and calcium ion levels in the neutrophils of patients with primary dysmenorrhea. Journal of Reproductive Immunology. 2013;100(2):87–92. doi: 10.1016/j.jri.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Dawood M. Y. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. The American Journal of Medicine. 1988;84(5):23–29. doi: 10.1016/0002-9343(88)90473-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng F., Cheng H., Cao J. H., et al. Exploration of TCM syndrome distribution characteristics of primary dysmenorrhea. Journal of Chinese Medicine. 2013;28(183):1194–1196. [Google Scholar]
- 7.Peng R. F., Yi D. Study progress on pharmacodynamics of DangGui and its active ingredients. Western Journal of Traditional Chinese Medicine. 2012;25(9):125–128. [Google Scholar]
- 8.Hua Y. Q., Su S. L., Duan J. A., Wang Q. J., Lu Y., Chen L. Danggui-Shaoyao-San, a traditional Chinese prescription, suppresses PGF2α production in endometrial epithelial cells by inhibiting COX-2 expression and activity. Phytomedicine. 2008;15(12):1046–1052. doi: 10.1016/j.phymed.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Xi X. Q., Ke Z., Rong Y. L. Progress of Chuanxiong volatile oil. Lishizhen Medicine and Materia Medica Research. 2007;18(6):1508–1510. [Google Scholar]
- 10.Yu G. J., Ao M. L. Analysis of the pharmacological effects of motherwort. Seek Medical and Ask the Medicine. 2012;10(6, article 791) [Google Scholar]
- 11.Lan J. X., Hao H., Chang L. Z. Review in research of radix Paeoniae rubra . Drug Evaluation Research. 2010;33(6):233–236. [Google Scholar]
- 12.Fang L., Zhu X. B., Zhu C. C., et al. The study on the mechanism of JQF for the treatment of primary dysmenorrheal. Chinese Traditional and Herbal Drugs. 2010;41(4):615–617. [Google Scholar]
- 13.Fang L., Zhu X. B., Yin J., et al. Mechanisms of JQF prescription on experimental dysmenorrhea in rats. Tianjin Journal of Traditional Chinese Medicine. 2010;27(1):46–49. [Google Scholar]
- 14.Sun X. M., Jiang G. Y., Lai X. Y., et al. Study on active ingredients of JQF prescription for treating dysmenorrhea. Journal of Tianjin University of Traditional Chinese Medicine. 2011;30(4):220–224. [Google Scholar]
- 15.Wei L., Wang M. L., Shen Z. X. Introduces the current development of TCM internationalization. The Asia-pacific Traditional Medicine. 2006;(12):24–26. [Google Scholar]
- 16.Chen Y. On the development of TCM internationalization. Journal of Traditional Chinese Medicine Management. 2006;20(9):820–822. [Google Scholar]
- 17.Jing L. Clinical observation of ‘Tongjing Decoction’ in periodically treating dysmenorrhea of cold-blood deficiency. Shanghai Journal of Traditional Chinese Medicine. 2011;45(1):57–59. [Google Scholar]
- 18.Li X. Y. Clinical observation on the cycle treatment with TCM ofprimary dysmenorrhea. Journal ofEmergency in Traditional Chinese Medicine. 2008;17(8):1075–1078. [Google Scholar]
- 19.Gu R. Y., Jing B. Q., Song Z. Y., et al. Observation of efficacy on primary dysmenorrhea treated by periodic therapy of Chinese herbal medicine. Journal of Sichuan of Traditional Chinese Medicine. 2012;30(12):90–92. [Google Scholar]
- 20.Li D. J., Jun J., Liu C. L., et al. Effect of Zinitongjingning to qi stagnation and blood stasis type primary dysmenorrhea of serum sex hormone, prostaglandin and calcium, magnesium. Clinical Journal of Traditional Chinese Medicine. 2012;24(10):949–952. [Google Scholar]
- 21.Zhang X. Q. Clinical observation on treating 81 cases of primary dysmenorrhea in TCM. Clinical Journal of Chinese Medicine. 2013;5(18):66–67. [Google Scholar]
- 22.He K., Liu J., Li Q., et al. Effects of Dan'e Fukang decocted extract on dysmenorrhea mice. Pharmacology and Clinics of Chinese Materia Medica. 2012;28(3):106–108. [Google Scholar]
- 23.Zhang L. B., Yan X. Q., Duan J. A., et al. Influence of the different extracts of Artemisiae argyi on primary dysmenorrheal model in mice. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18(12):205–208. [Google Scholar]
- 24.Li L. F., Xie L. J., Li G. F. Effects of Fufang Xiaojingtong Capsule on dysmenorrhea induced by oxytocin in rats. Chinese Traditional Patent Medicine. 2009;31(3):363–367. [Google Scholar]
- 25.Dai N., Fang L., Li Y.-B., Wang Y.-M., Yin J., Pu B.-C. Effect of Jingqian Zhitong Fang on serum sex hormone levels in women with primary dysmenorrhea. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/876431.876431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang L., Lai X. Y., Zhang Y. J., et al. The clinical observation of JQF for the treatment of primary dysmenorrheal. Chinese Archives of Traditional Chinese Medicine. 2011;29(11):2480–2481. [Google Scholar]
- 27.Fang L., Jiang G. Y., Dai N., et al. Clinical observation on therapeutic effect of administration of drug in treating primary dysmenorrhea during luteal regression stage. Journal of Tianjin University of Traditional Chinese Medicine. 2012;31(2):77–79. [Google Scholar]
- 28.Li J., Li X. C., Wu Y. X. Comparative study of the three methods of confirming estrous cycle of mice. Laboratory Animal Science. 2007;24(3):62–65. [Google Scholar]
- 29.Sun H.-Y., Cao Y.-X., Liu J., Gao J.-W., Ma M. The establishment of the dysmenorrhea model in mice. Chinese Pharmacological Bulletin. 2002;18(2):233–236. [Google Scholar]
- 30.Schmauss C., Yaksh T. L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta, and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. Journal of Pharmacology and Experimental Therapeutics. 1984;228(1):1–12. [PubMed] [Google Scholar]
- 31.Chen Q. Research Methods in Pharmacology of Chinese Materia Medica. Beijing, China: People's Medical Publishing; 2011. [Google Scholar]
- 32.Ji B., Zhang L.-F., Zhu J., et al. On building the dysmenorrhea model and assessment methods. Chinese Pharmacological Bulletin. 2008;24(6):711–714. [Google Scholar]
- 33.Feng Y. J. Obstetricsand Gynecology. Beijing, China: People's Medical Publishing House; 2010. [Google Scholar]
- 34.Shi X. Y. Modern Medical Laboratory Animal Science. Beijing, China: People's Military Medical Press; 1999. [Google Scholar]
- 35.Lumsden M. A., Kelly R. W., Baird D. T. Primary dysmenorrhoea: the importance of both prostaglandins E2 and F2α . British Journal of Obstetrics and Gynaecology. 1983;90(12):1135–1140. doi: 10.1111/j.1471-0528.1983.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 36.Nazar H., Usmanghani K. Clinical evaluation to assess the safety and efficacy of coded herbal medicine ‘Dysmo-off’ versus allopathic medicine ‘Diclofenac Sodium’ for the treatment of primary dysmenorrhea. Journal of Herbal Pharmacotherapy. 2006;6(1):21–39. doi: 10.1300/j157v06n01_03. [DOI] [PubMed] [Google Scholar]
- 37.Powell A. M., Chan W. Y., Alvin P., Litt I. F. Menstrual-PGF2α, PGE2 and TXA2 in normal and dysmenorrheic women and their temporal relationship to dysmenorrhea. Prostaglandins. 1985;29(2):273–289. doi: 10.1016/0090-6980(85)90208-4. [DOI] [PubMed] [Google Scholar]
- 38.Moya R. A., Moisa C. F., Morales F., Wynter H., Ali A., Narancio E. Transdermal glyceryl trinitrate in the management of primary dysmenorrhea. International Journal of Gynecology and Obstetrics. 2000;69(2):113–118. doi: 10.1016/S0020-7292(00)00185-5. [DOI] [PubMed] [Google Scholar]
- 39.Liao C.-H., Ho C.-T., Lin J.-K. Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes. Biochemical and Biophysical Research Communications. 2005;329(4):1306–1314. doi: 10.1016/j.bbrc.2005.02.110. [DOI] [PubMed] [Google Scholar]
- 40.Fenakel K., Lurie S. The use of calcium channel blockers in obstetrics and gynecology; a review. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1990;37(3):199–203. doi: 10.1016/0028-2243(90)90025-v. [DOI] [PubMed] [Google Scholar]