Abstract
Aspiration of gastric fluid into the lung mediates the development of obliterative bronchiolitis (OB) in orthotopic WKY-to-F344 rat pulmonary transplants that have been subjected to immunosuppression with cyclosporine. However, the contribution of ischemic time to this process remains unknown. In this study, the effect of long (n = 16) and short (n = 12) ischemic times (average of 6 h and of 73 min, respectively) on rat lung transplants receiving aspiration of gastric fluid was assessed. Long ischemic times (LIT) led to significantly (p < 0.05) greater development of OB (ratio of OB lesions/total airways = 0.45 ± 0.07, mean ± standard error) compared to short ischemic times (ratio = 0.19 ± 0.05). However, the development of OB was dependent on aspiration, as controls receiving aspiration with normal saline showed little development of OB, regardless of ischemic time (p < 0.05). These data suggest that LIT, while insufficient by itself to lead to OB, works synergistically with aspiration of gastric fluid to exacerbate the development of OB.
Keywords: Aspiration, gastric fluid, ischemia, obliterative bronchiolitis, pulmonary allograft, transplant
Introduction
Lung transplantation has been evolving into an effective therapeutic treatment for patients with end-stage lung diseases since the first success in 1983 (1). However, the long-term survival rate of pulmonary allografts is relatively low compared to other solid organ transplants (2), due in large part to the frequent occurrence of obliterative bronchiolitis (OB) in pulmonary allografts. This form of chronic rejection is characterized by submucosal fibroproliferation of the small airways, which in turn leads to luminal compromise and respiratory failure (3,4).
The role of innate immunity in the pathogenesis of OB has gained increasing attention, highlighting potential limitations in therapies targeted strictly at T cell responses. For example, nonalloimmune factors such as infection, ischemia-reperfusion (I-R) injury and gastroesophageal reflux (GER) are thought to activate the innate immune system, potentially leading to the upregulation of the adaptive immune system and eventual rejection of the pulmonary graft (5).
Several factors support the idea that GER and potentially activation of the innate immune response by GER are important factors mediating pulmonary allograft rejection. First, the incidence of reflux is higher after lung transplantation (6), and GER is correlated with poorer pulmonary function (7). Second, Palmer et al. reported a case in which fundoplication resulted in resolution of airway inflammation (8). Third, additional studies demonstrated that fundoplication for GER disease is associated with improved lung function in lung transplant recipients (8,9). Finally, the causal relationship between gastroesophageal reflux disease (GERD) and OB suggested by these clinical observations is supported by studies in rats demonstrating that OB can be reliably induced by aspiration of gastric fluid (10,11).
Ischemia-reperfusion (I-R) injury is another important nonalloimmune factor typically associated with allograft rejection. I-R injury of the pulmonary graft has been associated a higher risk of developing bronchiolitis obliterans syndrome in a clinical analysis (12), and a prolonged ischemic time has been correlated with poorer long-term survival after lung transplantation (13). However, the relationship between GERD, I-R injury and OB formation after lung transplant has not been clarified in the literature. It thus remains unknown whether I-R may play a role in modulating the injury associated with GER, or, conversely, whether GER might modulate the impact of I-R associated injury. In this study, we probe the idea that donor organ ischemic time may affect the development of OB in a rat model; we also ask whether that potential effect is modulated by chronic aspiration of gastric fluid. For this purpose, the development of OB was assessed in rat pulmonary allografts with either prolonged or short ischemic times (SIT) in combination with and without chronic aspiration of gastric fluid.
Material and Methods
Animals
Male Wistar Kyoto (WKY; RT1I) and Fischer 344 (F344; RT1Iv1) were purchased from Harlan Laboratories (Indianapolis, IN, USA). The rats weighed 250–300 g at the time of transplantation. All rats were housed in specific pathogen-free conditions in the animal care facilities at Duke University Medical Center in accordance with institutional guidelines. All animal care and procedures were approved by the Duke Institutional Animal Care and Use Committee.
Gastric fluid collection
Gastric fluid was collected from F344 rats using the following procedure. Each animal was anesthetized with inhaled isoflurane (5%) and orotracheally intubated with a 14-gauge catheter. Each rat was ventilated (60 breaths per min, tidal volume of 5 mL/kg) with 2–3% isoflurane for the remainder of the procedure. A small midline incision was made in the upper abdomen, the peritoneal cavity was entered, and the stomach and proximal duodenum were then identified. The stomach at the level of the pylorus was ligated with 5–0 silk suture and the abdomen was closed in two layers. This pyloric ligation procedure required approximately 10 min to perform for each rat. Eight to twelve hours later, the rat was again anesthetized. The abdominal sutures were removed, and the peritoneal cavity was entered. The distal esophagus at the level of the gastroesophageal junction was ligated with 5–0 silk suture and a gastrectomy was performed. The gastric fluid was collected from the stomach and stored at −80°C. Gastric fluid from approximately 30 rats were thawed, combined, and filtered through a 70-μm strainer (BD Biosciences, Bedford, MA, USA). The pH of the pooled gastric fluid was measured (pH = 2.5), and then the pooled gastric fluid was aliquoted and stored at −80°C until needed.
Study design and ischemic time
Left lungs from WKY (Allo) or F344 (Iso) rats were orthotopically transplanted into F344 rats using the nonsuture external cuff technique reported previously (14). The procedure entails making a left lateral thoracotomy at the 4th intercostal space, creating the anastomoses with a cuff at the left main bronchus, left pulmonary artery and left pulmonary vein, and administering cyclosporine (5 mg/kg, subcutaneously) three times per week beginning immediately after completion of the transplant. The immunosuppression was utilized to prevent acute rejection, which would otherwise occur in this model in the face of aspiration of gastric fluid (10,11). A total of 52 pulmonary graft recipients were utilized in this study. These animals were divided into six groups, as described in Table 1. Animals received allografts or isografts, long ischemic time (LIT) or SIT and aspiration with either gastric fluid or normal saline (Table 1). During the course of the study, it became apparent that those animals receiving isografts and those animals receiving normal saline aspiration showed little to no OB-like pathology (see "Results" section), regardless of other variables. With this in mind, the number of animals assigned to those groups was kept smaller than the number assigned to groups receiving allografts with aspiration (Table 1). Further, because little OB-related pathology was observed in groups receiving either isografts or aspiration with normal saline, an experimental group utilizing both isografts and aspiration with normal saline was deemed unnecessary.
Table 1.
Study design and groups
| Group | Cold ischemic time in min mean (SD) |
Warm ischemic time in min mean (SD) |
n |
|---|---|---|---|
| Allo LIT GF | 300 (0) | 60 (0) | 16 |
| Allo SIT GF | 57.9 (7.7) | 14.8 (4.0) | 12 |
| Allo LIT NS | 300 (0) | 60 (0) | 5 |
| Allo SIT NS | 57.9 (7.7) | 14.8 (4.0) | 7 |
| Iso LIT GF | 300 (0) | 60 (0) | 6 |
| Iso SIT GF | 102.2 (33.3) | 39.7 (22.3) | 6 |
Cold ischemic time was defined as the interval from the initiation of perfusion of Perfadex (Vitrolife, Kungsbacka, Sweden) at 4°C to the beginning of warm ischemic time recording. The standard warm ischemic time was recorded as the period between the removal of the graft from the ice and reperfusion of the graft in the recipient. In the WKY-to-F344 short ischemic time (Allo SIT) group (n = 19), the cold and warm ischemic times were 57.9 ± 7.7 min (mean ± SD) and 14.8 ± 4.0 min, respectively. For the long ischemic time (Allo LIT) group (n = 21), the cold ischemic time were maintained at 300 min. To reduce the side effect of prolong anesthesia to the recipient, the donor graft was put into a temperature-controlled chamber set at 37°C at the beginning of the warm ischemic time for 30–45 min. Once the recipient’s left pulmonary vessels and bronchus were well prepared, the graft was moved to the recipient for transplantation. The reperfusion of the donor graft was initiated once the warm ischemic time reached 60 min. In the F344-to-F344 short ischemic time (Iso SIT) group (n = 6), the cold and warm ischemic times were 102.2 ± 33.3 min and 39.7 ± 22.3 min, respectively. For the F344-to-F344 long ischemic time (Iso LIT) group (n = 6), the cold and warm ischemic times were controlled at 300 min and 60 min, the same as that in the Allo LIT group (Table 1). As described in the operative procedures, the donor lung was kept inflated during the entire ischemic time.
Of note was the observation that some animals experienced complications that were observed within 24 h of transplantation. These complications, predominantly associated with animals in the LIT group, are described in the “Results”. The affected animals were excluded from the study and are not counted in the 52 rats shown in Table 1.
Aspiration procedure
Transplanted rats utilized in the study (n = 52) received aspiration of 0.5 mL/kg fluid once weekly into the left (transplanted) lung for 8 weeks starting 1-week posttransplantation as previously described (10; Figure 1). The Allo LIT and SIT animals were subdivided into two groups, each sub-group receiving gastric fluid (Allo LIT GF, n = 16; Allo SIT GF, n = 12) or normal saline (Allo LIT NS, n = 5; Allo SIT NS, n = 7). The Iso LIT and SIT groups were both treated with gastric fluid (Iso LIT GF, n = 6; Iso SIT GF, n = 6). The aspiration procedure was conducted as follows: Rats were sedated and orotracheally intubated with a 14-gauge catheter. The rats were then placed in a left lateral decubitus and reverse Trendelenburg position at a 35°–40°angle. A small silastic catheter was inserted into the distal trachea, through which 0.5 mL/kg of pooled gastric fluid was injected. A small amount of air was then introduced through the silastic catheter and endotracheal tube to expel any remaining gastric fluid. Rats were sacrificed 1 week after the final aspiration event at 9–10 weeks posttransplant.
Figure 1. Time course of the experiment.

Transplanted rats received aspiration of 0.5 mL/kg gastric fluid or normal saline once weekly into the left (transplanted) lung for 8 weeks starting one week posttransplantation. The rats were sacrificed one week after the last aspiration.
Procurement of heart–lung block
Following 8 weeks of weekly aspiration, the heart and lungs of transplanted animals were removed en bloc. In brief, rats were anesthetized with inhaled isoflurane (5% in oxygen), and a midline sternolaparotomy was performed. The IVC was transected, and the RAA and LAA were excised. A small incision was created at the RVOT, and the lungs were flushed with 30 mL normal saline. The heart–lung block was then completely removed.
Histology
The left lung was separated into upper and lower halves, and was fixed in 10% neutral buffered formalin for at least 24 h, then processed into paraffin blocks for microtomy. Tissues were then sectioned at 5 μm thickness and mounted onto positively charged microslides (Erie Scientific Company, Portsmouth, NH, USA). Sections for histological analysis were then stained with hematoxylin and eosin or with Masson’s trichrome stain. All sections were assessed in a blinded fashion.
The extent of pulmonary fibrosis in specimens was numerically graded according to a scoring system as described previously (15). Both tissue sections (upper and lower) from each animal were systematically scanned under a microscope using a 10× objective lens. Each tissue section contained an average of 4–5 microscopic fields, and all fields in each section was assessed for severity of interstitial fibrosis and allotted a score between 0 and 8. The mean score of all the fields in the section was taken as the section score. Finally, the score of each left lung was obtained by averaging the section scores of two (upper and lower) sections.
OB was defined as dense fibrosis in the submucosa of membranous and respiratory bronchioles, resulting in partial or complete luminal occlusion (4). Every bronchiole of each tissue section was reviewed, and total number of bronchioles in each section was recorded. The pathology score was reported as the fraction of airways affected (the ratio of the number of OB lesions divided by the total number of bronchioles in each section).
Statistical analysis
Unpaired t-tests were performed using Prism 5.1 software (GraphPad Software, Inc., San Diego, CA, USA) and an alpha = 0.05 was used. Contingency tables were assessed using the program designed by Kirkman (16).
Results
Intraoperative and perioperative morbidity and mortality of the recipients
In this experiment, 36 orthotopic left lung transplantations were performed on rodents at prolonged cold and warm ischemic times (300 min and 60 min, respectively). There was no intraoperative morbidity and mortality. However, nine animals (6 out of 27 allografts and 3 out of 9 isografts) developed severe hemoptysis within 24 h after the operation. Autopsies revealed hemorrhagic consolidation of the left lung, with histologic evidence of parenchymal necrosis and diffuse alveolar hemorrhage. On the contrary, 25 recipients receiving donor grafts with SIT (see Table 1 for cold and warm ischemic times) had no intraoperative or perioperative complications or death. Analysis with a 2 × 2 contingency table revealed a significant difference in perioperative morbidity (hemoptysis) between the rats receiving the operations with LIT versus SIT (p = 0.008).
Gross morphology of allograft with chronic exposure to gastric fluid aspiration
As described in “Materials and Methods,” the recipients received either aspiration with gastric fluid or with normal saline once weekly for 8 weeks (Table 1). Shrinkage, discoloration and consolidation developed in the majority of the allografts with chronic exposure to gastric fluid (12 of 16, Allo LIT GF; 7 of 12, Allo SIT GF) (Figures 2A and B). Conversely, normal appearance and expansion of the left lung was noted in most of the animals (5 of 5, Allo LIT NS; 5 of 7, Allo SIT NS) treated with normal saline (Figures 2C and D). Analysis utilizing a 2 × 2 contingency table revealed a significant difference in the incidence of graft consolidation between the animals with aspiration of gastric fluid versus those with aspiration of normal saline (p = 0.005). However, no difference of gross morphology was noted as a function of ischemic time (Allo LIT GF vs. Allo SIT GF, p = 0.432; Allo LIT NS vs. Allo SIT NS, p = 0.470).
Figure 2. Gross morphology of lung allografts 9 weeks after transplantation.

Lungs were exposed to either long (A and C) or short (B and D) ischemic time, and to aspiration with either gastric fluid (A and B) or normal saline (C and D). In the recipients receiving chronic exposure of gastric fluid, most of the allografts (arrow) developed shrinkage, discoloration and consolidation (A, B). In the animals with normal saline treatment, the majority of the left lung grafts were grossly normal (C, D).
Obliterative bronchiolitis in the small airways of allografts
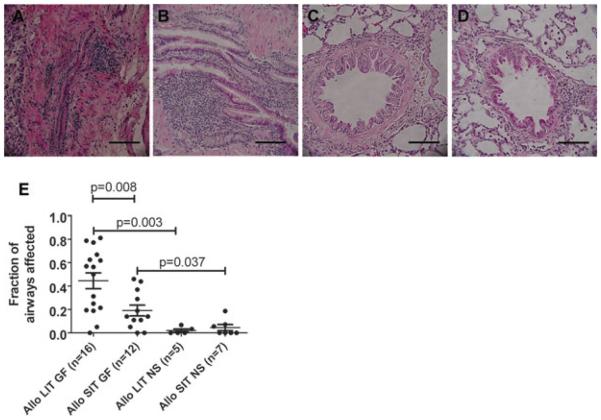
The classical histopathology of OB includes fibrotic proliferation of the submucosa, leading to narrowing or occlusion of the bronchioles with or without inflammatory cell infiltration (3,4). Consistent with this picture, submucosal fibrosis, epithelial damage and regional mononuclear cell infiltration caused near-complete obliteration of bronchioles in the allografts of animals in the Allo LIT GF group (Figure 3A). Similar pathology was observed in the allografts of Allo SIT GF rats (Figure 3B). However, there was a significant difference in the ratio of airways affected between the Allo LIT GF and the Allo SIT GF groups: The fraction of OB lesions/airway in the Allo LIT GF group was 0.45 ± 0.07 (mean ± SEM), compared to 0.19 ± 0.05 in the Allo SIT GF group (p = 0.008; Figure 3E). On the other hand, most bronchioles of animals (either LIT or SIT) receiving chronic exposure of normal saline were intact (Figures 3C and D), and OB-like lesions were rare in these groups: The ratio of airways affected were 0.02 ± 0.01 for the Allo LIT NS group (p = 0.003 vs. Allo LIT GF) and 0.04 ± 0.03 for the Allo SIT NS group (p = 0.037 vs. Allo SIT GF) (Figure 3E).
Figure 3. Obliterative bronchiolitis developed in the allografts with treatment of gastric fluid.
Lungs were exposed to either long (LIT; panels A and C) or short (SIT; panels B and D) ischemic time, and to aspiration with either gastric fluid (GF; panels A and B) or normal saline (NS; panels C and D). Sections were procured and stained with hematoxylin and eosin as described in the “Materials and Methods.” (A) Submucosal fibroproliferation, epithelial damage and regional mononuclear cell infiltration led to near-complete obliteration of bronchioles in an allograft of Allo LIT GF animals. (B) Fibrosis and inflammatory cell infiltration of the submucosa caused narrowing of small airways in one of the Allo SIT GF rats. (C, D) The epithelium and lumen remained normal in the Allo LIT and Allo SIT animals with treatment of normal saline (E) Quantitative analysis revealed significantly more OB lesions in Allo LIT GF grafts. The bar represents 50 μm.
Histological fibrosis of allograft parenchyma
Noticeable fibrosis and infiltration of inflammatory cells developed in the parenchyma of the allografts after chronic exposure to gastric fluid (Figures 4A and B) but not in most of the animals treated with normal saline (Figures 4C and D). As described in the “Materials and Methods” section, we utilized a semi-quantitative grading system (15) to assess the severity of fibrosis in the lung parenchyma of all allografts. The fibrosis score of the Allo LIT GF group was 5.37 ± 0.57 (mean ± SEM), which was similar to the score of the Allo SIT GF group (5.68 ± 0.53, p = 0.703), suggesting that ischemic time did not have a substantial impact on the severity of fibrosis. However, there was a significant difference between the rats treated with gastric fluid versus those treated with normal saline (Allo LIT GF vs. Allo LIT NS, p = 0.002; Allo SIT GF vs. Allo SIT NS, p = 0.011) (Figure 4E), indicating that aspiration of gastric fluid affected fibrosis.
Figure 4. Histological fibrosis in the parenchyma of the allografts with chronic exposure of gastric fluid.
Lungs were exposed to either long (LIT; panels A and C) or short (SIT; panels B and D) ischemic time, and to aspiration with either gastric fluid (GF; panels A and B) or normal saline (NS; panels C and D). Sections were procured and stained with hematoxylin and eosin as described in the “Materials and Methods.” Infiltration of inflammatory cells and severe fibrosis with narrowing of the airways developed in the (A) Allo LIT GF and (B) Allo STI GF grafts. The remnants of gastric fluid can be seen (B, arrow). Expansion of the alveoli and patent airways were noted in the majority of the LIT and SIT allografts with normal saline treatment (C, D). (E) Lung parenchyma in the allografts with chronic exposure of gastric fluid revealed significantly more fibrosis than that in the allografts with treatment of normal saline. The bar represents 100 μm.
The effect of chronic gastric fluid exposure to the isograft
We then tested the effect of gastric fluid aspiration on the isografts (Table 1). After treatment of gastric fluid for 8 weeks, the majority of the left lungs in both the Iso LIT GF and the Iso SIT GF groups developed consolidation and discoloration (Figure 5A). Further, marked fibrosis of the lung parenchyma was found in the isografts in both groups (Figures 5B and C). Fibrosis scores demonstrated that the severity of fibrosis in the isografts exposed to gastric fluid was similar to that seen in the allografts exposed to gastric fluid (Figure 5D). However, few OB-like lesions were seen in the isografts with gastric fluid exposure, regardless of ischemic time (Figure 5E). Further, no noticeable effect of long versus short ischemic time on parenchyma fibrosis and OB lesions was noted in the rats receiving isografts.
Figure 5. The effect of chronic gastric fluid exposure to the isograft.
Lungs were exposed to either long (LIT) or short (SIT) ischemic time and to aspiration with gastric fluid (GF). Representative LIT grafts are shown in panels A–C. Consolidation and discoloration were noted in the majority of isografts receiving gastric fluid aspiration (A). Severe fibrosis of the parenchyma was observed, but patent bronchioles with intact epithelium were still seen (B, hematoxylin and eosin stain; C, Masson trichrome stain). Severe parenchyma fibrosis with chronic exposure to gastric fluid developed in most isografts (D). Relatively few OB-like lesions were seen in isografts (E) and no noticeable difference in parenchyma fibrosis or in the prevalence of OB lesions was noted in between the groups with long versus short ischemic time (D and E). The bar represents 100 μm.
Discussion
GERD has a strong association with chronic rejection after lung transplantation (6–9,17), and studies in humans (8,9) as well as in rodent models (10,11) have supported the idea that GER is an important part of the milieu which causes OB. In this study, OB developed in minor-MHC-mismatched allografts after chronic, repetitive exposure of gastric fluid, but not in most allografts receiving aspiration with normal saline. However, a significantly higher fraction of airways affected by OB was noted in the allografts with prolonged operative ischemic times versus those which shorter times. Further, only occasional OB lesions were observed in the isografts exposed to gastric fluid, regardless of ischemic time.
The results of this study, summarized above, indicate that the alloimmune response is an important part of the milieu leading to OB. At the same time, consistent with previous studies cited above, aspiration of gastric fluid is also a critical and potentially necessary factor influencing the development of OB. This study contributes further to our understanding of the pathogenesis of OB, adding ischemic time to the list of factors contributing to the development of OB.
The approach used in this study has several advantages. These advantages include the reproducibility of the pathology, the similarity of the pathology to the OB seen in clinical practice and the relatively short time frame (10 weeks) in which results can be obtained. However, some aspects of the model do not necessarily recapitulate the situation seen in the clinic: The donor/recipient mismatch is relatively minor compared to that encountered in the clinic, and the degree of aspiration during a single event may exceed that typically found in the clinic. With this in mind, future studies might be directed at increasing the donor/recipient mismatch and the duration of aspiration while decreasing the net amount of aspirate in a given event. However, the extent to which such efforts will result in OB lesions reflecting those seen in the clinic remains unknown.
Another limitation of this study is that it does not address the importance of warm versus cold ischemic time. We selected a total ischemic time of 6 h for the LIT groups, which corresponds to the time at which graft survival appears to markedly decrease (13). We increased both the cold and warm ischemic times relative to that in the SIT (control) group (by factors of approximately 5 and 4, respectively) so that we can focus at this time on the differences in total ischemic time instead of the type of ischemia (i.e. cold versus warm) the organ is enduring. Thus, future studies will be necessary to determine the relative contributions of cold and warm ischemic time to the development of OB. Past experiments focused on ischemic time have generally focused on either warm or cold ischemic time, and thus the relative contributions of the two components have received little attention. For example, Waddell’s work evaluating the connection between I-R injury and MHC class II expression focused on warm ischemic time in a rat model (18). On the other hand, Serrick et al. exploited the effects of a prolonged cold ischemic time in a canine model (19). Thus, future studies in which the contributions of cold and warm ischemic times are simultaneously but individually addressed may be worthwhile.
When comparing the two groups of allografts receiving aspiration of gastric fluid, rats transplanted with prolonged ischemia times had a greater fraction of airways affected by OB compared to rats transplanted with shorter ischemia times (p = 0.008). The mechanism underlying this observation probably reflects an intersection between those mechanisms associated with I-R injury and those mechanisms associated with the fibrosis that is typical of OB. One potential point of intersection may involve exacerbation of the alloimmune response by I-R. Waddell et al. has shown that MHC expression increases in the donor lung as a result of I-R injury (18), and increased MHC expression may promote a greater alloimmune response from T cells that recognize them. Further, I-R injury could possibly also lead to an increase in cellular debris, from which antigens may be sequestered and presented by antigen presenting cells, thus stimulating the alloimmune response.
Although upregulation of the adaptive, allospecific immune response might be a critical factor in the exacerbation of OB by I-R, upregulation of the innate immune system by prolonged ischemic times may also promote OB. Indeed, upregulation of the innate immune response by I-R is well established. For example, TLR4 expression has been shown to be increased after I-R injury in kidney transplantation (20), and less I-R-mediated injury of the lung parenchyma developed in TLR4-deficient mice (21). During I-R injury, the endogenous ligands are released, causing upregulation of the TLR pathway, which promotes rejection by shaping the adaptive immune response (22). However, the results of the present study indicate that prolonged ischemic time per se is not sufficient to cause OB. This finding is consistent with clinical research studies from Hosenpud’s group which shows that prolonged ischemic time alone does not lead to poor pulmonary allograft survival times but that the combination of LIT plus advanced donor age negatively impacts graft survival (23,24).
Histological fibrosis of the pulmonary parenchyma developed in the rat allografts following the treatment of gastric fluid, although parenchymal fibrosis is not a typical manifestation of chronic rejection following clinical lung transplantation. However, a similar histopathology, including parenchymal fibrosis, was noticed in the isografts exposed to gastric fluid, indicating that gastric fluid aspiration induces the development of parenchyma fibrosis without the contribution of allogenecity in this rodent model. Consistent with this view, Appel et al. reported similar degrees of parenchymal fibrosis in the nontransplanted lungs of rats that received aspiration with gastric fluid for 8 weeks (25). Given that aspiration of GF in the absence of an alloimmune response can apparently cause parenchyma fibrosis but not OB, future studies aimed at elucidating the differences in the immunological microenvironments of the pulmonary parenchyma and the bronchioles are probably warranted.
In conclusion, this study demonstrates that chronic aspiration of gastric fluid reproducibly produces OB lesions in a rat orthotopic lung transplantation model. This induction is dependent on the alloimmune response, although non-OB fibrotic lesions can be induced by aspiration of gastric fluid in isografts. Further, prolonged ischemic time acts in concert with chronic aspiration, inducing more OB than aspiration alone. This research provides an important landmark for understanding the mechanisms associated with pulmonary allograft rejection, indicating that an interplay between alloimmunity, I-R, and aspiration of gastric fluid is involved in the pathogenesis.
Abbreviations
- F344
Fischer 344
- GER
gastroesophageal reflux
- GERD
gastroesophageal reflux disease
- GF
gastric fluid
- I-R
ischemia-reperfusion
- IVC
inferior vena cava
- LAA
left atrial appendage
- LIT
long ischemic time
- LL
left lower
- LU
left upper
- NS
normal saline
- OB
obliterative bronchiolitis
- RAA
right atrial appendage
- RVOT
right ventricular outflow tract
- SIT
shortschemic time
- WKY
Wistar Kyoto
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Davis RD, Jr., Pasque MK. Pulmonary transplantation. Ann Surg. 1995;221:14–28. doi: 10.1097/00000658-199501000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008. U.S. Dept of Health and Human Services; Rockville, MD: 2009. [Google Scholar]
- 3.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: Alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008;20:173–182. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–1693. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 7.Hadjiliadis D, Duane Davis R, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363–368. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SM, Miralles AP, Howell DN, Brazer SR, Tapson VF, Davis RD. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest. 2000;118:1214–1217. doi: 10.1378/chest.118.4.1214. [DOI] [PubMed] [Google Scholar]
- 9.Davis RD, Jr., Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533–542. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614–1621. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209–217. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 12.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1047. doi: 10.1016/s0003-4975(01)03606-2. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 13.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: A multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 14.Mizobuchi T, Sekine Y, Yasufuku K, Fujisawa T, Wilkes DS. Comparison of surgical procedures for vascular and airway anastomoses that utilize a modified non-suture external cuff technique for experimental lung transplantation in rats. J Heart Lung Transplant. 2004;23:889–893. doi: 10.1016/j.healun.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkman TW. Statistics to use. 1996 http://wwwphysicscsbsjuedu/stats/ Accessed March 26, 2007.
- 17.Reid KR, McKenzie FN, Menkis AH, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336:206–208. doi: 10.1016/0140-6736(90)91734-r. [DOI] [PubMed] [Google Scholar]
- 18.Waddell TK, Gorczynski RM, DeCampos KN, Patterson GA, Slutsky AS. Major histocompatibility complex expression and lung ischemia-reperfusion in rats. Ann Thorac Surg. 1996;62:866–872. doi: 10.1016/s0003-4975(96)00509-7. [DOI] [PubMed] [Google Scholar]
- 19.Serrick C, Giaid A, Reis A, Shennib H. Prolonged ischemia is associated with more pronounced rejection in the lung allograft. Ann Thorac Surg. 1997;63:202–208. doi: 10.1016/s0003-4975(96)00898-3. [DOI] [PubMed] [Google Scholar]
- 20.Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanotti G, Casiraghi M, Abano JB, et al. Novel critical role of Tolllike receptor 4 in lung ischemia-reperfusion injury and edema. Am J Physiol Lung Cell Mol Physiol. 2009;297:L52–L63. doi: 10.1152/ajplung.90406.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: The final frontier for lung transplantation. Chest. 2011;140:502–508. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 23.Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest. 2000;118:1255–1262. doi: 10.1378/chest.118.5.1255. [DOI] [PubMed] [Google Scholar]
- 24.Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. J Heart Lung Transplant. 1999;18:425–431. doi: 10.1016/s1053-2498(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 25.Appel JZ, 3rd, Lee SM, Hartwig MG, et al. Characterization of the innate immune response to chronic aspiration in a novel rodent model. Respir Res. 2007;8:87. doi: 10.1186/1465-9921-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]