Abstract
Unlike, calorie restriction, exercise fails to extend maximum life span, but the mechanisms that explain this disparate effect are unknown. We used a 24-wk protocol of treadmill running, weight matching, and pair feeding to compare the effects of exercise and calorie restriction on biomarkers related to aging. This study consisted of young controls, an ad libitum-fed sedentary group, two groups that were weight matched by exercise or 9% calorie restriction, and two groups that were weight matched by 9% calorie restriction + exercise or 18% calorie restriction. After 24 wk, ad libitum-fed sedentary mice were the heaviest and fattest. When weight-matched groups were compared, mice that exercised were leaner than calorie-restricted mice. Ad libitum-fed exercise mice tended to have lower serum IGF-1 than fully-fed controls, but no difference in fasting insulin. Mice that underwent 9% calorie restriction or 9% calorie restriction + exercise, had lower insulin levels; the lowest concentrations of serum insulin and IGF-1 were observed in 18% calorie-restricted mice. Exercise resulted in elevated levels of tissue heat shock proteins, but did not accelerate the accumulation of oxidative damage. Thus, failure of exercise to slow aging in previous studies is not likely the result of increased accrual of oxidative damage and may instead be due to an inability to fully mimic the hormonal and/or metabolic response to calorie restriction.
Keywords: energetics, obesity, energy balance
Calorie restriction provides a powerful and widely applicable intervention for attenuating many age-related diseases and extending maximal life span (41). Despite its well-documented benefits, limiting calorie intake (>30%) alone may not provide a practical long-term solution for maximizing human health and longevity. Instead, an alternative approach to energy deprivation could be to increase activity-related energy expenditure or to supplement moderate calorie restriction with exercise.
Several studies have reported that increased physical activity can improve mean life span presumably by reducing mortality risk from many age-related diseases, including cardiovascular disease, stroke, type 2 diabetes, and certain cancers (9, 17, 21, 27–29). In contrast, exercise and longevity studies in rodents (17, 21) and humans (32) have failed to document an exercise effect on maximum life span. For example, it was found in rats that exercise improved survival (mean life span) compared with sedentary ad libitum-fed controls, but did not result in life span extension (17–19, 21). Furthermore, when exercising rats were matched for body weight with food-restricted paired- weight sedentary rats, only the food-restricted rats had an increase in maximum life span (21).
Although the evidence clearly shows a greater benefit from calorie restriction compared with exercise on longevity, there remains a significant gap in the literature explaining this disparate effect. The effects of calorie restriction (40) and exercise (6) on age-related diseases and markers of aging have been exhaustively studied independently, while only a limited number have examined calorie restriction and exercise in tandem (26, 36). Although informative, these studies lack adequate control for changes in energy balance induced by calorie restriction and exercise for making definitive “head-to-head” comparisons. Therefore, using body weight as a proxy of energy balance, this study compared long-term exercise and calorie restriction under carefully-matched conditions on markers purportedly related to aging in mammals. Specifically, beneficial actions of calorie restriction have been attributed to an attenuation of oxidative damage (4), reductions in total body fat (3), improved endocrine and metabolic profiles (1), and alterations in the balance of cell death and survival (8). Therefore, this study assessed the effect of calorie restriction and exercise on body composition, markers of energy metabolism, oxidative stress, and hormesis.
The results show that compared with fully-fed controls, ad libitum-fed runners had significantly lower serum IGF-1 levels and less DNA damage, but no improvement in fasting insulin. Animals that were weight matched with ad libitum-fed runners using only mild calorie restriction had similar IGF-1 levels, but significantly lower insulin levels and stress-related proteins. Serum insulin levels were further reduced in mice on mild calorie restriction plus exercise, but stress-related proteins in liver and skeletal muscle from this group tended to be elevated compared with sedentary animals. Animals on moderate calorie restriction that were weight matched to calorie-restricted runners exhibited the greatest reduction in insulin levels, and most closely resembled young animals for measures of stress-related proteins. Overall, these data suggest that the failure of exercise in prior studies to extend life span is not likely due to an increased accrual of oxidative damage and may instead be due to an inability to fully mimic the physiologic response to calorie restriction.
METHODS
Animals
Male C57BL/6 mice were ordered at 9 wk of age from Charles River Laboratories (Wilmington, MA). Upon arrival, animals were group housed (4 per cage) and immediately placed in humidity-and temperature-controlled rooms on a reverse-phase light cycle (12:12-h light-dark: lights on at 2400 h, lights off at 1200 h) and provided a moderately high-fat diet (cat. no. D00101881, 35% of calories from fat; Research Diets, New Brunswick, NJ) and water ad libitum. To allow for animals to fully acclimate to the reverse light cycle, mice were group housed for 2 wk followed by 1 wk singly housed prior to beginning the experiment. All work was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Experimental design
Male C57BL/6 mice (n = 82) were assigned to one of six experimental groups matched for body weight and fat mass. Mice assigned to group 1 (young, n = 12) were killed at the beginning of the study; all mice in the five remaining groups (groups 2–6, n = 14 per group) underwent a specific dietary and/or exercise regimen for 24 wk with the aim of manipulating body mass via exercise and/or calorie restriction.
Mice assigned to group 2 were fed ad libitum and remained sedentary (AL/SED), while animals in group 3 were fed ad libitum but exercised (AL/EX). Mice in group 4 were fed a restricted amount of food to remain weight matched with group 3 but were sedentary for the entire experiment (WM3/SED). Animals assigned to group 5 were pair-fed to group 4, but exercised (PF4/EX), and animals in group 6 were fed a restricted amount of food to weigh the same as group 5, but remained sedentary throughout the study (WM5/SED).
Exercise training protocol
Mice assigned to groups 3 (AL/EX) and 5 (PF4/EX) exercised on a treadmill 5 days per wk for 24 wk under dim red light early in their dark cycle. Initially, runners were acclimated to the treadmill on three consecutive days at 8–10 m/min for 10–15 min. The mice then underwent a progressive training protocol consisting of a 3-min warm-up, followed by a training bout in which both duration and intensity were gradually increased over the first 6 wk until a peak speed of 15 m/min at an 8% grade for a maximum duration of 1 h was reached. For the remainder of the experiment, mice trained at a treadmill speed of 13–15 m/min at an 8% grade for 45–60 min. Both groups of runners followed the same training regimen such that the absolute workload for each was equal. To control for potential noise and handling stress during the exercise session, sedentary mice were housed in control lanes of similar dimensions, which were attached to the main framing of the treadmill apparatus, but did not exercise.
Animal feeding and weight matching
To induce weight gain, animals were fed a moderately high-fat diet (Research Diets, New Brunswick, NJ). All mice were weighed twice per week (every 3–4 days), and food given to ad libitum-fed animals was weighed and replaced with fresh diet at each weighing. Animals in groups where food intake was clamped were provided a weighed food pellet daily early in their dark cycle. To weight match animals in groups 3 and 4 and groups 5 and 6, respectively, food intake was typically adjusted by 50–100 milligrams twice per week. Animal hoppers and bedding were routinely checked for any remaining food, which was removed, weighed, and recorded.
Body composition
At baseline, 3 days prior to beginning the experiment, mice were anesthetized with isoflourane (2%), and body composition was assessed in vivo by dual-energy X-ray absorptiometry (DXA; GE-Lunar PIXImus, software version 1.45; Lunar, Madison, WI) as described previously (22, 31). Subsequent scans at 6, 12, 18, and 24 wk in the study were performed by quantitative magnetic resonance for the noninvasive determination of fat and lean mass (3-in-1 Composition Analyzer, Echo Medical Systems, Houston, TX) (39). Quantitative magnetic resonance scans were able to be performed without sedation in <90 s, thus avoiding the confounding effects of anesthesia on food intake, body weight, and activity.
Necropsy and tissue processing
At the end of 24 wk, mice did not exercise for 48 h, were fasted for a minimum of 8 h, and killed late in their light cycle by decapitation. The liver was rapidly removed, cut into smaller pieces, snap frozen in liquid nitrogen, and stored at −80°C. The gastrocnemius muscle was separated from the soleus, snap frozen in liquid nitrogen, and stored at −80°C. Blood was allowed to clot at room temperature, and serum was separated by centrifugation and stored at −80°C until further analysis.
Serum measures
Glucose was measured in duplicate using an Analox (Lunenberg, MA) glucose analyzer. Insulin was measured in duplicate using a high-sensitivity rat/mouse RIA (Millipore, Bellerica, MA), and IGF-1 was assessed in duplicate using a commercially-available RIA (Diagnostic System Laboratories, Webster, TX). The intra-assay coefficient of variation for the measurement of insulin and IGF-1 was 0.9, 8.4, and 1.2%, respectively.
Western blot analysis
For total protein extraction, frozen liver and skeletal muscle samples were dounce homogenized in an ice-cold lysis buffer of 50 mM Tris·HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.25% deoxycholate, 0.1% SDS, 5 mM EDTA, 100 μM PMSF, 0.1 μM okadaic acid, 1 mM orthovanadate, 50 mM NaF, 5 mM Na pyrophosphate, and complete EDTA-free protease inhibitor cocktail tablet (Roche Diagnostics, Penzberg, Germany). Lysates were then assayed for total protein content using the bicinchoninic acid protein assay (Sigma, St. Louis, MO) with BSA as a standard.
For immunoblotting, 20 μg of total protein from liver and muscle was loaded onto Bis-Tris 4–12% gradient midigels (Invitrogen, Carlsbad, CA), and separated by SDS-PAGE. Proteins were then semidry transferred (Novex semidry blotter; Invitrogen) at 20 V constant for 1 h onto PVDF membranes. All membranes were stained with Ponceau S (Sigma) to confirm equal loading and transfer. Membranes were then destained and blocked for 1 h with 5% milk in PBST at room temperature.
Membranes were incubated overnight at 4°C with either a rabbit polyclonal antibody to Sirtuin 1 (SIRT1) (1:2,000; Upstate Biotechnology, Lake Placid, NY), pAMPKThr172, (1:1,000; Cell Signaling, Beverly, MA), heat shock protein 25 (HSP25) (1:1,000; Assay Designs, Ann Arbor, MI), total HSP70 (1:1,000 Cell Signaling), inducible HSP70 (1:25,000; Assay Designs), a rabbit monoclonal antibody to Total AMPKα (1:1,000; Cell Signaling), or SOD2 (1:15,000; Fitzgerald, Concord, MA). In addition, skeletal muscle was incubated with a rabbit polyclonal antibody against uncoupling protein 3 (UCP3) (1:1,000; Abcam, Cambridge, MA).
For the detection of oxidative phosphorylation complex content from skeletal muscle mitochondria, membranes were incubated with a mouse monoclonal antibody cocktail (1:1,000, Mitosciences, Eugene, OR) designed for the simultaneous detection of each of the five complexes by detecting a single core subunit of complexes I–V, respectively. This antibody detects core subunits specific for complex I (ND6), II (FeS), III (Core 2), IV (CoxII), and V (α), which are labile when their corresponding complex is not assembled and hence are indicative of the amount of assembled complex. Additionally, a mouse monoclonal antibody against porin (1:1,000, Mitosciences) was used as an indicator of mitochondrial content.
After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody for 1 h, bands were visualized by chemiluminescence (Super West Dura kit, Pierce Biotechnologies, Rockford, IL) in a ChemiDoc imaging system (Bio-Rad, Hercules, CA). All images were captured with an attached 8-bit camera with the exception of the oxidative phosphorylation complexes (I–V) which were imaged with a 16-bit camera. Band densitometry was performed on all images using Bio-Rad Quantity One software.
Protein carbonyls
Total liver and skeletal muscle protein carbon-yls were determined as described previously with slight modifications (37). Briefly, samples were derivitized with 20 mM 2,4-dinitrophenyl hydrazine in 10% trifluoroacetic acid for 15 min at room temperature and then neutralized and reduced with 2M Tris/30% glycerol/20% 2-mercaptoethanol. For the determination of protein carbonyls, 10 μg of derivitized liver or skeletal muscle protein was separated by SDS-PAGE and semidry transferred onto polyvinylidene difluoride membranes. All membranes were stained with Ponceau S (Sigma) to confirm equal loading and transfer. After being blocked for 1 h with 5% milk in PBST at room temperature, membranes were incubated overnight at 4°C with a rabbit polyclonal anti-2,4-dinitrophenol anti body (1:500; Sigma). Following incubation with an anti-rabbit horse-radish perioxidase-conjugated secondary antibody for 1 h, bands were visualized by chemiluminescence using a Bio-Rad ChemiDoc imaging system with attached 16-bit camera and total carbonyls quantified using Bio-Rad Quantity One software.
Immunostaining for 8-OHdG
At necropsy, a small piece of liver was briefly rinsed in ice-cold PBS and immediately placed in ice-cold Pen-Fix fixative (Richard-Allan Scientific, Kalamazoo, MI) at 4°C for 10–12 h. Tissues were then processed through a series of graded alcohols and xylenes and embedded in paraffin. For immunostaining of 8-hydroxydeoxyguanosine (8-OHdG), 5-μm sections of fixed liver were mounted onto treated slides. Following rehydration, antigen retrieval was performed in a pressure cooker on high pressure in citric acid buffer (pH 6) for 10 min. At the completion of the pressure cooker retrieval, slides were cooled quickly with distilled water and a second retrieval step was performed with proteinase K digestion at 37°C for 15 min. Slides were treated with 3.0% H2O2 for 5 min to quench endogenous peroxidase activity followed by 15 min with avidin and 15 min with biotin, to block endogenous biotin. Liver sections were then blocked with goat anti-mouse IgG for 20 min (Jackson ImmunoResearch, West Grove, PA), postfixed in neutral-buffered formalin for 10 min, and blocked with preimmune goat serum (1%) for 20 min.
Following the blocking steps, sections were incubated for 1 h with a mouse monoclonal antibody against 8-OHdG (1:700, Northwest Life Science, Vancouver, WA). Deletes were included in the same run by omitting primary antibody from the staining procedure. Sections were then incubated with goat anti-mouse link (1:1,000, Jackson ImmunoResearch) for 20 min followed by a 20-min incubation with a biotin-streptavidin containing label (Signet, Dedham, MA). Finally, diaminobenzidine was added to the slides for 7 min for visualization of the antigen-antibody complex, and tissues were counterstained lightly with hematoxylin. Immunostaining intensity of 8-OHdG was scored on a scale of 0 (no staining) to 4 (intense staining) by a pathologist (W. E. Grizzle) blinded to the experimental treatments.
Statistics
Longitudinal measures for food intake, body weight, and body composition were assessed by repeated-measures ANOVA. When significant differences were observed for the model, post hoc analyses were performed using Bonferroni corrections (P ≤ 0.05). Tissue and serum biomarkers were assessed by one-way ANOVA. When significance was detected for the main effect, planned contrasts were carried out using Bonferroni corrections (P ≤ 0.05). When data were not normally distributed, analysis was performed by the Kruskal-Wallis procedure (HSP25, HSP70i, 8-OHdG), and post hoc comparisons were conducted using Dunnett's T3 corrections (P ≤ 0.05) when appropriate. Mitochondrial proteins in skeletal muscle, including UCP3 and oxidative phosphorylation complexes, were normalized to mitochondrial mass by calculating ratios to skeletal muscle porin. All analyses were performed using SPSS (SPSS, Chicago, IL).
RESULTS
Body weight
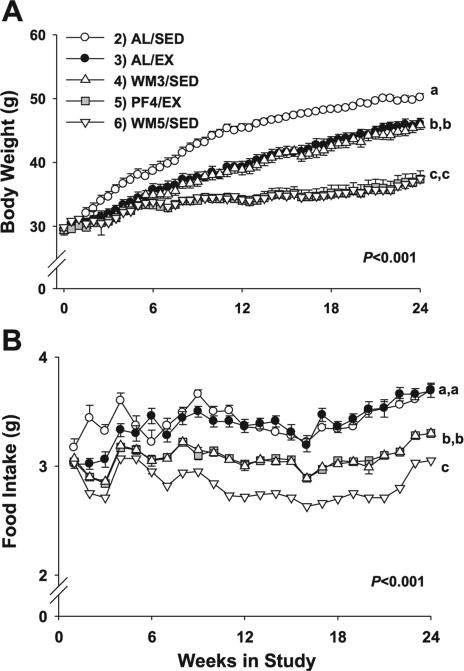
The results for body weight are shown in Fig. 1A. Body weights were significantly different among groups (group effect, P < 0.001; group × time interaction, P < 0.001). Planned contrasts among groups revealed that group 2 (AL/ SED) were significantly heavier than all other experimental groups, reaching nearly 50 g by the end of the study (Fig. 2A, P < 0.001). Ad libitum-fed runners in group 3 (AL/EX) were significantly lighter (~4 g) than AL/SED animals (P < 0.001), as were their weight-matched, sedentary counterparts (WM3/ SED). Animals in group 5 (PF4/EX), which were pair-fed to mice in group 4 (WM3/SED) but also exercised, ceased gaining weight by week 6, and maintained their body weight for the duration of the experiment, as did their weight-matched, sedentary counterparts (WM5/SED). Furthermore, PF4/EX and WM5/SED mice weighed significantly less than all other groups (P < 0.001), differing by nearly 13 g from AL/SED mice, and ~9 g less than the heavier weight-matched mice (AL/EX and WM3/SED) at the end of the study.
Fig. 1.
Body weight and food intake for mice subjected to a specific dietary and/or exercise intervention for 24 wk. Young, control group killed at beginning of study; AL/SED, ad-libitum fed/sedentary; AL/EX, ad-libitum fed/ exercised; WM3/SED, fed a restricted amount of food to remain weight matched with group 3/sedentary; PF4/EX, assigned to group 5 and pair-fed to group 4/exercised; WM5/SED, assigned to group 6 and fed a restricted amount of food to weigh the same as group 5/sedentary. Body weight results from the 24-wk weight-matching paradigm (A) and results for food intake over the 24-wk protocol (B) are shown. Values are means ± SE. Young animals in group 1 were killed at baseline and are not included in the figure. A significant main effect for group (P < 0.001) and group × time interaction (P < 0.001) was observed. a,b,cDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
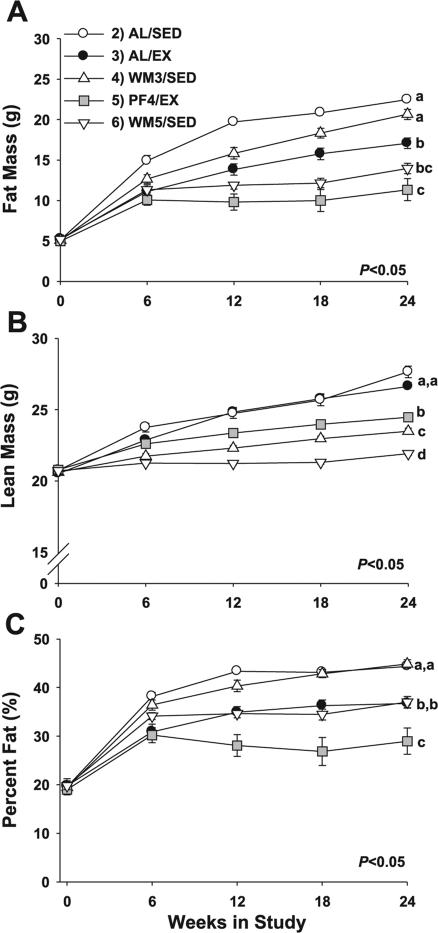
Fig. 2.
Longitudinal measures for body composition in mice subjected to a specific 24-wk dietary and/or exercise intervention. Measurements were made every 6 wk for fat mass (A), lean mass (B), and %fat (C). Values are means ± SE. Young animals in group 1 were killed at baseline and are not included in the figure. A significant main effect for group (P < 0.001) and group × time interaction (P < 0.001) was observed for each measure. a,b,cDifferent letters denote a significant difference between groups at 24 wk; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
Food intake
Repeated measures for food intake detected a significant main effect for group (P < 0.001) and group × time interaction (P < 0.001). When compared by groups, AL/SED and AL/EX mice, which were both ad libitum fed, consumed significantly more food across the study than other experimental groups (Fig. 1B, P < 0.001) but did not differ from each other (P = 0.99). The average ad libitum food intake for the AL/SED and AL/EX mice was ~3.4 g/day. To remain weight matched with the AL/EX group, WM3/SED mice consumed an average of 3.1 g/day of the same diet (~9% food restriction), which was significantly less than ad libitum-fed mice (P < 0.001). Furthermore, this same amount of food (3.1 g/day average) was consumed by their pair-fed counterparts that exercised (P = 0.99, PF4/EX). To remain weight matched with PF4/EX mice, WM5/SED animals consumed 2.8 g/day on average, which was an energy restriction of ~18% and was significantly less than all other groups (P < 0.001).
Body composition
Repeated measures for fat mass, lean mass, and percentage fat detected a significant main effect for group (P < 0.001) and group × time interaction (P < 0.001). Post hoc comparisons for fat mass at week 24 demonstrated the greatest levels in AL/SED mice, reaching nearly 23 g (Fig. 2A, P < 0.01), but lean mass was not different from AL/EX mice (Fig. 2B, P = 0.17). When the heavier weight-matched groups (AL/EX and WM3/SED) were compared, runners had significantly less fat mass at 24 wk (~3.5 g, P < 0.05) and more lean mass (~4 g, Fig. 2B, P < 0.001) than their 9% food-restricted counterparts (WM3/SED). When the lighter weight-matched groups were compared, the restricted runners (PF4/EX) had significantly more lean mass at week 24 (~2.5 g; Fig. 2B, P < 0.001) and tended to have less fat mass at 24 wk (~2.5 g; Fig. 3A, P = 0.36) than did their weight-matched counterparts who were sedentary. Based upon percentage fat, AL/SED and WM3/SED mice that were 9% restricted were the fattest at 24 wk (~45% fat, P < 0.05), while AL/EX animals (37% fat) were leaner than their weight-matched counterparts at 24 wk (Fig. 2C, P < 0.01). WM5/SED animals had a similar percentage fat (~37%) at the end of the experiment as AL/EX mice (P = 0.99), but their weight-matched counterparts (PF4/EX) that exercised were leaner at 24 wk (~29% fat, P < 0.01).
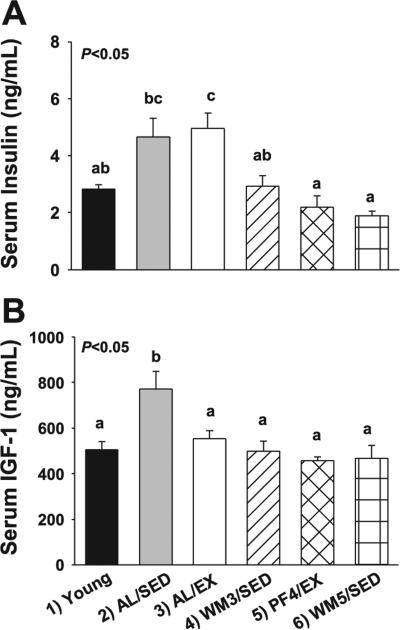
Fig. 3.
Serum measures in young mice and animals subjected to a specific dietary and/or exercise intervention for 24 wk. Fasting serum insulin (A) and fasting serum IGF-1 (B) are shown. Bars are means ± SE. a,b,cDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
Insulin, IGF-1, glucose and AMPK
Fasting serum insulin levels differed significantly among groups (Fig. 3A, group effect P < 0.001). The greatest concentrations were observed in AL/SED and AL/EX mice. A significantly lower fasting insulin level was observed in WM3/SED animals that were weight matched to AL/EX animals by a ~9% food restriction, but did not exercise (P < 0.05). Even lower levels were observed in PF4/EX mice and their weight-matched food-restricted counterparts (WM5/SED), which was significant from some groups (P < 0.05).
Fasting serum IGF-1 levels differed significantly among groups (Fig. 3B, group effect P < 0.001). IGF-1 concentrations were greatest in the AL/SED group compared with other experimental groups (Fig. 3B, P < 0.05). However, no significant differences were observed among AL/EX, WM3/SED, PF4/EX, and WM5/SED animals. Furthermore, no differences were observed among groups for serum glucose levels (group effect, P = 0.41, data not shown) or for tissue total AMPK (liver, P = 0.81; muscle, P = 0.57), phosphorylated AMPKThr172 (pAMPKThr172; liver, P = 0.24; muscle, P = 0.72) or the pAMPKThr172-to-AMPK ratio (liver, P = 0.93; muscle, P = 0.72) (data not shown).
HSPs and SIRT1
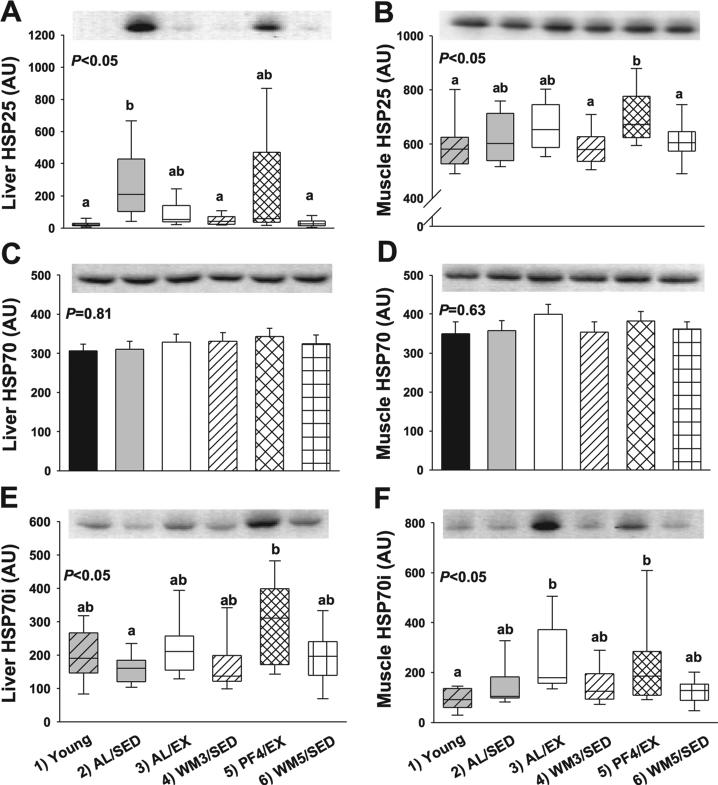
Results for HSPs in the liver are shown in Fig. 4. Liver HSP25 levels differed significantly among groups (Fig. 4A, group effect P < 0.001). Liver HSP25 was significantly elevated in AL/SED and PF4/EX animals compared with other groups (Fig. 4A, P < 0.05). Meanwhile, young mice and WM5/SED animals had the lowest expression of liver HSP25. In contrast, HSP70 in the liver was not different among groups (Fig. 4C, P = 0.81), but inducible HSP70 (HSP70i) in liver was significantly different among groups (Fig. 4E, group effect P < 0.01). The greatest expression of liver HSP70i was observed in lean runners (PF4/EX), but this was only significantly different from AL/SED mice (P < 0.05).
Fig. 4.
Protein measures related to hormesis in liver and skeletal muscle from young mice and animals subjected to a specific dietary and/or exercise intervention for 24 wk. Liver heat shock protein 25 (HSP25) (25 kDa; A), muscle HSP25 (B), liver total HSP70 (70 kDa; C), muscle total HSP70 (D), liver inducible HSP70 (HSP70i; E), and muscle HSP70i (70 kDa; F) are shown. Bars are means ± SE. Box plots (A, B, E, F) represent 1st to 3rd quartile range (box); line in box indicates median value, and whiskers indicate 5th–95th percentile range. AU, arbitrary units. a,bDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
Results for HSPs in the skeletal muscle are shown in Fig. 4. Skeletal muscle HSP25 levels differed significantly among groups (Fig. 4B, group effect, P < 0.01). Skeletal muscle HSP25 was significantly elevated in lean runners (P < 0.05) as compared some groups, while the lowest expression of skeletal muscle HSP25 was observed in young WM3/SED and WM5/SED mice (P < 0.05). HSP70 in skeletal muscle was not significantly different among groups (Fig. 4D, P = 0.63), but muscle HSP70i significantly differed among groups (Fig. 4F, group effect, P < 0.001). The greatest expression of muscle HSP70i was observed in AL/EX mice (P < 0.05), while young and WM5/SED mice had the lowest expression of HSP70i in skeletal muscle (P < 0.05). SIRT1 expression in the liver (P = 0.81) and skeletal muscle (P = 0.56) was unaltered by the various interventions (data not shown).
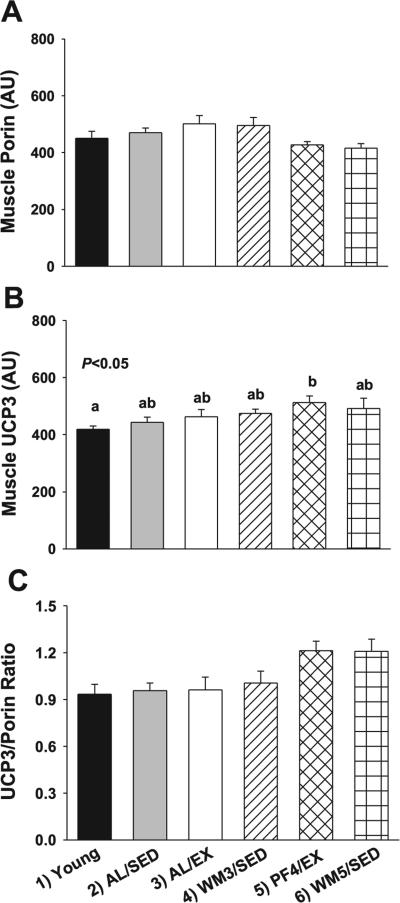
Porin, UCP3, and oxidative phophorylation complexes
A significant main effect for group was observed for skeletal muscle porin (Fig. 5A, P < 0.05), which serves as an indirect measure of mitochondrial mass. However, no significant differences were observed among groups after planned contrasts, although numerically greater levels were observed in AL/EX animals that tended to differ from WM5/SED mice (P = 0.07). Likewise, a significant main effect for group was observed for skeletal muscle UCP3 (Fig. 5B, P < 0.05), and planned contrasts detected a significant difference between young and PF4/EX mice (P = 0.05). However, after normalizing to porin, a significant main effect was observed for UCP3 (Fig. 5C, P < 0.01), but UCP3 only tended to be elevated in PF4/EX and WM5/SED mice.
Fig. 5.
Raw and adjusted values for porin and UCP3 protein expression in skeletal muscle from young mice and animals subjected to a specific dietary and/or exercise intervention for 24 wk. Skeletal muscle porin protein content (31 kDa) as an indirect indicator of mitochondrial content (A), and UCP3 in skeletal muscle (34 kDa) before (B) and after (C) normalizing to porin. Bars are means ± SE. a,bDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
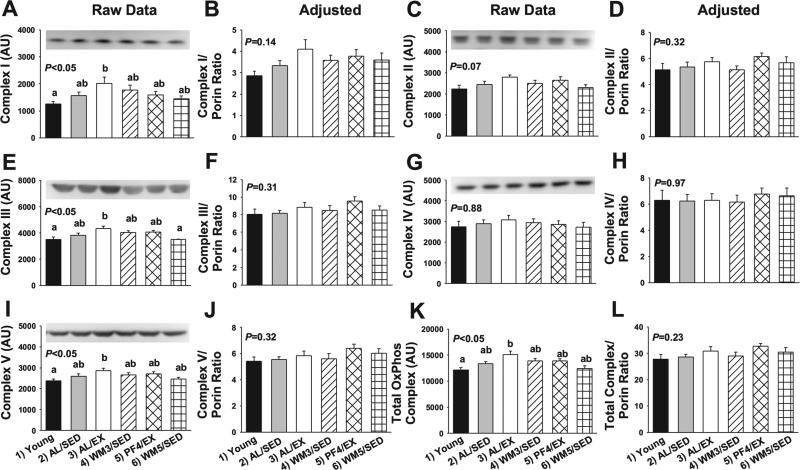
Expression for the electron transport chain complexes in skeletal muscle are presented in Fig. 6. A significant main effect was observed for complex I (NADH dehydrogenase, Fig. 6A, P < 0.05), complex III (ubiquinol-cytochrome c oxidoreductase, Fig. 6E, P < 0.01), complex V (ATP synthase, Fig. 6I), and the sum of complexes I–V (Fig. 6K, P = 0.001) per milligram protein. The greatest expression was observed in AL/EX mice, while young animals and WM5/SED mice, which were 18% restricted, tended to have the lowest expression (P < 0.05). However, differences in electron transport chain complexes were no longer significant after normalizing to porin (Fig. 6, P > 0.05).
Fig. 6.
Raw and adjusted values for the mitochondrial electron transport chain complexes in skeletal muscle from young mice and animals subjected to a specific dietary and/or exercise intervention for 24 wk. Complex I content (ND6, 20 kDa) before (A) and after (B) normalizing to porin, complex II content (FeS, 35 kDa) before (C) and after (D) normalizing to porin, complex III content (Core 2, 42 kDa) before (E) and after (F) normalizing to porin, complex IV content (CoxII, 25 kDa) before (G) and after (H) normalizing to porin, complex V content (α, 55 kDa) before (I) and after (J) normalizing to porin, and total complex content before (K) and after (L) normalizing to porin. Bars are means ± SE. a,bDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
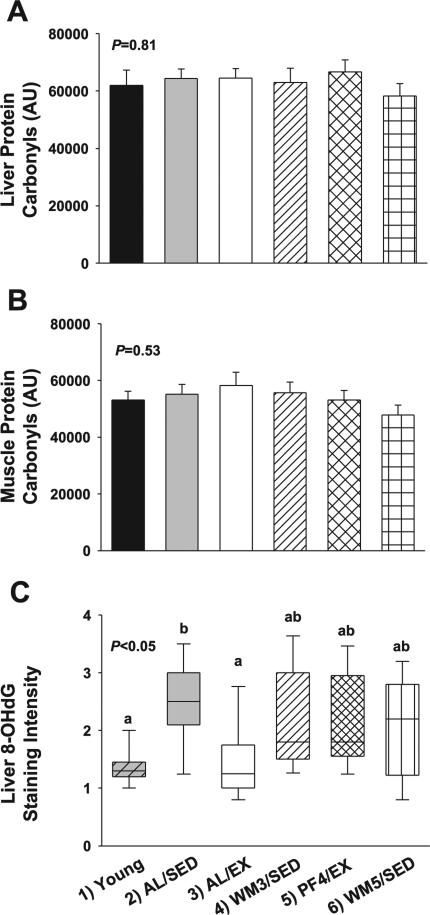
Oxidative stress markers
No significant differences were observed for total protein carbonyls in the liver (Fig. 7A, P = 0.81) or skeletal muscle (Fig. 7B, P = 0.53) among groups, although a small numeric reduction was observed in both liver and muscle from the WM5/SED group. A significant difference was observed in liver 8-OHdG immunostaining among groups (Fig. 7C, group effect, P < 0.01, planned contrasts, P < 0.05). AL/SED mice had the greatest numerical mean staining intensity (~2.5), and this was significantly greater than young and AL/EX animals, which had a mean staining intensity of 1.35 and 1.45, respectively. However, there were no significant differences among groups for SOD2 protein content in liver (P = 0.39) or skeletal muscle (P = 0.96; data not shown).
Fig. 7.
Measures of oxidative stress in liver and skeletal muscle from young mice and animals subjected to a specific dietary and/or exercise intervention for 24 wk. Liver protein carbonyls (A), muscle protein carbonyls (B), and liver 8-OHdG staining intensity (C) are shown. Bars are means ± SE. Box plots (C) represent the 1st to 3rd quartile range (box); the line in the box indicates median value, and whiskers indicate 5th–95th percentile range. a,bDifferent letters denote a significant difference between groups; groups not sharing a common letter are different from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment.
DISCUSSION
Exercise has been shown to protect from many age-related diseases but fails to extend maximum life span in rodents (16, 17, 21). Since no prior biomarker study has controlled for body weight and energy balance changes with calorie restriction and/or exercise, potential mechanisms responsible for the disparate effects of calorie restriction, and exercise on longevity remain poorly understood. Therefore, the goal of this study was to directly compare long-term exercise and calorie restriction under carefully matched conditions using representative bio-markers believed to be predictive of the aging process. The results of this study indicate that at the same body weight, runners were significantly leaner than their weight-matched counterparts that were calorie restricted but sedentary. This is in agreement with recent human studies showing that despite a similar body mass index, individuals engaged in high-volume running are leaner than those consuming a low-calorie diet (10, 12).
Since a reduction in adiposity may be an important mediator of calorie restriction, this would presumably be an advantage for the health and longevity of exercised rodents and humans. However, this introduces an interesting paradox since the wheel-running rats in the longevity studies by Holloszy (16–19, 21) were observed to be leaner than their weight-matched, food-restricted sedentary counterparts (personal communication). Indeed, it was the calorie-restricted rats and not the wheel runners that had an extension in life span. Thus, it is possible that some harmful byproduct(s) or consequence(s) associated with exercise “interferes” with the beneficial effects of being lean. Alternatively, calorie restriction may extend life span by a mechanism(s) independent of leanness, and exercise may fail to activate these critical pathways to the same scope or magnitude.
To test the hypothesis that exercise is associated with greater levels of stress-related proteins and oxidative damage than calorie restriction, we measured HSPs, protein carbonyls, and 8-OHdG. Runners (AL/EX and PF4/EX) had elevated HSP25 and HSP70i in skeletal muscle, and HSP25 was elevated in PF4/EX mice in liver compared with sedentary animals. The robust increase in HSPs might suggest greater oxidative stress, need for cellular protection, and/or damage with exercise training, and hence a greater need for protein turnover compared with mild and moderate calorie-restricted animals.
However, no difference was observed in liver and muscle total protein carbonyls, although levels did tend to be numerically reduced in animals that were calorie restricted ~18% (WM5/SED). Studies examining differences in protein carbon-yls with short- or long-term exercise and/or calorie restriction have been mixed with some documenting a significant reduc- tion (25, 43, 44), while others have observed no significant effect of treatment (15, 35). It is possible that the lack of differences in carbonyls from this study was due to the age of the animals (12 and 36 wk old), which likely have greater capacity for protein turnover than older mice. In addition, previous studies have shown a reduction in carbonyls with >30% calorie restriction (44), while the greatest restriction achieved in this study was ~18%. Animals were also fed a high-fat diet, and the stress associated with this level of fat intake (35%) may have masked some of the benefits associated with calorie restriction and exercise.
The assessment of a second stress marker, 8-OHdG, which is indicative of DNA damage, was low in young animals and surprisingly low in AL/EX animals, whereas the greatest levels were observed in fully fed, obese, sedentary mice (AL/SED). These findings suggest that DNA damage increased with age, was accelerated by obesity, was slowed by mild-to-moderate calorie restriction and/or exercise but was completely blunted by exercise training in the heavy runners but not the lean runners. The disparate effect of exercise training on DNA damage may have been due to relative differences in the intensity of the exercise training on defense mechanisms and/or DNA repair capacity. Previous studies have demonstrated a reduction in DNA damage (2, 15, 33) and improved DNA base excision repair capacity with vigorous exercise training (33, 34). Indeed, despite being heavier, AL/EX mice performed the same absolute workload as PF4/EX animals, such that these animals were exercising at a greater intensity than PF4/EX mice.
An additional training adaptation observed in AL/EX mice was an increase in electron transport chain content per milli-gram protein in skeletal muscle compared with other experimental groups. However, this increase appeared to be due to variation in mitochondrial content since no differences were observed after adjusting for porin. Nevertheless, an increase in the metabolic machinery within the mitochondria may have provided added protection from oxidative stress to AL/EX mice by increasing the capacity for electron transport and ATP production.
Although the exact function of UCP3 is controversial, evidence suggests that UCP3 is elevated in response to calorie restriction and may play a role in limiting free radical production (4, 5). We observed a small numeric increase in UCP3 content in WM5/SED mice, but this was not significant. However, UCP3 levels in skeletal muscle were significantly elevated in PF4/EX mice compared with young animals. To our knowledge, this is the first study to show that exercise and calorie restriction in combination increase UCP3 content in skeletal muscle, an adaptation that may be present to protect against an increased efficiency of electron transport through the chain when ATP demand is low. In contrast, SOD2 protein content was not affected in this study by calorie restriction or exercise in liver and skeletal muscle, although changes in SOD2 activity or other antioxidant enzymes are possible. Indeed, several studies have noted increased expression and/or activities of antioxidant enzymes including catalase and glutathione peroxidase with exercise training (23, 24, 30).
These data demonstrate that at the same relative energy deficit, exercise is associated with increased levels of HSPs but not with greater oxidative damage than calorie restriction. Therefore, this result does not suggest that the failure of exercise to extend maximum life span is due to a greater accumulation of oxidative damage, although potential differences in older animals or under more robust calorie restriction cannot be ruled out. This is in agreement with the finding of Holloszy (17) showing that exercise combined with 30% food restriction did not interfere with the ability of calorie restriction to extend life span. Furthermore, life span was not extended in wheel-running male rats provided an antioxidant-supplemented diet (16), although the impact of the diet on oxidative stress levels was not measured.
To determine whether the inability of exercise to extend longevity is due to a failure to fully mimic the hormonal, metabolic, or hormetic response to calorie restriction, we measured several markers indicative of these pathways. When comparing weight-matched animals, calorie restriction tended to result in more favorable changes to the insulin/IGF-1 axis than exercise. In contrast, no difference was observed among groups for total AMPK or pAMPKThr172. Likewise, SIRT1, which has been associated with the hormetic response to fasting and food restriction, was unaltered by calorie restriction or exercise in this study.
Recently, Fontana and colleagues (10–12) have examined systemic biomarkers in individuals on a selfimposed low-calorie or low calorie-low protein diet vs. individuals engaged in high-volume exercise for several years. Despite a similar body mass index (10, 12) or fat content (11), long-term calorie restriction and exercise resulted in disparate effects on various parameters related to aging, including thyroid function, systemic inflammation, and growth factors. For instance, individuals engaged in voluntary calorie restriction were found to have lower levels of serum T3 and TNF-α levels than exercisers or a control group consuming a typical Western diet (11). In another study, individuals consuming a low calorie-low protein diet were shown to have a more favorable cancer risk profile than endurance runners or controls, including lower serum IGF-1 and greater levels of dehydroepiandrosterone sulfate and sex-hormone-binding globulin (10). Furthermore, a low-calorie vegan diet resulted in lower blood pressure than exercisers (12). Taken together, the present investigation, coupled with these related studies in humans suggest that at the same relative energy deficit, exercise fails to completely mimic the beneficial changes associated with calorie restriction.
Additional studies are needed to determine whether the failure of exercise to mimic the benefits of calorie restriction and extend life span is due to exercise itself or if a reduction in food intake per se is distinct from an increase in energy expenditure. Along these lines, we found that under clamped food intake conditions, increasing energy expenditure via thermoregulation, rather than exercise, resulted in smaller, leaner mice that had less cancer incidence and progression (22). Similarly, it was shown that compared with controls, rats exposed to cool water for 4 h/day, 5 days/wk consumed 44% more food, but weighed ~20–25% less, and had less sarcoma formation (20). However, the relative energy deficit imposed on these animals did not increase mean or maximum life span compared with controls (20). Therefore, the available evidence demonstrates that an increase in energy expenditure can attenuate disease risk (20, 22) but does not presently support the notion that an energy expenditure-induced energy deficit slows the rate of biological aging (20).
Although rodents appear to benefit more from calorie restriction than exercise, no well-controlled long-term studies currently exist on the potential of these interventions to extend human life span, making it premature to conclude that calorie restriction will prove more beneficial for human longevity than exercise. Along these lines, the CALERIE study (7, 15) has recently reported that 6 mo of calorie restriction or calorie restriction plus exercise resulted in ~10% weight loss were equally effective at reducing fasting insulin, core body temperature, and DNA damage and stimulating skeletal muscle mitochondrial biogenesis. Furthermore, an important distinction to be made is that unlike rats and mice, humans often die of cardiovascular complications, including heart disease and stroke, while this is a rare cause of death in rodents (38). Since exercise has well-known benefits for cardiovascular health (14, 42), the effect of exercise on human longevity may prove to be much more impressive than in animal models.
Perspectives and Significance
Rodent (17) and human studies (32) suggest that increasing energy expenditure via exercise fails to mimic the life-prolonging benefit of consuming fewer calories. Since the disparate effect of calorie restriction and exercise on aging remains poorly understood, we performed a carefully controlled experiment designed to compare biomarkers relevant to aging among adult sedentary, calorie-restricted, and exercised mice. These results showed that mild and moderate calorie restriction were more effective at lowering fasting IGF-1 and insulin levels than exercise, and animals on moderate calorie restriction most closely resembled young animals for measures of hormones and stress-related proteins. In contrast, exercised animals were leaner than calorie-restricted mice and exhibited greater tissue levels of HSPs and oxidative phophorylation complex content in skeletal muscle mitochondria. However, exercise did not exacerbate tissue oxidative damage levels. Overall, these data are in agreement with a prior study showing no effect of an antioxidant-supplemented diet on life span in wheel-running rats (16), suggesting that oxidative damage is not the culprit. The disparate effect of calorie restriction and exercise on insulin and IGF-1 levels is also in line with human studies showing a unique metabolic profile in individuals engaged in long-term calorie restriction vs. exercise (10–12). Therefore, we speculate that the failure of exercise to extend life span in prior studies may be due to an inability to fully mimic the hormonal or metabolic response to calorie restriction rather than an oxidative damage-mediated interference. It remains to be determined whether the inability of exercise to fully mimic calorie restriction is inherent to exercise itself or whether the life-prolonging action of calorie restriction is mediated by pathways independent of changes in energy balance and body composition.
ACKNOWLEDGMENTS
The authors thank Dr. Mark B. Cope for technical assistance.
GRANTS
This study was supported, in part, by a University of Alabama at Birmingham (UAB) Center for Aging Pilot/Feasibility Grant and the UAB Clinical Nutrition Research Unit/National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-56336. D. M. Huffman is supported by National Cancer Institute Predoctoral Training Grant CA-47888.
REFERENCES
- 1.Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asami S, Hirano T, Yamaguchi R, Itoh H, Kasai H. Reduction of 8-hydroxyguanine in human leukocyte DNA by physical exercise. Free Radic Res. 1998;29:581–584. doi: 10.1080/10715769800300621. [DOI] [PubMed] [Google Scholar]
- 3.Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci. 1999;54:B89–B96. doi: 10.1093/gerona/54.3.b89. discussion B97–B88. [DOI] [PubMed] [Google Scholar]
- 4.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–E438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 5.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Bronikowski AM, Carter PA, Morgan TJ, Garland T, Jr, Ung N, Pugh TD, Weindruch R, Prolla TA. Lifelong voluntary exercise in the mouse prevents age-related alterations in gene expression in the heart. Physiol Genomics. 2003;12:129–138. doi: 10.1152/physiolgenomics.00082.2002. [DOI] [PubMed] [Google Scholar]
- 7.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 9.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27:2103–2107. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84:1456–1462. doi: 10.1093/ajcn/84.6.1456. [DOI] [PubMed] [Google Scholar]
- 11.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 12.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007;10:225–234. doi: 10.1089/rej.2006.0529. [DOI] [PubMed] [Google Scholar]
- 14.Gaesser GA. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr Diab Rep. 2007;7:14–19. doi: 10.1007/s11892-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 15.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloszy JO. Longevity of exercising male rats: effect of an antioxidant supplemented diet. Mech Ageing Dev. 1998;100:211–219. doi: 10.1016/s0047-6374(97)00140-1. [DOI] [PubMed] [Google Scholar]
- 17.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70:1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO, Smith EK. Effects of exercise on longevity of rats. Fed Proc. 1987;46:1850–1853. [PubMed] [Google Scholar]
- 20.Holloszy JO, Smith EK. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory”. J Appl Physiol. 1986;61:1656–1660. doi: 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- 21.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 22.Huffman DM, Johnson MS, Watts A, Elgavish A, Eltoum IA, Nagy TR. Cancer progression in the transgenic adenocarcinoma of mouse prostate mouse is related to energy balance, body mass, and body composition, but not food intake. Cancer Res. 2007;67:417–424. doi: 10.1158/0008-5472.CAN-06-1244. [DOI] [PubMed] [Google Scholar]
- 23.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 24.Ji LL. Exercise-induced modulation of antioxidant defense. Ann NY Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci. 2006;61:211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- 27.Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, Aunola S, Keinanen-Kiukaanniemi S, Laakso M, Valle TT, Ilanne-Parikka P, Louheranta A, Hamalainen H, Rastas M, Salminen V, Cepaitis Z, Hakumaki M, Kaikkonen H, Harkonen P, Sundvall J, Tuomilehto J, Uusitupa M. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 28.Lee IM, Hsieh CC, Paffenbarger RS., Jr Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA. 1995;273:1179–1184. [PubMed] [Google Scholar]
- 29.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 30.McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 32.Pekkanen J, Marti B, Nissinen A, Tuomilehto J, Punsar S, Karvonen MJ. Reduction of premature mortality by high physical activity: a 20-year follow-up of middle-aged Finnish men. Lancet. 1987;1:1473–1477. doi: 10.1016/s0140-6736(87)92218-5. [DOI] [PubMed] [Google Scholar]
- 33.Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 34.Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- 35.Selsby JT, Judge AR, Yimlamai T, Leeuwenburgh C, Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fisher 344 rats. Exp Gerontol. 2005;40:37–42. doi: 10.1016/j.exger.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- 37.Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 38.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007;137:1078–1086. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 39.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 40.Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J Nutr. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- 41.Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Charles C. Thomas; Springfield, IL: 1988. [Google Scholar]
- 42.Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 43.Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci USA. 1992;89:9112–9116. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000;14:1825–1836. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]