Abstract
Polychlorinated biphenyls (PCBs) are among the most widespread and persistent pollutants in the global environment. Coplanar and noncoplanar PCBs have been shown to cause congener-specific apoptosis mediated neurotoxicity in rats. Very few, if any, such studies have been reported on human renal cell toxicity. The authors report here caspase-dependent or caspase-independent renal toxicity, as measured by apoptotic death induced by PCBs, depending on the planarity of congeners PCB-77 (coplanar) and PCB-153 (noncoplanar) in human kidney cells (HK2) in vitro. The authors have combined morphological and biological techniques to discover the relevance of apoptosis in renal proximal tubule cell death induced by these two PCB congeners. Treatment with both PCB congeners caused accelerated apoptosis in a time- and concentration-dependent manner. Based on our findings using human kidney (HK2) cells, there was more apoptosis-mediated loss of cell viability by non–ortho-substituted PCB-77 when compared to PCB-153. A significant increase of caspase-3 expression through immunoblot studies showed the involvement of apoptosis by PCB-77 compared to none by PCB-153. The broad-spectrum caspase inhibitor z-VAD-fmk showed increased cell death when treated by PCB-153, but not by PCB-77, confirming that caspase inhibitor induced a switch in the mode of cell death. It is reasonable to assume that apoptotic cell death in the renal proximal tubule cells treated by PCBs may have both caspase-dependent and caspase-independent pathways.
Keywords: Apoptosis, Caspase, Human Proximal Tubule Cell Culture, PCB-77, PCB-153
Poly chlorinated biphenyls (PCBs) are a category or family of chemical compounds, formed by adding 1 to 10 chlorine atoms to biphenyl (C12H10). There are 209 congeners of PCBs, depending on the positions of these chlorine molecules. PCB congeners are also grouped into coplanar and noncoplanar, which decide specificity of their biological activations (Chen and Bunce 2004; Gafni, Wong, and Pessah 2004; Howard et al. 2003; Machala et al. 2003; Patterson et al. 1994). Caspases, a family of specific cysteine proteases, have long been considered as the major executioner of all programmed cell death. However, increasing evidence suggest that programmed cell death can occur independent of caspase activation (Broker, Kruyt, and Giaccone 2005). The caspase activation may be started by (1) an intrinsic pathway involving mitochondrial activation (for example, from neurotoxins, ceramide, and other cytotoxic agents) or (2) an extrinsic pathway through binding specific protein ligands to transmembrane receptors belonging to the tumor necrosis factor (TNF) superfamily of death receptors (DRs) (Thonel and Eriksson 2005). TNF family death receptors activate apoptosis by recruiting a few adapters, signaling, and effector proteins in a complex called the death-inducing signaling complex (DISC). The assembly of the DISC allows binding of the bipartisan adapter protein FADD that recruits and allows self-activation of caspase-8. Activation of the initiator caspase (caspase-8) eventually leads to activating downstream effector caspases (caspase-7, -6, and -3) that eventually cause the death of the cell, thus completing the caspase-dependent cell-death pathway. The intrinsic pathway can also activate the effector caspases. Because of cellular stress, mitochondrial membrane may selectively become permeabilized, leading to the release of cytochrome c. Cytochrome c and the activation of the apical caspase-9 and Apaf-1 form the apopto-some, which is ATP dependent. This leads to direct activation of effector caspases. Thus, the mitochondrial activation loop has the potential to activate the death receptor, which can activate the caspase-dependent cell-death pathway. Caspase-9 can link with mitochondria leading to the caspase-protease network (Li et al. 1997). Caspase-8–mediated activation of BID protein cleavage may account for crosstalk between death receptor (extrinsic) and mitochondrial (intrinsic) pathways that lead to apoptosis. Mitochondria-derived reactive oxygen species (ROS) can lead directly to the caspase-independent cell-death pathway. For example, it has been shown that TNF-induced necrosis-like cell death is mediated by mitochondria-derived ROS (Schulze-Osthoff et al. 1992). Release of the apoptosis-inducing factor (AIF) from the mitochondrial intermembrane space can lead to caspase independence (Joza et al. 2001). Bcl-2, a human antiapoptotic member, is localized in the outer mitochondrial membrane, serving to preserve mitochondrial integrity. Cell-death signals from organelles such as lysosomes are involved in necrotic and autophagic cell death. Mitochondria-derived ROS can lead to more lysosomal membrane permeabilization, which can release cell-death proteases such as cathepsins (Zhao et al. 2003). Endoplasmic reticulum (ER) stress can induce permeabilization of the mitochondrial membrane and, thus, activate caspase-dependent or caspase-independent cell-death pathways (Jaattela 2004).
In 1989, a kidney cancer was reported in the utility workers exposed to PCB (Shalat et al. 1989). Similarly, an experiment that exposed rats to a low level of PCB 118 (10,000 ppb) concluded that kidney accumulates PCB 118 and also confirmed hisotopathological changes in kidney (Chu et al. 1995).
This study reports the renal toxic potential of PCBs and their relevance in apoptosis. We have evaluated the difference in effects of both noncoplanar versus coplanar PCBs. This was done by using various methods, such as the median lethal concentration (LC50) test, DNA fragmentation test, fluorescence staining of condensed chromatin structure, and Western blot for caspase-3 expression.
Materials And Methods
Test Chemicals and Cell Line
Noncoplanar PCB-153 (2, 2′,4, 4′,5,5′-hexachlorobiphenyl) and coplanar PCB-77 (3, 3′, 4, 4′-Tetrachlorinated biphenyl) are products of Ultra Scientific (North Kingstown, RI) and were 98.9% pure. HK2 (human kidney 2; catalog no. CRL-2190) (ATCC, VA) is a proximal tubular cell (PTC) line derived from a normal human kidney. The cells were immortalized by transduction with human papilloma virus 16 (HPV-16) E6/E7 genes (Ryan et al. 1994). A total of 2 × 106 HK2 cells were cultured in each 10-cm-diameter tissue culture plate. After reaching 80% to 90% confluence, HK2 cells were treated with PCBs at different time points. Hoechest (33342) dye and staurosporine were obtained from Sigma Chemicals, St. Louis, MO, USA. A 20-mM solution of the caspase inhibitor z-VAD-FMK (Enzyme Systems Products, Livermore, CA, U.S.A.) was prepared in sterile dimethyl sulfoxide (Sigma Chemical).
Median Lethal Concentration (LC50)
Median lethal concentration (LC50) tests were performed after 24 h of PCB-153 and PCB-77 treatments. A total of 2 × 106 HK2 cells in 10-cm-diameter cell culture plates were grown in 5 ml complete growth medium with different concentrations of PCB-153 (0, 17.2, 34.5, 69, 138, and 276μM) and PCB-77 (0, 6.25, 12.5, 25, 50, 70, and 100 μM) in three replicates along with three control wells for each PCB concentration. After 24 h, the media were removed; the cells were washed with 1 × phosphate-buffered saline (PBS) and stained with 0.05% Trypan Blue to count blue (dead) and white (live) cells and recorded separately for each plate by Trypan Blue exclusion method (Fresh-ney 1987). The GraphPad Prism (San Diego, CA) software was used to estimate the median lethal dose for both PCB-153 and PCB-77.
DNA Fragmentation
The harvested cell materials were pelleted by centrifugation for 5 min and resuspended in 300 μl buffer containing 10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 25 mM EDTA, 0.5% sodium dodecyl sulfate (SDS), and 0.5 mg/ml proteinase K and incubated at 37°C overnight. DNA was precipitated with ethanol, resuspended in 200 μl Tris-EDTA buffer at pH 7.5 containing 50 μg/ml RNase, and incubated at 37°C for an additional 2 h. Five micrograms of DNA samples was then electrophoresed on 2% agarose gel containing ethidium bromide (Duttaroy et al. 1998) (see Figure 1).
Figure 1.
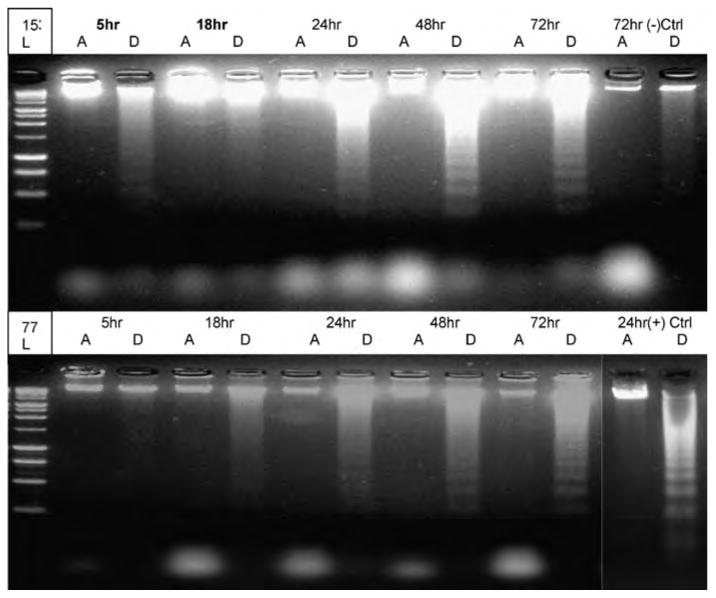
DNA fragmentation was tested with electrophoresis agarose gel at different time points (5, 18, 24, 48, and 72 h) with PCB-153 70 μM (upper gel) and PCB-77 40 μM (lower gel) treatment. DNA fragments appeared at 24 and 48 h for PCB-153 and PCB-77. Positive control (lower-right corner) was HK2 cells treated with 30 nM staurosporine (STS) for 24 h; negative control (upper-right corner) was HK2 cells without any PCB or STS treatment for 72 hours. A = attached cells; D = detached cells, L = ladder.
Fluorescence Microscopy
The bis-benzimidazole dye, Hoechst 33342, has a high affinity for AT-containing DNA by virtue of its minor groove–binding properties. HK2 cells were growing in the four-well-chamber slide with PCB-153 and PCB-77 treatments. When the desired expression times were reached, the chamber and medium were removed; the cells were washed with cold (4°C) 1× PBS, put into cold (−20°C) pure methanol for 20 min at room temperature, then washed with 1× PBS and incubated with Hoechst 33342 (10 μg/ml in PBS) for 15 min at room temperature. The cells were then washed with 1× PBS and mounted in glycerol: water (90:10 v/v) under cover glass. The images were captured with a digital camera under ultraviolet (UV) light. The visual standard of chromatin staining (Filipovic, Meng, and Reeves 1999) was used for this test. All of the samples had 1 ml ker-atinocyte medium and about 30,000 HK2 cells. The samples were washed with cold PBS, fixed with pure methanol, stained with Hoechst 33342, and captured with a digital camera under UV light (Filipovic, Meng, and Reeves 1999). Several controls (see Figure 2A) were used in this study: (1) negative control had only medium and cells; (2) positive controls had two different toxic levels of staurosporine (STS), 30 nM and 200 nM; (3) DMSO, which was used as a solvent for PCBs, STS, and z-VAD-fmk, was added to the medium at 60 μl as another control; and(4)z-VAD-fmk(z-val-ala-asp-[ome]-fluroromethylketone), which is the inhibitor for all caspases, was added to the medium at 60 μM.
Figure 2.
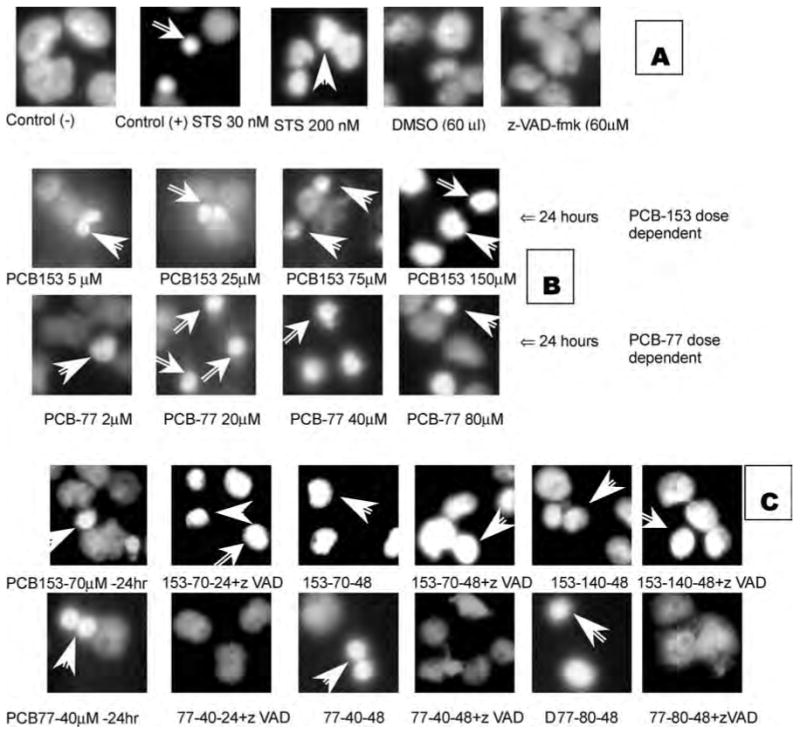
Chromatin fluorescence staining for control cells at 24 h. Arrows indicate highly condensed chromatin (A). Chromatin fluorescence staining for HK2 apoptotic cells treated with dose-dependent PCB-153 and PCB-77 at 24 h. Arrows show highly condensed chromatin (B). Hoechst 33258 fluorescence staining of HK2 apoptotic cells impelled by PCB-153 70/140 μM and PCB-77 40/80 μM at 24 and 48 h with or without caspase inhibitor z-VAD-fmk. Arrows show highly condensed chromatin (C). The number represented in C is the PCB used-concentration-duration + with/without zVAD incubation. For example, 153-140-48 means PCB-153 was used at concentration of 140 μM for the duration of 48 h.
Immunoblotting Studies
Monolayer HK2 cells (2 × 106) were grown in 100 × 20-mm tissue culture dishes up to 70% to 80% confluence; PCB-153 or PCB-77 was added into the dishes in required concentration and for required time exposure. After removal of the medium, 400 to 500 μl of cell lysis buffer was used to scrap cells and centrifuged at 14000 rpm for 30 min at 4°C. The protein estimation was done using a protein Assay Kit (Pierce-23225). The Invitrogen 1.0 × 10-mm well, 4% to 20% Tris-glycine gel (precasted), was used and run at 120 volts for the best resolution. Membrane trans blot was done using the polyvinylidene difluoride (PVDF) membrane (Immobilon P, Milipore; 0.45 μm). The membrane was soaked in 100% methanol for a while and placed in the Hoffer (Invitrogen) transfer cassette following the manufacturer's orientation and run overnight at 22 V with constant stirring at 4°C. Western blotting was done by the following protocol of Sambrook and Russell (2001). Briefly, after washing, the membrane was blocked with 5% nonfat dry milk (Blotto/Invitrogen) for at least 1 h. The primary antibody was added to the membrane and kept on gentle shaking for at least 2 to 3 h The secondary antibody was added over the membrane with gentle shaking for 1 h. After washing, the membranes were placed in plastic wrap, completely soaked with Enhanced Chemiluminescence (ECL) Plus. Western blotting detection system (RPN2132/Amersham Pharmacia) was used according to the manufacturer's instruction. In the darkroom, KODAK Biofilm MR was placed on the membrane with different exposure times and developed in a Kodak automatic developer.
Results
LC50 Tests
LC50 is the toxicant concentration that would cause death in 50% of the test organisms. LC50 was tested at 24 h for HK2 cells with exposure to PCB-153 and PCB-77, which were 80 μM (29 ppm) and 40 μm (12 ppm), respectively. It shows that at 24 h, PCB-77 is more toxic to HK2 cells than PCB-153.
DNA Fragmentation Analysis
DNA fragmentation can distinguish the apoptotic cell death from other forms of cell death. In Figure 1, dead HK2 cells showed DNA smear at 5 h and 18 h and DNA fragments at 24, 48, and 72 h. Among 24, 48, and 72 h, the 48-h DNA fragments were most obvious at PCB-153 treatment. At 72 h, DNA fragments were more prominent in PCB-77 treatment. It clearly indicates that complete disintegration of heterochromatin structure in later time points with PCB-153.
Fluorescence Staining for Apoptotic Cells
Both control of DMSO and z-VAD-fmk did not show any effect on HK2 cells, which was the same as the negative control. It means that these two reagents were not toxic to HK2 cells. In 30 nM STS positive control, apoptotic cells were stained with Hoechst 33342 as small condensed dots; in 200 nM STS positive control, apoptotic cells were stained as large highly condensed dots. It shows that when compared to a lower concentration of STS, higher concentration caused HK2 cell apoptosis to be more effective, and more DNA were stained by Hoechst dye (Figure 2A). Most of the different concentrations of PCB-153 PCB-77 induced apoptosis in a concentration-dependent manner with small bright dots (Figure 2B). Only PCB-153 at 150 μM had larger highly condensed dots like the ones induced by 200 nM STS. When the caspase inhibitor was added (Figure 2C), PCB-153 and PCB-77 showed significant differences. PCB-153 caused HK2 cellular apoptosis both with and without the caspase inhibitor z-VAD-fmk, which even increased apoptosis; PCB-77 caused HK2 cellular apoptosis but was prevented by z-VAD-fmk. Cande et al. (2002) made a similar observation.
Apoptotic cells at each group also were counted and compared to each other (Figure 2C). It was apparent that (1) PCB-153 and PCB-77 caused HK2 cellular apoptosis in a dose-dependent manner; (2) PCB-153 caused HK2 cell apoptosis at 24 h and was not inhibited but increased by z-VAD-fmk, a well-known caspase inhibitor; (3) PCB-77 caused HK2 cell apoptosis at 24 h and was inhibited by z-VAD-fmk.
Immunoblotting Studies
These results suggest that inhibition of the caspase-dependent effect on PCB-153–induced cell death might have switched the death program from a classical caspase-dependent pathway to a caspase-independent pathway. Activation of the apoptotic initiator caspases (caspase-8) is a molecular event that constitutes one of the earlier steps to the entry of extrinsic apoptotic cascade (Chen et al. 2005), whereas activation of effector caspases (caspase-3) is deemed as the point of no return from the commitment to an apoptotic cell death program. We have proceeded to examine the PCB-77– and PCB-153–induced caspase-8 and caspase-3 protein expression pattern. We expected that if PCB-153 is not dependent on the “activation” of effector caspase-3 to exert cell death effects, then compared to PCB-77-induced caspase expression, PCB-153 would not be able to induce a comparable level of caspase-3 expression. As can be seen from Figure 3, as expected, PCB-153 failed to induce any caspase-3 expression. The PCB-153– and PCB-77–induced caspase-3 expression patterns have also been independently verified in a recent article (Sanchez-Alonso et al. 2003).
Figure 3.
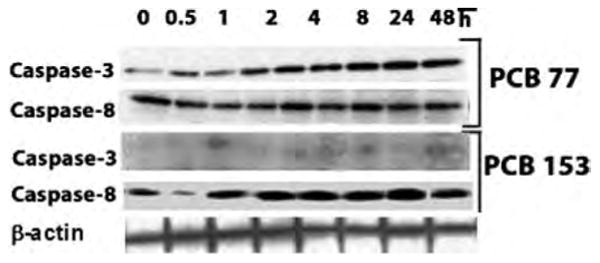
Time-dependent HK2 cell caspase-3 and caspase-8 protein expression with PCB-77 and PCB-153 treatments. When comparing PCB-77 and PCB-153 treatments, an obvious difference can be seen in caspase-3, but not in caspase-8. It is also clear that caspase-3 expression in PCB-77 treatment is time dependent. Caspase-3 has a molecular weight of 17 kDa, and caspase-8 has a molecular weight of 10 kDa.
Discussion
DNA fragmentation, a hallmark of apoptosis, was clearly shown in the HK2 cells under both PCB -153 and PCB -77 treatments. The apoptosis was caused by both PCB-153 and PCB-77, but with two distinct mechanisms. The two main mechanisms of apoptosis are the death receptor pathway and the mitochondria pathway, and caspase may be involved in apoptosis. If so, this investigation gives some insight as to whether PCB-induced apoptosis in HK2 cells is caspase-dependent or caspase-independent.
In PCB-153 studies, Howard et al. (2003) observed that noncoplanar PCB-47 significantly increased DNA fragmentation in hippocampal but not cortical neurons, and this effect was blocked by the caspase inhibitor z-VAD-fmk. In our HK2 cells treated with noncoplanar PCB-153, DNA fragmentation and chromatin condensation were observed, but the effect was not blocked by the caspase inhibitor z-VAD-fmk. It suggests that PCB-153 did induce apoptosis and that this apoptosis is caspase independent. The difference between hippocampal and HK2 might be due to the tissue/cell specificity.
In HK2 cells treated with PCB-153, DNA fragmentation and chromatin condensation were not inhibited, but were enhanced by the caspase inhibitor z-VAD-fmk; caspase-3 expression in time-dependent study did not show any significant difference. It suggests that PCB-153–induced HK2 cell apoptosis was caspase independent (Figure 4).
Figure 4.
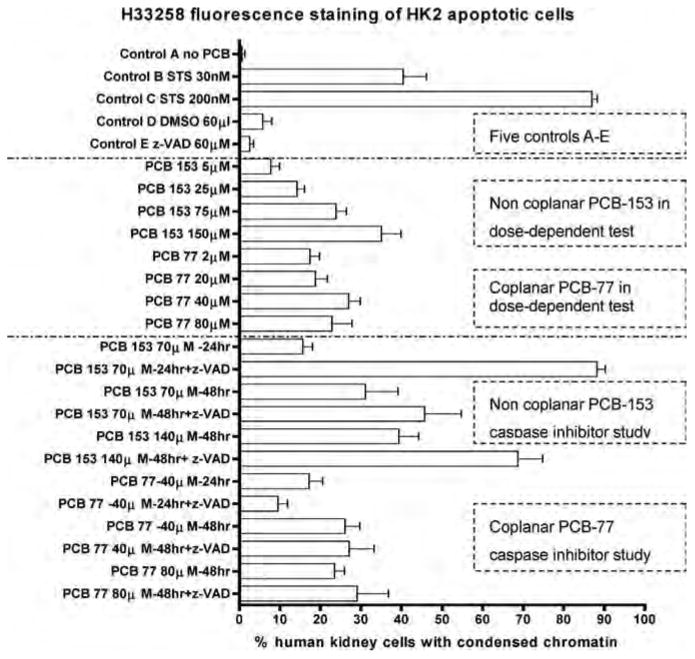
Quantitative Hoechst 33342 fluorescence staining of HK2 apoptotic cells induced by PCB-153 and PCB-77. The white bars show the percentage of HK2 cells with condensed chromatin. STS (staurosporine) is the known apoptosis-causing chemical; z-VAD-fmk (z-val-ala-asp-(ome)-fluroromethylketone) is the inhibitor for all caspases. It shows that both PCB congeners induced apoptosis in HK2 cells in a dose-dependent manner. However, z-VAD-dmk did not inhibit; instead, it enhanced PCB-153–induced apoptosis, but inhibited PCB-77–induced cells.
In HK2 cells treated with PCB-77, at least two biological processes: caspase-3 expression increasing in both a time- and dose-dependent manner; DNA fragmentation and chromatin condensation, which was inhibited by caspase inhibitor z-VAD-fmk. It suggests that PCB-77-induced HK2 cells apoptosis was caspase dependent (Figure 4). It has been shown that caspase inhibition can switch the mode of cell death not through apoptosis but by the involvement of necrosis, depending upon cell lines in vitro (Prabhakaran et al. 2004). This explains our observation that cell death induced by PCB-153 was enhanced by the caspase inhibitor z-VAD-fmk. It was observed that a high concentration of z-VAD-fmk (> 100 μM) enhanced neutrophil apoptosis, and lower concentrations (1 to 30 μM) completely blocked apoptosis (Cowburn et al. 2005).
Acknowledgments
The work was carried out at the Department of Biology, Howard University, Washington, DC. It was supported partly by NTH/SCORE (S06 GM 08016-33) grant to SKD. The authors are grateful to Professor H. Ullah, Department of Biology, Howard University, for productive discussion on the role of caspases and for use of his fluorescence microscope. The authors thank Dr. E. Hoffman, Professor and Director, Center of Genetic Medicine, Children's National Medical Center, Washington, DC, for use of the instruments for DNA and protein measurements.
References
- Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: A review. Clin Cancer Res. 2005;11:31–55. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Interaction between halogenated aromatic compounds in the Ah receptor signal transduction pathway. Environ Toxicol. 2004;19:480–489. doi: 10.1002/tox.20053. [DOI] [PubMed] [Google Scholar]
- Chen YQ, De S, Moses L, Hoffman E, Ghosh S, Dutta SK. Poly-chlorinated biphenyls (PCBs) induced gene expression profiling in human kidney cells. Proceedings of the 105th ASM General Meeting, Atlanta, Georgia. 2005:427. [Google Scholar]
- Chu I, Villeneuve DC, Yagminas A, Lecavalier P, Hakansson H, Ahlborg UG, Valli VE, Kennedy SW, Bergman A, Seegal RF, et al. Toxicity of PCB 77 (3,3′,4,4′-tetrachlorobiphenyl) and PCB 118 (2,3′,4,4′5-pentachlorobiphenyl) in the rat following subchronic dietary exposure. Fundam Appl Toxicol. 1995;26:282–292. doi: 10.1006/faat.1995.1099. [DOI] [PubMed] [Google Scholar]
- Cowburn AS, White JF, Deighton J, Walmsley SR, Chilvers ER. z-VAD-fmk augmentation of TNF alpha-stimulated neutrophil apoptosis is compound specific and does not involve the generation of reactive oxygen species. Blood. 2005;105:2970–2972. doi: 10.1182/blood-2004-07-2870. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Bourbeau D, Wang XL, Wang E. Apoptosis rate can be accelerated or decelerated by overexpression or reduction of the level of elongation factor-1 alpha. Exp Cell Res. 1998;238:168–176. doi: 10.1006/excr.1997.3819. [DOI] [PubMed] [Google Scholar]
- Filipovic DM, Meng X, Reeves WB. Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol. 1999;277:F428–F436. doi: 10.1152/ajprenal.1999.277.3.F428. [DOI] [PubMed] [Google Scholar]
- Freshney R. Culture of animal cells: A manual of basic technique. New York: Wiley-Liss; 2005. [Google Scholar]
- Gafni J, Wong PW, Pessah IN. Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) amplifies ionotropic glutamate receptor signaling in embryonic cerebellar granule neurons by a mechanism involving ryanodine receptors. Toxicol Sci. 2004;77:72–82. doi: 10.1093/toxsci/kfh004. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190:72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Multiple cell death pathways as regulators of tumor initiation and progression. Oncogene. 2004;23:2746–2756. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wake-ham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-l/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Machala M, Blaha L, Vondracek J, Trosko JE, Scott J, Upham BL. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: Inhibitory potencies and screening for potential mode(s) of action. Toxicol Sci. 2003;76:102–111. doi: 10.1093/toxsci/kfg209. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Jr, Todd GD, Turner WE, Maggio V, Alexander LR, Needham LL. Levels of non-ortho-substituted (coplanar), mono- and di-ortho-substituted polychlorinated biphenyls, dibenzo-p-dioxins, and dibenzofurans in human serum and adipose tissue. Environ Health Perspect. 1994;102:195–204. doi: 10.1289/ehp.94102s1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K, Li L, Borowitz JL, Isom GE. Caspase inhibition switches the mode of cell death induced by cyanide by enhancing reactive oxygen species generation and PARP-1 activation. Toxicol Appl Pharmacol. 2004;195:194–202. doi: 10.1016/j.taap.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: Laboratory manual. 3d. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sanchez-Alonso JA, Lopez-Aparicio P, Recio MN, Perez-Albarsanz MA. Apoptosis-mediated neurotoxic potential of a planar (PCB 77) and a nonplanar (PCB 153) polychlorinated biphenyl congeners in neuronal cell cultures. Toxicol Lett. 2003;144:337–349. doi: 10.1016/s0378-4274(03)00238-8. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff KA, Bakker C, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions: Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- Shalat SL, True LD, Fleming LE, Pace PE. Kidney cancer in utility workers exposed to polychlorinated biphenyls (PCBs) Br J Ind Med. 1989;46:823–824. doi: 10.1136/oem.46.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonel AD, Eriksson JE. Regulation of death receptors: Relevance in cancer therapies. Toxicol Appl Pharmacol. 2005;207:123–132. doi: 10.1016/j.taap.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–3786. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]


