Abstract
IMPORTANCE
In vitro and animal model data suggest that intraoperative preservation solutions may influence endothelial function and vein graft failure (VGF) after coronary artery bypass graft (CABG) surgery. Clinical studies to validate these findings are lacking.
OBJECTIVE
To evaluate the effect of vein graft preservation solutions on VGF and clinical outcomes in patients undergoing CABG surgery.
DESIGN, SETTING, AND PARTICIPANTS
Data from the Project of Ex-Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) study, a phase 3, multicenter, randomized, double-blind, placebo-controlled trial that enrolled 3014 patients at 107 US sites from August 1, 2002, through October 22, 2003, were used. Eligibility criteria for the trial included CABG surgery for coronary artery disease with at least 2 planned vein grafts.
INTERVENTIONS
Preservation of vein grafts in saline, blood, or buffered saline solutions.
MAIN OUTCOMES AND MEASURES
One-year angiographic VGF and 5-year rates of death, myocardial infarction, and subsequent revascularization.
RESULTS
Most patients had grafts preserved in saline (1339 [44.4%]), followed by blood (971 [32.2%]) and buffered saline (507 [16.8%]). Baseline characteristics were similar among groups. One-year VGF rates were much lower in the buffered saline group than in the saline group (patient-level odds ratio [OR], 0.59 [95% CI, 0.45-0.78; P < .001]; graft-level OR, 0.63 [95% CI, 0.49-0.79; P < .001]) or the blood group (patient-level OR, 0.62 [95% CI, 0.46-0.83; P = .001]; graft-level OR, 0.63 [95% CI, 0.48-0.81; P < .001]). Use of buffered saline solution also tended to be associated with a lower 5-year risk for death, myocardial infarction, or subsequent revascularization compared with saline (hazard ratio, 0.81 [95% CI, 0.64-1.02; P = .08]) and blood (0.81 [0.63-1.03; P = .09]) solutions.
CONCLUSIONS AND RELEVANCE
Patients undergoing CABG whose vein grafts were preserved in a buffered saline solution had lower VGF rates and trends toward better long-term clinical outcomes compared with patients whose grafts were preserved in saline- or blood-based solutions.
Despite advances in medical management and surgical techniques, vein graft failure (VGF) remains a common complication after coronary artery bypass graft (CABG) surgery.1,2 To preserve endothelial integrity after harvesting, the vein is temporarily stored in a preservation solution. Considerable variation exists among surgeons and hospitals in the choice of preservation solution. Preservation solutions range from physiologic saline to autologous blood to solutions that contain ions for pH buffering, antioxidants, high-molecular-weight molecules, and an ionic composition similar to that of intracellular fluid. Although in vitro and animal models have suggested that these vein graft preservation solutions may have important differences, they have not been compared in clinical studies.3 We performed an observational comparative-effectiveness evaluation of various vein graft preservation solutions using data from a large randomized clinical trial of patients undergoing CABG surgery. We described the patterns of use of vein graft preservation solutions in clinical practice. We then compared VGF rates at 1 year and long-term clinical outcomes after CABG surgery among patients whose grafts were treated with various preservation solutions.
Methods
Study Population
The study population consisted of participants from the Project of Ex-Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) trial. The design, primary results, and long-term follow-up data have been published previously.4-7 In short, PREVENT IV was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial of ex vivo treatment of vein grafts with the E2F transcription factor decoy edifoligide in patients undergoing CABG surgery. The trial enrolled 3014 patients at 107 US sites from August 1, 2002, through October 22, 2003. Eligibility criteria for the trial included age from 18 to 80 years and first isolated CABG surgery for coronary artery disease with at least 2 planned vein grafts. Exclusion criteria included prior cardiac surgery or planned concomitant valve surgery, nonatherosclerotic causes of coronary artery disease, or life expectancy of less than 5 years. The institutional review boards of participating medical centers approved the PREVENT IV protocol, and all patients gave written informed consent. The primary results of the trial showed no difference in VGF (odds ratio [OR], 0.96 [95% CI, 0.80-1.14; P = .66]) and in any of the secondary clinical end points between the treated (edifoligide) and control (placebo) group.
Intraoperative Vein Graft Harvest and Preservation
After harvesting, vein grafts were temporarily stored in a preservation solution, after which the PREVENT IV protocol dictated that veins be placed on a trough and inserted into a fluorinated ethylene polypropylene tube attached to a pressure syringe (a pressure-mediated delivery system [Corgentech Inc]) with the active drug (edifoligide in buffered saline solution) or placebo (buffered saline solution). Drug delivery was facilitated by applying low (6 psi) nondistending pressure to the vein in a sealed tube for 10 minutes. After the vein was treated within the pressure device, vein grafts were removed from the tubing, rinsed, and placed in preservation solution at the discretion of the surgeon until surgical implantation. Other than the administration of the study drug, all drugs, solutions (including those used for initial flushing), and interventions were left to the surgeon's discretion.
In the present analysis, vein graft preservation solutions were categorized into the following groups by investigators at Duke Clinical Research Institute who were blinded to outcomes data: (1) saline, (2) buffered saline, and (3) blood (eFigure in the Supplement).
Angiographic and Clinical Outcomes
The first 2400 patients enrolled in PREVENT IV were assigned to an angiographic cohort scheduled to return, per protocol, for angiography 12 to 18 months after surgery. Patients in the angiographic cohort who underwent angiography for clinical reasons and had VGF before the programmed angio-graphic follow-up were exempt from additional angiography. All angiograms were analyzed at the PERFUSE Angiographic Core Laboratory, with all analysts blinded to any clinical or procedural information. We defined VGF as a stenosis of the vein graft diameter of 75% or greater.
Clinical outcomes included 5-year all-cause mortality, myocardial infarction (MI), and revascularization. Clinical outcomes were assessed annually to 5 years through direct mail and telephone contact with the trial participants. For patient-reported events, medical records were collected and events were adjudicated by an independent clinical events committee using prespecified criteria.4 Five-year follow-up was complete in 95.1% of patients; 2.0% of patients withdrew consent for participation, and 2.9% of patients could not be located.7
Statistical Analysis
Patients were categorized according to the vein graft preservation solution category (saline, buffered saline, blood, or other/unknown solution) used during CABG surgery. Continuous baseline and surgical characteristics were summarized according to the vein graft preservation solution categories using medians and interquartile ranges. We compared characteristics using a Kruskal-Wallis test. Categorical baseline and surgical characteristics, in-hospital care, adverse events through 30 days, and concomitant medications were summarized by vein graft preservation solution as frequencies and percentages and compared using the Pearson χ2 or exact test.
Logistic regression assessed the relationship between vein graft preservation solutions and VGF at the patient and individual graft levels. For the graft-level analysis, we used generalized estimating equation methods to account for correlation among multiple grafts within individuals. Results were adjusted for covariates, including weight, duration of index surgery, use of endoscopic vein graft harvesting, quality of the target artery, use of a composite graft, and use of cardiopulmonary bypass during surgery. For the patient-level analysis, we also adjusted results for the worst target artery quality, any use of composite grafts, and any use of endoscopic vein graft harvesting.
Kaplan-Meier estimated event rate curves and Cox proportional hazards regression assessed the relationship between vein graft preservation solution and the composite outcome of death, MI, or revascularization; the composite outcome of death or MI; and death alone. Adjustment covariates included age, sex, preoperative ejection fraction, history of congestive heart failure, history of diabetes mellitus, baseline creatinine clearance, history of chronic lung disease, history of atrial flutter/fibrillation, MI within 30 days, endoscopic vein graft harvesting, internal mammary artery, worst target artery quality, and MI during the index CABG procedure. For the end points of death and MI and death alone, the model also included preoperative cardiogenic shock and any prior MI. For the composite end points of death or MI and death, MI, or revascularization, duration of cardiopulmonary bypass and the worst graft quality were also included. Linearity and proportional hazards assumptions were tested for all applicable covariates, and appropriate transformations were applied when a significant violation was detected. No violations were applicable to vein graft preservation solutions.
The main analysis included other/unknown preservation solutions as a nuisance level in models; however, results are not specifically reported for this category. Instead, for each analysis, we made an overall comparison across the 3 primary categories of vein graft preservation solution (χ2 test with 2 df) and individual 2-way comparisons. All analyses were performed using commercially available software (SAS, version 9.2; SAS Institute Inc).
Results
Vein Graft Preservation Solutions
A description of the various types and combinations of vein graft preservation solutions for the overall PREVENT IV cohort (3014 patients) and the angiographic cohort is provided in the eTable in the Supplement. Most of the patients had grafts that were preserved in saline solution (1339 [44.4%]), followed by blood (971 [32.2%]) and buffered saline (507 [16.8%]) solutions. In 197 patients, we could not determine whether the grafts were preserved in a saline-, buffered saline–, or blood-based solution. These preservation solutions were classified as other/unknown.
Patient Demographics and Operative Characteristics by Preservation Solution Group
Baseline clinical and operative characteristics are described by vein graft preservation solution for the entire PREVENT IV cohort (Table 1). Patient characteristics among the 3 preservation solution groups were similar. Patients in the buffered saline group more frequently had 3-vessel and/or left main coronary artery disease compared with the other groups. Patients in the buffered saline group also had worse vein graft and target artery quality, more endoscopic graft harvesting, more use of cardiopulmonary bypass, and longer duration of surgery but fewer emergent procedures and less use of composite grafts with multiple targets compared with the other groups.
Table 1.
Baseline and Intraoperative Characteristics Among the Vein Graft Preservation Solution Groupsa
| Characteristic | Vein Graft Preservation Solution Group | ||||
|---|---|---|---|---|---|
| Saline (n = 1339) | Buffered Saline (n = 507) | Blood (n = 971) | Unknown (n = 197) | P Valueb | |
| Age, median (25th-75th percentile), y | 63 (56-71) | 62 (55-69) | 64 (56-71) | 65 (57-71) | .05 |
| Female sex | 281/1339 (21.0) | 97/507 (19.1) | 201/971 (20.7) | 50/197 (25.4) | .67 |
| White race | 1229/1339 (91.8) | 459/507 (90.5) | 882/971 (90.8) | 170/197 (86.3) | .60 |
| BMI, median (25th-75th percentile) | 28.9 (26.1-32.8) | 28.7 (25.9-32.9) | 28.9 (26.0-32.3) | 28.7 (25.8-32.0) | .82 |
| CHF | 135/1339 (10.1) | 45/507 (8.9) | 94/971 (9.7) | 18/197 (9.1) | .74 |
| Current smoker | 306/1339 (22.9) | 103/507 (20.3) | 227/971 (23.4) | 54/197 (27.4) | .09 |
| Chronic lung disease | 195/1339 (14.6) | 72/507 (14.2) | 178/971 (18.3) | 30/197 (15.2) | .03 |
| Hypertension | 1004/1339 (75.0) | 379/507 (74.8) | 725/971 (74.7) | 155/197 (78.7) | .98 |
| Hypercholesterolemia | 996/1339 (74.4) | 407/507 (80.3) | 744/970 (76.7) | 152/197 (77.2) | .03 |
| Diabetes mellitus | 512/1339 (38.2) | 180/507 (35.5) | 361/971 (37.2) | 86/197 (43.7) | .55 |
| Renal failure | 25/1339 (1.9) | 12/507 (2.4) | 23/971 (2.4) | 5/197 (2.5) | .66 |
| Prior events | |||||
| MI | 572/1339 (42.7) | 218/507 (43.0) | 404/971 (41.6) | 79/197 (40.1) | .83 |
| MI within 30 d | 263/1339 (19.6) | 100/507 (19.7) | 206/971 (21.2) | 40/197 (20.3) | .62 |
| Percutaneous coronary intervention | 353/1339 (26.4) | 130/507 (25.6) | 252/971 (26.0) | 45/197 (22.8) | .94 |
| Stroke | 79/1339 (5.9) | 30/507 (5.9) | 52/971 (5.4) | 4/197 (2.0) | .84 |
| Cancer | 114/1339 (8.5) | 42/506 (8.3) | 72/971 (7.4) | 19/197 (9.6) | .62 |
| Ejection fraction, median (25th-75th percentile), % | 50 (40-60) | 51 (40-60) | 50 (40-60) | 55 (45-60) | .25 |
| CrCl, median (25th-75th percentile), mL/min/1.73 m2 | 86.9 (69.9-110.8) | 91.9 (72.9-114.6) | 88.7 (69.0-112.3) | 85.9 (62.2-108.3) | .17 |
| Left main or 3-vessel disease | 597/1338 (44.6) | 246/506 (48.6) | 373/970 (38.5) | 80/197 (40.6) | <.001 |
| Status of procedure | <.001 | ||||
| Emergent/salvage | 46/1337 (3.4) | 4/506 (0.8) | 28/971 (2.9) | 11/197 (5.6) | |
| Urgent | 575/1337 (43.0) | 263/506 (52.0) | 507/971 (52.2) | 116/197 (58.9) | |
| Elective | 716/1337 (53.6) | 239/506 (47.2) | 436/971 (44.9) | 70/197 (35.5) | |
| No. of vein grafts | .19 | ||||
| 1 | 41/1339 (3.1) | 12/507 (2.4) | 25/971 (2.6) | 3/197 (1.5) | |
| 2 | 817/1339 (61.0) | 302/507 (59.6) | 575/971 (59.2) | 112/197 (56.9) | |
| 3 | 410/1339 (30.6) | 173/507 (34.1) | 303/971 (31.2) | 74/197 (37.6) | |
| ≥4 | 71/1339 (5.3) | 20/507 (3.9) | 68/971 (7.0) | 8/197 (4.1) | |
| Multiple distal targets | 444/1339 (33.2) | 125/507 (24.7) | 401/971 (41.3) | 75/197 (38.1) | <.001 |
| Endoscopic vein harvest | 777/1335 (58.2) | 341/506 (67.4) | 544/965 (56.4) | 91/195 (46.7) | <.001 |
| IMA graft use | 1230/1339 (91.9) | 480/507 (94.7) | 891/971 (91.8) | 182/197 (92.4) | .09 |
| Worst target artery | <.001 | ||||
| Good | 581/1337 (43.5) | 174/507 (34.3) | 437/970 (45.1) | 101/196 (51.5) | |
| Fair | 471/1337 (35.2) | 200/507 (39.4) | 347/970 (35.8) | 64/196 (32.7) | |
| Poor | 285/1337 (21.3) | 133/507 (26.2) | 186/970 (19.2) | 31/196 (15.8) | |
| Worst vein graft | <.001 | ||||
| Good | 937/1338 (70.0) | 331/507 (65.3) | 720/971 (74.2) | 146/196 (74.5) | |
| Fair | 343/1338 (25.6) | 136/507 (26.8) | 204/971 (21.0) | 43/196 (21.9) | |
| Poor | 58/1338 (4.3) | 40/507 (7.9) | 47/971 (4.8) | 7/196 (3.6) | |
| Surgery duration, median (25th-75th percentile), min | 231 (194-271) | 239 (205-275) | 227 (187-270) | 224 (192-271) | .003 |
| Use of CPB | 973/1339 (72.7) | 430/507 (84.8) | 802/971 (82.6) | 172/197 (87.3) | <.001 |
| CPB duration, median (25th-75th percentile), min | 100 (82-124) | 104 (81-123) | 97 (75-121) | 93 (76-121) | .002 |
| Use of edifoligide (vs placebo) | 675/1339 (50.4) | 242/507 (47.7) | 489/971 (50.4) | 100/197 (50.8) | .56 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHF, congestive heart failure; CPB, cardiopulmonary bypass; CrCl creatinine clearance; IMA internal mammary artery; MI, myocardial infarction.
SI conversion factor: To convert CrCl to milliliters per second per square meter, multiply by 0.0167.
Unless otherwise indicated, data are expressed as number/total number (percentage).
P value represents differences among the saline, buffered saline, and blood groups only.
Postoperative Course Up to 30 Days
Table 2 shows the in-hospital care and adverse events through 30 days. Overall, times for receiving mechanical ventilation and stay in the intensive care unit setting were similar among the vein graft preservation solution groups. Early adverse events, including stroke and reoperation for bleeding, were also similar among the groups. Postoperative use of secondary prevention medications, such as aspirin, β-blockers, and statins, tended to be higher in the buffered saline group compared with the saline and blood groups.
Table 2.
In-Hospital Care and Adverse Events Through 30 Days Among Patients With Various Vein Graft Preservation Solutionsa
| Characteristic | Vein Graft Preservation Solution Group | P Valueb | |||
|---|---|---|---|---|---|
| Saline (n = 1339) | Buffered Saline (n = 507) | Blood (n = 971) | Unknown (n = 197) | ||
| ICU stay, median (25th-75th percentile), h | 26 (22-47) | 25 (22-49) | 26 (22-47) | 26 (22-46) | .91 |
| Ventilator duration, median (25th-75th percentile), h | 7 (5-14) | 7 (5-13) | 8 (5-13) | 8(5-15) | .65 |
| Length of hospital stay, median (25th-75th percentile), d | 6 (5-8) | 6 (5-8) | 6 (5-8) | 6 (5-8) | .01 |
| Clinical events | |||||
| Stroke | 22/1339 (1.6) | 9/507 (1.8) | 12/971 (1.2) | 3/197 (1.5) | .65 |
| Bleeding requiring reoperation | 37/1339 (2.8) | 14/507 (2.8) | 20/971 (2.1) | 5/197 (2.5) | .53 |
| Atrial fibrillation | 348/1339 (26.0) | 126/507 (24.9) | 266/971 (27.4) | 41/197 (20.8) | .55 |
| Renal failure | 43/1339 (3.2) | 15/507 (3.0) | 35/971 (3.6) | 6/197 (3.0) | .78 |
| Pneumonia | 37/1339 (2.8) | 8/507 (1.6) | 21/971 (2.2) | 4/197 (2.0) | .29 |
| Adult respiratory distress syndrome | 11/1339 (0.8) | 3/507 (0.6) | 12/971 (1.2) | 0 | .41 |
| Mediastinitis | 7/1339 (0.5) | 5/507 (1.0) | 7/971 (0.7) | 2/197 (1.0) | .54 |
| Pulmonary embolism | 9/1339 (0.7) | 2/507 (0.4) | 5/971 (0.5) | 1/197 (0.5) | .75 |
| Peri-index CABG MI | 1213/1339 (90.6) | 466/507 (91.9) | 867/971 (89.3) | 173/197 (87.8) | .25 |
| Second CABG | 2/1339 (0.1) | 2/507 (0.4) | 0 | 1/197 (0.5) | .13 |
| Percutaneous coronary intervention | 7/1339 (0.5) | 0 | 1/971 (0.1) | 1/197 (0.5) | .13 |
| Medications at 30 d | |||||
| Aspirin | 1196/1330 (89.9) | 472/507 (93.1) | 864/960 (90.0) | 173/192 (90.1) | .09 |
| Clopidogrel/ticlopidine | 301/1330 (22.6) | 92/507 (18.1) | 222/960 (23.1) | 39/192 (20.3) | .07 |
| Warfarin | 107/1330 (8.0) | 52/507 (10.3) | 97/960 (10.1) | 13/192 (6.8) | .15 |
| ACE/ARB inhibitor | 559/1331 (42.0) | 216/507 (42.6) | 389/960 (40.5) | 84/192 (43.8) | .69 |
| β-Blocker | 1028/1331 (77.2) | 434/507 (85.6) | 742/960 (77.3) | 150/192 (78.1) | <.001 |
| HMG-CoA reductase inhibitor | 962/1331 (72.3) | 393/507 (77.5) | 693/960 (72.2) | 131/192 (68.2) | .05 |
| Nitrates | 77/1330 (5.8) | 41/507 (8.1) | 58/960 (6.0) | 26/192 (13.5) | .18 |
| Digoxin | 113/1330 (8.5) | 41/507 (8.1) | 90/960 (9.4) | 18/192 (9.4) | .65 |
| Other antiarrhythmic drugs | 231/1330 (17.4) | 60/507 (11.8) | 128/960 (13.3) | 26/192 (13.5) | .003 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CABG, coronary artery bypass graft; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; ICU, intensive care unit; MI,myocardial infarction.
Unless otherwise indicated, data are expressed as number/total number (percentage).
P value represents differences among the saline, buffered saline, and blood groups only.
VGF at 1-Year Scheduled Angiographic Follow-up
Angiographic follow-up was available for 1828 patients with 4343 vein grafts. As shown in Figure 1, patient- and graft-level rates of VGF were significantly lower among grafts preserved in a buffered saline solution compared with those preserved in a saline solution (patient level, 35.2% vs 46.1%; OR, 0.59 [95% CI, 0.45-0.78; P < .001]; graft level, 19.3% vs 27.0%; OR, 0.63 [95% CI, 0.49-0.79; P < .001]). Patient- and graft-level rates of VGF were also significantly lower among grafts preserved in a buffered saline solution compared with those preserved in a blood-based solution (patient level, 35.2% vs 44.0%; OR, 0.62 [95% CI, 95% CI, 0.46-0.83; P = .001]; graft level, 19.3% vs 27.0%; OR, 0.63 [95% CI, 0.48-0.81; P < .001]). Patient- and graft-level rates of VGF were similar between grafts treated with saline or blood solutions (patient level, 46.1% vs 44.0%; OR, 0.95 [95% CI, 0.76-1.20; P = .69]; graft level, 27.0% vs 27.0%; OR, 1.00 [95% CI, 0.82-1.22; P = .99]).
Figure 1.
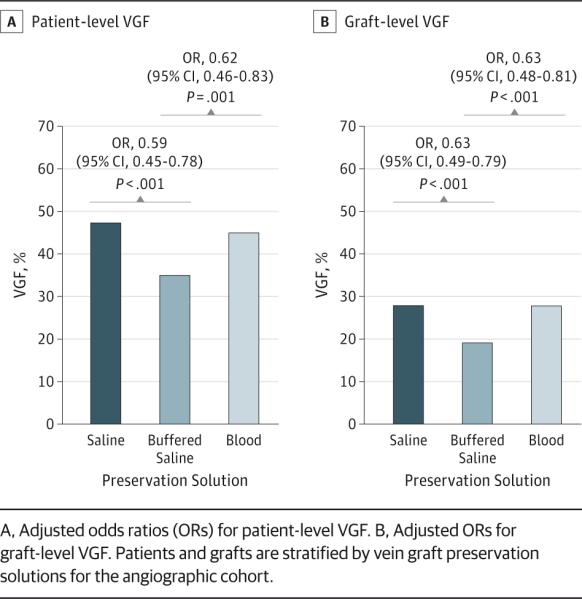
Unadjusted Vein Graft Failure (VGF) Rates According to Vein Graft Preservation Solutions
Long-term Clinical Follow-up
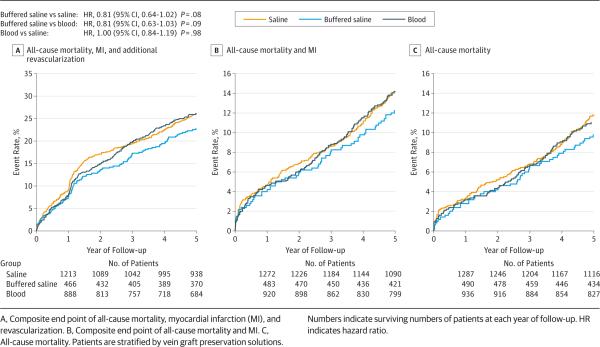
The Kaplan-Meier event curves and the multivariable adjustment model for the composite of death, MI, and revascularization are displayed in Figure 2 and Table 3, respectively. We found a nonsignificant trend toward lower rates of death, MI, or revascularization in patients who had vein grafts preserved in buffered saline solutions compared with those preserved in saline (hazard ratio, 0.81 [95% CI, 0.64-1.02; P = .08]) and blood (0.81 [0.63-1.03; P = .09]) solutions. As shown in Table 3, this trend was not evident for the composite of death or MI or for death alone during the 5-year follow-up period.
Figure 2.
Kaplan-Meier Event Curves for Long-term Clinical Outcomes According to Vein Graft Preservation Solutions
Table 3.
Adjusted HRs for 5-Year Clinical Events Among Vein Graft Preservation Solution Groups
| Vein Graft Preservation Solution Group | Outcome | |||||
|---|---|---|---|---|---|---|
| Death, MI, or Revascularization | Death or MI | Death | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Blood vs saline | 1.00 (0.84-1.19) | .98 | 0.91 (0.71-1.16) | .43 | 0.86 (0.66-1.13) | .28 |
| Buffered saline vs saline | 0.81 (0.64-1.02) | .08 | 0.85 (0.61-1.17) | .31 | 0.78 (0.54-1.11) | .17 |
| Buffered saline vs blood | 0.81 (0.63-1.03) | .09 | 0.93 (0.66-1.30) | .68 | 0.90 (0.62-1.31) | .59 |
Abbreviations: HR, hazard ratio; MI, myocardial infarction.
Discussion
Coronary artery bypass graft surgery relieves angina and improves survival in selected patients with coronary artery disease. Although CABG surgery has been much studied, many of the individual components of this procedure have not. The principal mechanisms thought to limit the benefits of CABG surgery are progression of atherosclerosis and graft failure. Although the mechanisms of VGF are not completely understood, endothelial damage during vein graft harvesting and implantation is known to be an important cause of acute and intermediate VGF.8,9 Although significant attention has been given to various harvesting techniques to minimize trauma,10 intraoperative graft preservation solutions have not been investigated in recent clinical studies. The lack of evidence is illustrated by a lack of consensus and variation in the use of vein graft preservation solutions. We investigated the association among 3 types of intraoperative vein graft preservation solution and vein graft patency and clinical outcomes after CABG surgery. We found that patients whose vein grafts were stored in a preservation solution that contained buffered saline had better vein graft patency rates and trends toward improved clinical outcomes during a 5-year follow-up. As a negative control, postoperative events that are unlikely to be mediated by graft failure—such as stroke, reoperation for bleeding, mediastinitis, and postoperative atrial fibrillation—were indeed all similar across groups. This similarity suggests that our findings are less likely to be due to confounding factors, such as surgical skill, and more likely due to the choice of vein graft preservation solution.
To our knowledge, the only other similar human in vivo study in patients undergoing CABG surgery was performed 3 decades ago.11 In 1982, Catinella et al11 showed that VGF rates at the 10-day follow-up were significantly higher for grafts preserved in autologous blood compared with a buffered saline solution (20% vs 7%; P < .01). Although the sample size was smaller and a different follow-up interval was chosen, these findings are aligned with the angiographic observations from our analysis.
A number of ex vivo studies have investigated the effect of intraoperative solutions on vein graft endothelial function using saphenous vein graft segments. Although Gundry et al12 showed that storage in a saline solution had deleterious effects on endothelial function compared with preservation in autologous blood, other studies13-18 aiming to substantiate these findings have shown conflicting results. Other studies9,15,16,19-23 have investigated the effect of storage in various buffered saline solutions on vessel wall tension and endothelial function in discarded vein graft segments and overall have shown improved endothelial function after preservation in a buffered saline storage solution compared with saline- and/or blood-based media.
Although VGF may result from a multitude of factors, the marked differences we found among patients who had grafts treated with buffered solutions compared with saline-and blood-based solutions in 1-year VGF rates suggest that graft preservation solutions are important. Endothelial integrity seems to play a crucial role in the ability of vein grafts to adapt quickly to hemodynamic forces, shear stress, and radial wall stress of a high-pressure, pulsatile arterial system.24 Adaptation of vein grafts with functional endothelium occurs via early lumen dilation followed by subsequent wall thickening and stiffening of the vascular wall. Anything that causes injury to the fragile endothelial layer of vein graft conduits, whether vein graft harvesting, preservation media, excessive manipulation in preparation for bypass, or ischemia and reperfusion injury, results in an inflammatory response within the vessel wall, loss of endothelial functioning, and thereby vasoactive impairment to the response to hemodynamic changes.25-30 This endothelial pathway may then result in early VGF, neointimal hyper-plasia, and subsequent VGF and potential adverse clinical outcomes.27,31,32
This study was a retrospective, nonrandomized, observational analysis of clinical trial data. Models were developed to adjust for multiple covariates associated with each outcome, and early postoperative events were similar across groups, making confounding less likely. The potential for unmeasured confounding still exists, however, especially because covariates associated with the decision to use a particular graft preservation solution may have been provider based and not collected in this study. Information on (cardiac) cause of death was not available. The use of a pressure-mediated delivery system was mandated per protocol and might affect the generalizability of our results. However, because this device was used in all patients, it is unlikely to have affected the relationship between preservation solution and outcome. Total duration of exposure to the storage solution and temperature of the solution were not documented, nor were differences in distension pressure during flushing, which may have affected vein graft patency. The potential effect of additives to preservation solutions could not be explored because of sample size limitations. Finally, the PREVENT IV trial enrolled patients during 2002 and 2003, and treatment patterns, including preferences for and methods of vein graft preservation, may have evolved over time.
Conclusions
Patients undergoing CABG surgery whose vein grafts were preserved intraoperatively in a buffered saline solution had lower rates of VGF and a trend toward improved clinical outcomes compared with patients whose grafts were preserved in blood-or saline-based solutions. These hypothesis-generating findings may have important implications for the care of patients undergoing CABG surgery and should be further investigated in adequately sized randomized clinical studies.
Supplementary Material
Acknowledgments
Dr Alexander has received grants from Bristol-Myers Squibb, Merck, and Regado Biosciences; travel support from Bristol-Myers Squibb; and consulting fees from Bristol-Myers Squibb, Pfizer, Merck, AstraZeneca, Boehringer-Ingelheim, Ortho-McNeil-Janssen Pharmaceuticals, PolyMedix, Regado Biosciences, Bayer, and Daiichi Sankyo. Dr Brophy has received grant 2RO1 HL070715 from the National Institutes of Health (NIH) to study prevention of vein graft spasm and has a financial interest in Vasoprep Inc. Drs Alexander, Williams, and Ferguson are supported in part by grant U01-HL088953 from the NIH Cardiothoracic Surgical Trials Network. Dr Peterson has served as principal investigator of the Data Analytic Center for the American Heart Association Get With the Guidelines; has received research grants from Johnson & Johnson, Eli Lilly, and Janssen Pharmaceuticals; and has served as a consultant to Boehringer Ingelheim, Johnson & Johnson, Medscape, Merck, Novartis, Ortho-McNeil-Janssen, Pfizer, Westat, the Cardiovascular Research Foundation, WebMD, and United Healthcare. Dr Califf reports receiving research funding from the Bristol-Myers Squibb Foundation and Novartis and consulting or other services (including continuing medical education [CME] or other non-CME) from Bayer, Bristol-Myers Squibb Foundation, CV Sight, LLC, DSI-Lilly, Gambro, Heart.org, Janssen R&D, LLC, Kowa, and Novartis. Dr Harrington has received research funding from Abbott Laboratories, Abiomed, Allied Clinical Management Cardiovascular, and Aastrom Biosciences. Dr Lopes has served as a consultant for and is a member of the boards of Boehringer-Ingelheim and Bristol-Myers Squibb and has received grants from AstraZeneca, Boehringer-Ingelheim, and Daiichi Sankyo.
Funding/Support: This study was supported in part by Somahlution, Inc.
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00042081
Author Contributions: Dr Lopes had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Studyconceptanddesign:Harskamp,Alexander,Lopes. Acquisition, analysis, or interpretation of data: All authors.
Draftingofthemanuscript:Harskamp,Schulte,Williams. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Schulte.
Obtained funding: Lopes.
Administrative, technical, or material support: Harskamp, Williams, Schulte.
Study supervision: Alexander, Brophy, Mack, Peterson, Gibson, Kouchoukos, Harrington, Lopes.
Additional Contribution: Peter Hoffmann, BA, Duke Clinical Research Institute, provided excellent editorial assistance. He did not receive compensation from the sponsor for this role.
Conflict of Interest Disclosures: No other disclosures were reported.
Correction: This article was corrected on August 6, 2014, to fix the subtitle.
REFERENCES
- 1.Deb S, Cohen EA, Singh SK, Une D, Laupacis A, Fremes SE, RAPS Investigators Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol. 2012;60(1):28–35. doi: 10.1016/j.jacc.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257(5):824–833. doi: 10.1097/SLA.0b013e318288c38d. [DOI] [PubMed] [Google Scholar]
- 3.Tsakok M, Montgomery-Taylor S, Tsakok T. Storage of saphenous vein grafts prior to coronary artery bypass grafting: is autologous whole blood more effective than saline in preserving graft function? Interact Cardiovasc Thorac Surg. 2012;15(4):720–725. doi: 10.1093/icvts/ivs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JH, Hafley G, Harrington RA, et al. PREVENT IV Investigators Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JH, Ferguson TB, Jr, Joseph DM, et al. PREVENT IV Investigators The Project of Ex-vivo Vein Graft Engineering via Transfection IV (PREVENT IV) trial: study rationale, design, and baseline patient characteristics. Am Heart J. 2005;150(4):643–649. doi: 10.1016/j.ahj.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Lopes RD, Mehta RH, Hafley GE, et al. Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) Investigators Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation. 2012;125(6):749–756. doi: 10.1161/CIRCULATIONAHA.111.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes RD, Williams JB, Mehta RH, et al. Edifoligide and long-term outcomes after coronary artery bypass grafting: Project of Ex-vivo Vein Graft Engineering via Transfection IV (PREVENT IV) 5-year results. Am Heart J. 2012;164(3):379–386. e1. doi: 10.1016/j.ahj.2012.05.019. doi:10.1016/j.ahj.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamm P, Juchem G, Milz S, Reichart B. Continuous graft perfusion: optimizing the quality of saphenous vein grafts. Heart Surg Forum. 2002;5(suppl 4):S355–S361. [PubMed] [Google Scholar]
- 9.Wilbring M, Tugtekin SM, Zatschler B, et al. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur J Cardiothorac Surg. 2011;40(4):811–815. doi: 10.1016/j.ejcts.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg. 2002;73(4):1189–1195. doi: 10.1016/s0003-4975(02)03425-2. [DOI] [PubMed] [Google Scholar]
- 11.Catinella FP, Cunningham JN, Jr, Srungaram RK, et al. The factors influencing early patency of coronary artery bypass vein grafts: correlation of angiographic and ultrastructural findings. J Thorac Cardiovasc Surg. 1982;83(5):686–700. [PubMed] [Google Scholar]
- 12.Gundry SR, Jones M, Ishihara T, Ferrans VJ. Optimal preparation techniques for human saphenous vein grafts. Surgery. 1980;88(6):785–794. [PubMed] [Google Scholar]
- 13.Bush HL, Jr, Jakubowski JA, Curl GR, Deykin D, Nabseth DC. The natural history of endothelial structure and function in arterialized vein grafts. J Vasc Surg. 1986;3(2):204–215. doi: 10.1067/mva.1986.avs0030204. [DOI] [PubMed] [Google Scholar]
- 14.Lawrie GM, Weilbacher DE, Henry PD. Endothelium-dependent relaxation in human saphenous vein grafts: effects of preparation and clinicopathologic correlations. J Thorac Cardiovasc Surg. 1990;100(4):612–620. [PubMed] [Google Scholar]
- 15.Chester AH, O'Neil GS, Tadjakarimi S, Borland JA, Yacoub MH. Effect of peri-operative storage solution on the vascular reactivity of the human saphenous vein. Eur J Cardiothorac Surg. 1993;7(8):399–404. doi: 10.1016/1010-7940(93)90002-s. [DOI] [PubMed] [Google Scholar]
- 16.Santoli E, Di Mattia D, Boldorini R, Mingoli A, Tosoni A, Santoli C. University of Wisconsin solution and human saphenous vein graft preservation: preliminary anatomic report. Eur J Cardiothorac Surg. 1993;7(10):548–552. doi: 10.1016/1010-7940(93)90055-g. [DOI] [PubMed] [Google Scholar]
- 17.Weiss DR, Juchem G, Kemkes BM, Gansera B, Nees S. Extensive deendothelialization and thrombogenicity in routinely prepared vein grafts for coronary bypass operations: facts and remedy. Int J Clin Exp Med. 2009;2(2):95–113. [PMC free article] [PubMed] [Google Scholar]
- 18.Dumanski A, Sopel M, Pelczar M, Szłapka M, Kustrzycki W, Zabel M. Influence of pressure on the endothelium of the saphenous vein coronary artery bypass graft. In Vivo. 2007;21(5):785–789. [PubMed] [Google Scholar]
- 19.Cavallari N, Abebe W, Mingoli A, et al. Functional and morphological evaluation of canine veins following preservation in different storage media. J Surg Res. 1997;68(2):106–115. doi: 10.1006/jsre.1996.4981. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez AM, Wooldridge TA, Boerboom LE, Olinger GN, Almassi GH, Rusch NJ. Comparison of saphenous vein graft relaxation between Plasma-Lyte solution and normal saline solution. J Thorac Cardiovasc Surg. 1994;107(6):1445–1453. [PubMed] [Google Scholar]
- 21.Hickethier T, Dämmrich J, Silber RE, Finster S, Elert O. Ultrastructural investigations for reducing endothelial cell damage of vein grafts during CABG-operation and practical consequences. J Cardiovasc Surg (Torino) 1999;40(1):71–76. [PubMed] [Google Scholar]
- 22.Thatte HS, Biswas KS, Najjar SF, et al. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg. 2003;75(4):1145–1152. doi: 10.1016/s0003-4975(02)04705-7. [DOI] [PubMed] [Google Scholar]
- 23.Wilbring M, Tugtekin SM, Zatschler B, et al. Preservation of endothelial vascular function of saphenous vein grafts after long-time storage with a recently developed potassium-chloride and N-acetylhistidine enriched storage solution. Thorac Cardiovasc Surg. 2013;61(8):656–662. doi: 10.1055/s-0032-1311549. [DOI] [PubMed] [Google Scholar]
- 24.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009;50(5):1063–1070. doi: 10.1016/j.jvs.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 26.Ehsan A, Mann MJ, Dell'Acqua G, Tamura K, Braun-Dullaeus R, Dzau VJ. Endothelial healing in vein grafts: proliferative burst unimpaired by genetic therapy of neointimal disease. Circulation. 2002;105(14):1686–1692. doi: 10.1161/01.cir.0000013775.02396.93. [DOI] [PubMed] [Google Scholar]
- 27.Tsui JC, Souza DS, Filbey D, Karlsson MG, Dashwood MR. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel “no-touch” technique: potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35(2):356–362. doi: 10.1067/mva.2002.121072. [DOI] [PubMed] [Google Scholar]
- 28.Dashwood MR, Savage K, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80(3):939–944. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pharmacologic relaxation of vein grafts is beneficial compared with pressure distention caused by upregulation of endothelial nitric oxide synthase and nitric oxide production. J Thorac Cardiovasc Surg. 2006;132(4):925–932. doi: 10.1016/j.jtcvs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82(4):1458–1464. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Davies MG, Klyachkin ML, Dalen H, Massey MF, Svendsen E, Hagen PO. The integrity of experimental vein graft endothelium: implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7(2):156–165. doi: 10.1016/s0950-821x(05)80756-x. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y, Suehiro S, Becker AE, Kinoshita H, Ueda M. Role of endothelial cell denudation and smooth muscle cell dedifferentiation in neointimal formation of human vein grafts after coronary artery bypass grafting: therapeutic implications. Heart. 2000;83(1):69–75. doi: 10.1136/heart.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.